Abstract
Emerging scientific advances in microbial research linking estrogens and the gut-skin microbiome in reference to dermal health are featured in this narrative review of journal reports and reviews from January 2018 through February 2022. Background information on advances in microbial research along with defining the microbiota and microbiome is presented in brief. The development of and factors that influence the gut microbiome in health and disease as well as the intrinsic and extrinsic factors influencing the skin microbiome and skin aging are summarized. New information on the development and changes of organ microbiomes have exposed similarities between skin and gut structure/function, microbial components/diversity/taxonomy and how they impact the immune response for combating disease and enhancing wellness. Estrogens promote health and support homeostasis in general and directly impact dermal health. Moreover, the gut, based upon the level of the microbial enzyme β-glucuronidase, which regulates estrogen’s enterohepatic recirculation, constitutes a gut-skin microbial axis. This axis revolves around the systemically available estrogen to support immune function, counteract inflammation and oxidative stress, and decrease the risk of hormone-dependent skin cancers. These data support the direct effect of estrogens on skin health and the interaction of diet on dermal health via effects on the gut microflora. Finally, the potential for bioactive botanicals containing phytoestrogens or selective estrogen receptor modulators (SERMs) to evade the effects of gut β-glucuronidase expressing flora is proposed that may have a positive impact on skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this narrative review? |
The emerging field of microbiomics has focused on the gut. This has put into relief the gut-skin axis, which is implicated in dermal health and wellness. This overview focuses on the emerging evidence of the past 4 years and provides an update that examines the direct actions of estrogens on skin and the connection to estrogens in the gut-skin microbiome axis, which affect immune function, regulating inflammation and oxidative stress to augment skin health |
What was learned from the review? |
In addition to the direct actions of estrogens on skin, as a substrate for bacteria, estrogens contribute to gut microbiome diversity. Estrogen metabolism by gut bacteria affects not only cutaneous health, but also hormone-dependent disorders/cancers, especially after menopause |
Diet is paramount in maintaining the gut-skin axis, in part through plant SERMs. Therefore, supplementation with bioactive botanicals (phytoestrogens, SERMs) may be employed to boost general health and improve dermal parameters, especially during aging and after menopause |
Introduction
The purpose of this narrative overview is to provide updated perspectives on the gut microbiome with emphasis on estrogen's direction actions on the skin and the estrobolome (gut-skin axis) in dermal health using foundational figures and graphics that depict recent literature results covering the last 4 years. This overview updates the concepts and principles of: (1) the gut microbiome in a focused manner (along with a brief summary of how the gut microbiome influences human disease/wellbeing, including skin conditions), while (2) incorporating the direct systemic chemical messenger effects and indirect actions of estrogens. The estrobolome (via β-glucuronidase) is illustrated to illuminate the complex interactions between and among steroid hormones and organ systems, (3) outlining how the gut-skin axis enhances dermal repair by regulating immune function and controlling inflammation, and (4) the influence of dietary intake, especially plant-based, SERM-containing food sources that can alter the gut-skin axis are briefly addressed.
Journal articles and reviews (with emphasis over the last 4 years) from January 2018 through February 2022 were interrogated using the keywords: diet, estrogens, estrobolome, isoflavonoids, gut microbiome, gut-skin axis, phytoestrogen, skin, and/or keyword combinations. The following databases were utilized: PubMed, Science Direct and Scopus and Google Scholar. Background references included topics on aging, dietary intake, estrobolome, estrogens, gut-skin axis, isoflavonoids, microbiome, phytochemicals, phytoestrogens, polyphenols, skin, and/or combinations. This article is based on previously conducted studies and does not contain new data/results (studies) of human participants or animals performed by the authors.
Advances in Microbial Research
Our understanding of the microbiome has been facilitated by the evolution of various technologies and tools, such as DNA content (metagenomics), RNA expression (metatranscriptomics), protein expression (metaproteomics) and small molecule metabolites (metabolomics) over the last 2 decades, which have revealed the influence of the microbiome on human health and disease (Fig. 1) [1,2,3,4].
Tools used in microbial research. DNA panel: define: Who is there, and what can they do? RNA panel: define: What is the response, and what pathways are activated? Protein panel: define: what are the host interactions, and what proteins are produced? Metabolites panel: define: what are the metabolites and their function? Adapted from [3] with permission
The history of microbiome research from the 1670 discovery of microorganisms by Anthony van Leuwenhoek, the “Father of Microbiology,” until the present day [of the Human Microbiome Project (HMP) sponsored by the National Institutes of Health (NIH), a 2007 extension of the Human Genome Project)] highlights how a person’s microbiome is critical for immune system development, function, and homeostasis for health [3,4,5]. This is to say, various studies have shown the human gut microbiome to be a potential controller of wellness and disease, including skin disorders such as psoriasis and atopic dermatitis [5,6,7,8], whereas dysbiosis (altered composition of microbes has a cascading impact on the immune system) can contribute to the susceptibility to infectious disease and chronic illness among human organ systems [4,5,6,7,8]. For example, dysbiosis is associated with increased oxidative stress, inflammation, and damage to DNA repair mechanisms [4,5,6,7,8]. The results from the Human Microbiome Project revealed that there are approximately 2,000,000 microbial genes, approximately 100 times the 20,000 human genes [4,5,6,7,8]. From another perspective, the human body contains trillions of microorganisms outnumbering human cells by ten to one. Because of their small size, microorganisms make up only about 1–3% of the body's mass (in a 200-pound adult, that is 2–6 pounds of bacteria) [4,5,6,7]. Among the microbes present in humans there are ∼1400 known species of human pathogens (including viruses, bacteria, fungi, protozoa, and helminths), and although this may seem like a large number, human pathogens account for much less than 1% of the total number of microbial species on the planet [9]. Finally, it is well established that estrogens have a direct positive impact on skin health. However, as reported in a later section, the influence of estrogens involved in the estroblome of the gut microbiome via β-glucuronidase action impacts the skin microbiome [10, 11]. This estrogen metabolism in turn benefits dermal health by enhancing immune function and wound healing and counteracting inflammation as well as protecting against cancer risk [10, 11].
The Microbiota and Microbiome
The term “microbiota” originated from the ancient Greek using the combination “micro” (small) and “biota,” which means the living organisms in a particular location. “Microbiome” is also from the ancient Greek, combining “micro” (small) with the term “bios” (life); modifying the ending to “ome” classifies species living in a specific location [3, 4, 6].
Often the labels microbiota and microbiome are used interchangeably, but these two terms have subtle differences (see recent review for updated microbiome definitions) [3]. In general, “microbiota” refers to the microorganisms according to different kingdoms (Prokaryotes: Bacteria, Archaea; Eukaryotes: Protozoa, Fungi, etc.) found within a specific environment [3,4,5,6,7]; see Fig. 2. For example, bacteria, archaea, viruses, and fungi are found among the skin and gut microbiota [4,5,6,7,8]. The skin and gut microbiome is usually dominated by bacteria, which are 3.5 billion years old [5,6,7,8], being the first members of the family tree of life of all living things on Earth [12, 13]. In general, the gut microbiome is more abundant and diverse compared to aggregate cutaneous tissue sites [4,5,6,7,8]. However, archaea (similar to bacteria, but having unique cell membrane, flagella, and intron characteristics) have been reported in skin. Older or pre-pubertal individuals (> 60 or < 12 years of age) displayed the highest abundance of Archaea [14]. Viruses have a special taxonomic classification because they are not plants, animals, or prokaryotic bacteria. They are very diverse and generally classified by phenotypic characteristics such as morphology, nucleic acid type (DNA or RNA), mode of replication, host organisms, and type of disease they cause [15].
The microbiota and microbiome. Diagram representing the microbiome containing both the microbiota (community of microorganisms) and their “theater of activity” (structural elements, metabolites/signal molecules, and the surrounding environmental conditions). Biome: a well-defined habitat with distinct bio-physio-chemical properties. Adapted from [3] with permission
“Microbiome” refers to the collection of microbial genes (genomes) represented in a specific environment [3, 4, 6]. This covers the whole spectrum of molecules produced by the microorganisms including “their theater of activity” composed of (1) microbial structural elements such as proteins/peptides, lipids, polysaccharides, and nucleic acids, (2) mobile genetic elements, and (3) microbial metabolites (e.g., signaling molecules, toxins, organic molecules) among the environmental conditions [3]; see Fig. 2. Finally, the field of microbiome research started with microbial ecology, and many investigative fields (agriculture, food science, biotechnology, mathematics, various areas of biology, and medicine) have utilized different or sometimes similar terminology to describe novel findings, mechanisms, and concepts [3,4,5,6,7].
Development of and Regulation of the Gut Microbiome
Traditionally, the “microbiome” has been addressed in the context of the gut flora [16]. For example, Fig. 3 displays the factors that influence the founding gut microbiota, starting with gestation through adulthood [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Before birth, a sterile environment exists, which elucidates the germ-free mouse model [39]. The mode of delivery at birth is through the vagina’s flora or through a sterile abdominal wound that may lead to seeding by the mother’s skin flora [7, 17, 24, 35]. Also, other environmental influences (at/after delivery care/physical contact) will determine the initial gut microbiota (founder species). Early dietary intake such as breast vs. bottle formula [17, 24, 39] and other physical contact [4, 5, 33, 35, 38] builds up the gut microbiome through 6 months of age (Fig. 3). During infancy, new strains usually outcompete the resident flora leading to diversity of the early microbiota. Diet and xenobiotics such as antibiotics are primary influences.

Adapted from National Academies of Sciences, Engineering, and Medicine (US) [18] with permission
Development of and factors that influence the gut microbiome. There are variations in the gut microbiome in its composition and function among different aged populations. Moreover, over the human life span, the gut microbiome changes as influenced by multiple factors as shown in this figure. For example, age and diet play primary roles in the variation observed in the gut microbiome (while water and nutrient availability drive the site-specific community states of the skin microbiome). Other factors such as genetics, gender, socioeconomic status, disease state, geography, pregnancy, and environmental exposures can also play a role in shaping the composition and function of the human gut microbiome.
By 3 years of age, the gut microbiome foundation is recognized as having alpha (number of types of microbes in a single sample) and beta (how different types are distributed among samples) diversity [4, 17, 35, 38]. For example, under similar environments and dietary intakes, ethnic background contributes to differences in alpha diversity, but not beta diversity of the gut microbiota in school children [40]. In adulthood, the microbial community continues to change because of diet, hormones, environmental factors, etc. [20,21,22,23, 25, 27,28,29, 31, 34, 36, 41]. However, this occurs at a slower rate compared to childhood [5, 17, 35], where highly distinct microbiota differentiates (Fig. 3). With aging, especially in the elderly, there is a substantially different gut microbial community with lower diversity compared to younger adults [5, 17, 35]. These changes occur especially after menopause [22, 28, 31] and can lead to age-related disorders like osteoporosis and osteopenia [34].
The Gut Microbiome in Health and Disease Interacts with Other Sites
Briefly, the gut microbiome has been implicated in brain [4, 5, 33, 35, 42,43,44], cardiovascular [33, 35, 45], respiratory [46,47,48], and skin health and dermal diseases [6,7,8, 19, 30, 32] (Fig. 4). Additionally, the following conditions or disorders are associated with the gut microbiome: aging [49], allergic and immune [4, 5, 17, 19], cancer [4, 11, 22, 33, 35], diabetes and metabolic syndrome [5, 17, 33, 35], gastrointestinal disorders [5, 33, 35], infection [4, 5, 26], and rheumatoid arthritis [33, 50] (Fig. 4).
Skin Aging and the Skin Microbiome (Intrinsic and Extrinsic Influencing Factors)
Skin aging, determined by intrinsic (chronological) and extrinsic (exposure to ultra-violet light or photo-aging), along with the importance of estrogen in maintaining dermal health has been reviewed in detail elsewhere [51,52,53,54,55,56,57,58,59]. Research on the skin microbiome is an emerging area of study [6,7,8, 19, 30], and more is known about the microbiome of the gut than the skin. Recent reports suggest the skin microbiome has both intrinsic and extrinsic factors that modulate the expression and maintenance of the dermal microbiota [60,61,62]. The intrinsic parameters shaping the skin microbiome include age, genetics, gender, immunity, hormones, sleep, stress, and metabolism [60,61,62]. For example, the skin site is a major determinant of the skin microbiome in healthy skin. Also, the skin microbiome at the same anatomical skin site from different individuals is more similar than the skin microbiome at different skin sites of the same individual [6, 8, 63]. Conversely, the extrinsic factors modulating the skin microbiome include many of the extrinsic elements of skin aging like sun-exposure, hygiene, beauty routine (cosmetics, etc.), and availability of nutrients for skin microbial organisms [60,61,62,63].
Similarities of the Skin and Gut Microbiome (Structure/Function, Microbial Components Including Major Bacteria Phyla, and Immune Advantage)
The similarities between the skin and gut include: (1) surface area (25 m2 vs 30 m2), (2) microbial cell abundance (1012 vs 1014 microbial cells), (3) tissue-cell types, (4) rapid cellular turnover (10–14 vs 3–5 days), (5) rich vascular and highly innervated structures for a bidirectional link between the exogenous environment that are involved in barrier as well as immune and neuroendocrine functions [7, 8, 30, 64]. In contrast to the stratified squamous epithelium of the skin (which contains keratin in the stratum corneum, which is a formidable barrier to most microorganisms at a pH around 4.7–5.5), the intestinal barrier is composed of a single layer of columnar epithelial cells (Fig. 5) [7, 8, 65]. However, this single layer of intestinal epithelial cells (IECs) or enterocytes is made of diverse cell types with properties of absorption, secretion, and immune function [7, 8]. For example, intestinal goblet cells secrete mucus to form a protective barrier between the intestinal epithelium and luminal contents from pathogenic agents, while also maintaining a healthy relationship between the intestinal tissue and bacteria. This coating is beneficial to metabolizing (fermenting) certain food molecules such as isoflavonoids and short chain fatty acids (SCFAs) as a source of hormonal communications and energy, respectively (Fig. 5) [7, 8, 66].
Similarities between the skin and gut microbiome (structure/function, microbial components, and immune advantage). The gut microbiome represents the colon in this figure. T-helper (Th) cells provide helper functions to other cells of the immune system; regulatory T (Treg) cells (a specialized subpopulation of T cells that act to suppress immune response); B cells (B-lymphocyte, hormonal immunity); IgA (immunoglobulin A, first line of defense in the resistance against infection via inhibiting bacterial and viral adhesion)
In brief, the skin (1) provides a physical barrier, preventing water loss and modulating body temperature and sensory information via mechanoreceptors, and it supports vitamin D synthesis; (2) provides immune function [via Langerhans epidermal, dermal and gut dendritic, T-help (Th), Treg, and B cells], and (3) produces antimicrobial peptides/proteins and mast cells, which form the first line of defense against antigens, bacteria, and parasites (Fig. 5) [7, 8, 67]. The expression profiles of the major bacteria phyla between the skin and gut microbiome (colon) are distinct, as shown in Fig. 5 [35, 61]. Finally, the details of the skin and gut microbiota have been reported elsewhere [4, 7, 8, 30, 32, 35, 61] including the presence of mites on the skin [63].
Estrogens Supporting Homeostasis and the Beneficial Interaction of the Skin and Gut Microbiomes
Estrogens induce and regulate many important functions. Several functions include: metabolic homeostasis, cell proliferation and death, liver protein expression, lipid metabolism, energy balance, glucose metabolism, immune and cardiovascular regulation, gonadotropin feedback, gametogenesis and reproduction, brain-neuronal development/memory processing and repair/neurodegeneration, and bone growth/maintenance. This review focuses on estrogen and the gut and skin microbiome’s effects on dermal health [11, 51, 55, 59, 68,69,70].
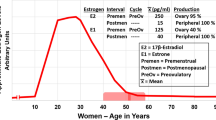
For example, 17β-estradiol is the most potent sex steroid hormone in controlling many aspects of health and wellbeing [11, 51, 55, 59, 68, 69]. In women, physiological levels of circulating 17β-estradiol are first seen at puberty and peak in the late 20s. Thereafter, estrogen levels exhibit wide swings during the decade preceding menopause and then decline to near zero after menopause [11, 51, 71]. Thereafter, the estrogen, estrone, which is seven times less potent than 17β-estradiol [11, 68, 69], is produced from the post-menopausal ovary, which secrets androstenedione. This estrone biosynthesis from androstenedione occurs in peripheral sites like adipose tissue [11, 51, 71].
The estrogen receptors are the primary effectors in estrogen action in the skin via the systemic circulation. Estrogens can bind estrogen receptor alpha or estrogen receptor beta to regulate gene expression of protein [11, 51, 69]. Also, the membrane-bound estrogen receptor known as a G protein-coupled seven transmembrane (GPR30) receptor is present in skin (but not well characterized) and directly affects intracellular signaling cascades [11, 51, 69]. Notably, among these estrogen receptors, estrogen receptor beta is more prevalent and predominates in skin cells in keratinocytes and other dermal structures [51, 53, 55, 59]. Finally, an updated perspective on the role of estrogens in skin aging has been reviewed by Lephart and Naftolin (in press, Clinical, Cosmetic and Investigational Dermatology, 2022).
Since the estrogens are subject to gut flora regulated metabolism and excretion as described in the next section, estrogen plays an important role in the gut, which affects all organs in the body including the skin. The gut microbial enzyme(s) regulates not only hormonal signaling involved in female health, disorders, and cancers, but estrogens also modulate the signals of the gut-skin axis (bacterial-host interactions) [7, 8, 10, 21, 22].
Microbiome- Gut-Skin Axis and Influence on Skin Health (Estrogens and the Estrobolome)
There are multiple reports and reviews on the gut-skin axis, especially describing the skin and gut microbiome and their roles in dermatological conditions (also see Fig. 3) [5,6,7,8, 30, 72, 73]. For example, the dysbiosis of the skin microbiome may trigger the pathogenic potential of dermal disorders and disease such as acne, atopic dermatitis, and psoriasis, which have been reported [7, 8, 35, 61]. The role of estrogens in the initial concept called the estrobolome was first elucidated by Adlercreutz’s laboratory in the 1960s through the 1980s. Briefly, the gut microbiota regulates estrogen metabolism, thereby contributing to the proportions of recirculated vs excreted estrogens and estrogen metabolites [74, 75]. As early as 2011, Plottel and Blaser proposed the estrobolome as the aggregate of enteric bacterial gene products that were capable of metabolizing estrogens [76].
More recently, it was shown that estrogens regulate the gut microbiome in a positive manner by increasing the diversity of the gut microbiota and augmenting the enzymes that metabolize estrogens [10, 29, 76]. For example, the gut microbiota contains some bacterial species that express β-glucuronidase activity. This enzyme is involved in estrogen metabolism [77, 78]; see (Fig. 6).
Gut microbial beta (β)-glucuronidase enzymes metabolize and recycle estrogens. Like all steroid hormones, estrogens readily diffuse across the cell membrane. When they reach the liver, during phase II metabolism, UDP-glucuronosyltransferase enzymes (UGTs) conjugate the steroids, making them water-soluble compounds. These conjugated steroids are then excreted in the bile, entering the small intestine from which they pass into the stool. Alternatively, the gut microbial β-glucuronidase enzymes deconjugate steroids, which allows them to enter the gut and be recirculated in the blood. This figure depicts the so-called enterohepatic recirculation of 17beta (β)-estradiol. Other estrogens such as estrone (and estriol) can go through the same sequence. See text and Fig. 7 for further detail. Recycling allows unbound estrogens to be recirculated through the systemic circulation. Thus, increased gut microbial β-glucuronidase enzymatic activity potentially provides a protective role in preventing disease and enhancing health. Adapted from [10] with permission
It has been reported that fecal β-glucuronidase is negatively proportional to the total estrogen levels in the circulation (Fig. 7) [78]. Also, in experimental animal studies, 17β-estradiol supplementation changed the gut microbiota [79].
Thus, estrogens induce gut microbiome diversity, thereby decreasing β-glucuronidase availability and increasing estrogen excretion. On the other hand, low estrogen levels decrease gut microbiome diversity, which increases β-glucuronidase activity and re-circulation of the estrogens [10, 77, 78] (Fig. 6). This has powerful implications for systemic homeostasis in organs such as the skin. For example, estrogen maintains protective epidermal keratinization, maintains hydration in the subdermal tissues, and enhances the immune response [51,52,53,54, 59, 79, 80]. Therefore, an estrobolome enriched in β-glucuronidase enzymes that promote estrogen metabolism deconjugation reactions may result in greater absorption of free estrogens that may cause a greater risk of hormone-dependent cancers and other diseases (Fig. 6) [10, 22, 77, 78, 81,82,83]. Notably, one of the prominent roles of the human microbiome in the regulation of endogenous estrogens defines the most important risk factor in breast cancer development especially in postmenopausal women [78]. Also, in postmenopausal women, microbial diversity in the distal gut was positively associated with the ratio of hydroxylated estrogen metabolites to parent estrogens (such as catechol estrogens). As well, phytoestrogen consumption to modify the gut microbiome may provide an alternative to current estrogen-deficient conditions [22, 28, 82, 83].
Diet, Phytoestrogens, the Gut-Skin Axis, and Improved Dermal Health
Since the gut microbiome has an immense impact on health and disease, the diet plays a fundamental role in shaping microbiome composition and function. This suggests a food regimen designed toward better feeding or enhancing the gut microbiome [84]. A diet rich in fat or protein has been found to be associated with higher fecal β-glucuronidase activity, whereas fiber consumption decreased its activity [77, 78]. Plants are a good source of fiber. They also contain non-steroidal compounds that interact with the estrogen receptors and produce estrogen-like effects (selective estrogen receptor modulators; SERMs). Phytoestrogens or polyphenolic compounds that are similar to 17β-estradiol also have a bidirectional interaction with the gut microbiome [85, 86]. For example, the gut microbiome can metabolize them to more or less potent SERMs, which enhance the diversity of the gut microbiome [85,86,87]. Strategies to achieve significant biological active concentrations of phytoestrogens in plasma include increasing plant-based dietary intake or/and nutraceutical supplementation to improve the gut microbiome and health [88].
The significant advancements in this emerging field of plant substitution of hormonal agents suggest that plant SERMs may enhance the gut-skin axis and enhance skin health. Various reports suggest a variety of ways this may be accomplished including improved immune function by counteracting inflammation and oxidative stress as shown in Fig. 7 [7, 8, 30, 64,65,66, 75, 89]. This link between nutrition and venerated skin health includes vitamin D’s mechanism of action and its effects on the skin [90,91,92].
Figure 8 suggests ways to establish and maintain a healthy gut and skin microbiome. Several clinical studies have shown how various dietary, physical, and behavioral factors can establish and maintain a healthy gut and skin microbiome. For example, the link between the gut and skin microbiome in the expression of dermal disorders and disease has been well established [5,6,7,8, 35]. Moreover, the intakes of different diets promoting the gut and skin microbiomes [84, 89, 93] suggest a positive impact on health, in general, and for certain body organs ([33]; see Fig. 4). Finally, the various behavioral factors such as sleep, stress, and exercise that can influence the gut-skin axis to enhance health benefits have also been reported [60,61,62,63].
Moreover, several studies have provided evidence for the influence of dietary intake on health. We have long been told to eat our fruits and vegetables, but according to a recent 2017 report from the Center for Disease Control and Prevention (CDC), just 9% of America adults get their daily recommended servings of vegetables, and only 12% get the recommended amount of fruit [94]. The goal is to keep your skin healthy by consuming a diet to maintain a diverse gut microbiome that can utilize estrogens (even during menopause) and/or potentially use botanical active ingredients to supplement and boost dermal health [51, 70, 93,94,95,96,97,98]. This is especially fitting for post-menopausal women since phytoestrogens have been shown to be safe and improve bone mineral density along with markers of cardiovascular risk without adverse effects on breast, endometrial, and colorectal cancers [99,100,101] and intervascular clotting [51, 102].
Conclusions
This narrative review updates the role of estrogens in the gut-skin microbiome in reference to dermal health and skin aging (using reports and reviews published in the last 4 years from January 2018 through February 2022). Adequate estrogen is necessary for maintaining skin health. The gut microbiome regulates the level of circulating estrogen via enterohepatic circulation. As well, estrogens regulate the gut-skin axis by increasing gut microbiome diversity to insure the uptake of bile-excreted estrogen from the gut. Finally, the importance of dietary intake of SERM-rich foods and plant-derived compounds in maintaining healthy skin in women is recommended, especially with aging and after menopause.
References
Jo H-Y, Kennedy EA, Kong HH. Research techniques make sample: Bacterial 16S Ribosomal RNA gene sequencing in cutaneous research. J Invest Dermatol. 2016;136:e23–7.
Yao H, Song J, Liu C, et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE. 2010;5: e13102.
Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. https://doi.org/10.1186/s40168-020-00875-0.
Gilbert J, Blaser MJ, Caporaso JG, et al. Current understanding of the human microbiome. Nature Med. 2018;24:392–400. https://doi.org/10.1038/nm.4517.
Kho ZY, Lal SK. The human gut microbiome a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
Mazur M, Tomczak H, Lodyga M, et al. The microbiome of the human skin and its variability in psoriasis and atopic dermatitis. Adv Dermatol Allergol. 2021;38:205–9.
Sinha S, Lin G, Ferenczi K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021;39:829–39.
De Pessemier B, Grine L, Debaere M, et al. Gut-skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353. https://doi.org/10.3390/microorganisms9010353.
Editorial. Microbiology by numbers. Nat Rev Microbiol. 2011;9:628. https://doi.org/10.1038/nrmicro2644.
Ervin SM, Li H, Lim L, et al. Gut microbial β-glucuronidases reactive estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294:18586–99.
Adams S. Estrobolome disparities may lead to developing biomarkers that could mitigate cancer risk. J Nat Cancer Inst. 2016;108:djw130. https://doi.org/10.1093/jnci/djw130.
Science Alert. Here’s the tiny human twig in the tree of life. 2014. https://www.sciencealert.com/here-s-the-tiny-human-twig-in-the-tree-of-life. Accessed 16 Dec 2021.
Hug LA, Baker BJ, Anantharaman K, et al. A new view of the tree of life. Nature Microbiol. 2016;1:1–6. https://doi.org/10.1038/NMicrobiol.2016.48.
Moissl-Eichinger C, Probst AJ, Birarda G, et al. Human age and skin physiology shape diversity and abundance of Archaea on skin. Sci Rep. 2017;7:4039. https://doi.org/10.1038/s41598-017-04197-4.
Simmonds P, Aiewsakun P. Virus classification – where do you draw the line? Arch Virol. 2018;163:2037–46.
Prados-Bo A, Casino G. Microbiome research in general and business newspapers: How many microbiome articles are published and which study designs make the news the most? PLoS ONE. 2021;16(4):e04249835. https://doi.org/10.1371/journal.pone.0249835.
Sekirov I, Russell SL, Antunes CM, Finlay BB. Gut microbiome in health and disease. Physiol Rev. 2010;90:859–904.
National Academies of Sciences, Engineering, and Medicine: Division of Earth and Life Sciences; Board on Life Sciences; Board on Environmental Studies and Toxicology. Committee on advancing understanding of the implications of: environmental chemicals, the human microbiome, and health risk: a research strategy. Washington: National Academies Press; 2017. https://doi.org/10.17226/24960.
Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, Levin M, Etten EV, Horwitz P, Kozyrskyi A, Campbell DE. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 2017;10:29. https://doi.org/10.1186/s40413-017-0160-5.
Levy G, Solt I. The human microbiome and gender medicine. Gender Genome. 2018;1:123. https://doi.org/10.1177/2470289718811764.
Garcia-Gomez E, Gonzalez-Pedrajo B, Camacho-Arroyo I. Role of sex steroid hormones in bacterial-host interactions. Biomed Res. 2013. https://doi.org/10.1155/2013/928290.
Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ. Associations of the fecal microbiome with urinary estrogens and estrogens metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99:4632–40.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Hoashi M, Meche L, Mahal LK, Bakacs E, Nardella D, Naftolin F, Bar-Yam N, Dominguez-Bello MG. Human milk bacterial and glycosylation patterns differ by delivery mode. Reproductive Sci. 2016;23:902–7.
Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72.
Khanna S, Pardi DS. Clinical implications of antibiotic impact on gastrointestinal microbiota and Clostridium difficile infection. Expert Rev Gastroenterol Hepatol. 2016;10:1145–52.
Orlich MJ, Siapco G, Jung S. Vegetarian diets and the microbiome. In: Mariotti F, editor. Vegetarian and plant-based diets in health and disease prevention. London: Academic Press-Elsevier; 2017. p. 429–61. https://doi.org/10.1016/B978-0-12-803968-7.00024-1.
Vieira AT, Castello PM, Ribeiro DA, Ferreira CM. Influence of oral and gut microbiome in the health of menopausal women. Front Microbiol. 2017;8:1884. https://doi.org/10.3389/fmicb.2017.01884.
Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormones is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201.
Ellis SR, Nguyen M, Vaugh AR, Notay M, Burney WA, Sandhu S, Sivamani RK. The skin and gut microbiome and its role in common dermatological conditions. Microorganisms. 2019;7:550. https://doi.org/10.3390/microorganisms7110550.
Mayneris-Perxachs J, Arnoriaga-Rodriguez M, Luque-Cordoba D, Priego-Capote F, Perez-Brocal V, Moya A, Burokas A, Maldonado R, Fernandez-Real J-M. Gut microbiome steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8:136. https://doi.org/10.1186/s40168-010-00913-x.
Sfriso R, Claypool J. Microbial reference frames reveal distinct shifts in the skin microbiota after cleansing. Microorganisms. 2020;8:1634. https://doi.org/10.3390/microorganisms8111634.
Ju W. The gut microbiome and its impact on the brain. 4.1 pressbooks, neuroscience: Canadian 1st edition open textbook. Toronto: University of Toronto; 2020.
Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus. 2021;5: e10452. https://doi.org/10.1002/jbm4.10452.
Goodman S. Medical Cell Biology, Chapter 15, the microbiome. London: Academic Press; 2021. https://doi.org/10.1016/B978-0-12-817927-7.09993-2.
Tanes C, Bittinger K, Gao Y, Friedman ES, Nessel L, Paladhi UR, Chau L, Panfen E, Fischbach MA, Braun J, Xavier RJ, Clish CB, Li H, Bushman FD, Lewis JD, Wu GD. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29:394–407.
van der Merwe M. Gut microbiome changes induced by a diet rich in fruits and vegetables. Internat J Food Sci Nutr. 2012;72:665–9.
Sprockett D, Fukami T, Relman DA. Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol. 2018;15:197–205.
Kennedy EA, King KY, Baldridge MT. Mouse microbiota models: Comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. https://doi.org/10.3389/fphys.2018.01534.
Liu K, Zhang Y, Li Q, et al. Ethnic differences shape the alpha and but not beta diversity of gut microbiota from school children in the absence of environmental differences. Microorganisms. 2020;8:254. https://doi.org/10.3390/microorganisms8020254.
Hussain T, Murtaza G, Kalhoro DH, et al. Relationship between the gut microbiome and host-metabolism: Emphasis on hormones related to reproductive function. Animal Nutr. 2021;7:1–10.
Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer’s disease. J Alzheimer’s Dis. 2017;58:1–15.
Boziki MK, Kesidou E, Theotokis P, et al. Microbiome in Multiple Sclerosis: Where are we, what we know and do not know. Brain Sci. 2020;10:234. https://doi.org/10.3390/brainsci10040234.
Zhang F, Yue L, Fang X, et al. Altered gut microbiota in Parkinson’s disease patients/healthy spouses and its association with clinical features. Parkinsonism Related Dis. 2020;18:84–8.
Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–70.
Vaughan A, Frazer ZA, Hansbro PM, Yang IA. COPD and the gut-lung axis: the therapeutic potential of fibre. J Thorac Dis. 2019;11:S2173–80.
Frati F, Salvatori C, Incorvaia C, et al. The role of the microbiome in asthma: the Gut-lung axis. Int J Mol Sci. 2019;20:123. https://doi.org/10.3390/ijms20010123.
Thibeault C, Suttorp N, Opitz B. The microbiome in pneumonia: from protection to predisposition. Sci Transl Med. 2021;13(576):eaba0501. https://doi.org/10.1126/scitranslmed.aba0501.
Liu S, D’Amico D, Shankland E, et al. Effect of urolithin a supplement on muscle endurance and mitochondrial health in older adults, a randomized clinical trial. JAMA Netw Open. 2022;5: e2144279. https://doi.org/10.1001/jamanetworkopen.2021.44279.
Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–78.
Lephart E, Naftolin F. Menopause and the skin: old favorites and new innovation in cosmeceuticals for estrogen-deficient skin. Dermatol Ther (Heidelb). 2021;11:53–69.
Bonté F, Girard D, Archambault J-C, et al. Chapter 10, skin changes during aging. In: Harris JR, Korolchuk VI, editors., et al., Biochemistry and cell biology of aging: part II clinical sciences, subcellular biochemistry. Singapore: Springer Nature; 2019. p. 249–80.
Lephart ED. Skin aging and oxidative stress: equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31:36–54.
Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transpl. 2018;27:729–38.
Lephart ED. A review of the role of estrogen in dermal aging and facial attractiveness in women. J Cosmet Dermatol. 2018;17:282–8.
Huang AH, Chien AL. Photoaging: a review of the current literature. Current Dermatol Rep. 2020;9:22–9.
Dyer JM, Miller RA. Chronic skin fragility of aging: current concepts in pathogenesis, recognition, and management of dermatoporosis. J Clin Aesthet Dermatol. 2018;11:13–8.
Sparavigna A. Role of the extracellular matrix in skin aging and dedicated treatment- state of the art. Plast Aesthet Res. 2020;7:14. https://doi.org/10.20517/2347-9264.2019.73.
Wilkinson HN, Hardman MJ. A role for estrogen in skin ageing and dermal biomechanics. Mech Ageing Dev. 2021;197: 111513. https://doi.org/10.1016/j.mad.2021.111513.
Moskovicz V, Gross A, Mizrahi B. Extrinsic factors shaping the skin microbiome. Microorganisms. 2020;8:1023. https://doi.org/10.3390/microorganisms8071023.
Skowron K, Bauza-Kaszewska J, Kraszewska Z, et al. Human skin microbiome: impact of intrinsic and extrinsic factors of skin microbiota. Microorganisms. 2021;9:543. https://doi.org/10.3390/microorganisms9030543.
Dimitriu PA, Iker B, Malik K, Leung H, Mohn WW, Hillebrand GG. New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. MBio. 2019;10(4):e00839-e919.
Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiome. Genome Res. 2008;18:1043–50.
Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459. https://doi.org/10.3389/fmicb.2018.01459.
Lambers H, Piessens S, Bloem A, et al. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Internat J Cosmet Sci. 2006;28:359–70.
Baky MH, Elshaded M, Wessjohann L, Farag MA. Interactions between dietary flavonoids and the gut microbiome: a comprehensive review. Br J Nutr. 2021. https://doi.org/10.1017/S0007114521003627.
Boxberger M, Cenizo V, Cassir N, La Scola B. Challenges in exploring and manipulating the human skin microbiome. Microbiome. 2021;9:125. https://doi.org/10.1186/s40168-021-01062-5.
Blakemore J, Naftolin F. Aromatase: contributions to physiology and disease in women and men. Physiology (Bethesda). 2016;31:258–69.
Santen RJ, Simpson E. History of estrogen: Its purification, structure, synthesis, biological actions, and clinical implications. Endocrinol. 2019;160:605–25.
Ceccarelli I, Bioletti L, Peparini S, et al. Estrogens and phytoestrogens in body functions. Neurosci Biobehav Rev. 2022;132:648–63.
Stanczyk FZ. Production, clearance, and measurement of steroid hormones. Glob Libr Womens Med. 2009. https://doi.org/10.3843/glowm.10278.
Chen G, Chen Z, Fan X, et al. Gut-brain-skin axis in psoriasis: a review. Dermatol Ther (Heidelb). 2021;11:25–38.
Mann EA, Bae E, Kostyuchek D, et al. The gut microbiome: human health and inflammatory skin diseases. Ann Dermatol. 2020;32:265–72.
Adercreutz H. Studies on oestrogen excretion in human bile. Acta Endocrinol. 1962;42:1–220.
Adlercreutz H, Jarvenpaa P. Assay of oestrogens in human feces. J Steroid Biochem. 1982;17:639–45.
Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–35.
Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9:631552. https://doi.org/10.3389/fcell.2021.631522.
Parida S, Sharma D. The microbiome-estrogen connection and breast cancer risk. Cells. 2019;8(12):1642. https://doi.org/10.3390/cells8121642.
Kim Y-S, Kim T-H, Park ES, Fadiel A, Naftolin F. Ezrin expression and activation in hypertrophic and keloid scar. Gynecol Reprod Endocrinol Metab. 2020;1:29–36.
Naftolin F. Prevention during the menopause is critical for good health: skin studies support protracted hormone therapy. Fertil Steril. 2005;84:293–4.
Song C-H, Kim N, Nam RH, et al. β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. 2020. Sci Rep. https://doi.org/10.1038/s41598-020-69112w.
Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. https://doi.org/10.1186/1479-5876-10-253.
Baker JM, Al-Nakkah L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. https://doi.org/10.1016/j.maturitas.2017.06.025.
Ercolini D, Fogliano V. Food design to feed the human gut micrbiome. J Agric Food Chem. 2018;66:3754–8.
Hameed ASS, Rawat PS, Meng X, Liu W. Biotransformation of dietary phytoestrogens by gut microbes: a review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotech Adv. 2020;43: 107576. https://doi.org/10.1016/j.biotechadv.2020.207576.
Dey P. Gut microbiota in phytopharmacology: a comprehensive review of concepts, reciprocal interactions, biotransformations and mode of actions. Pharma Res. 2019;147: 104367. https://doi.org/10.1026/j.phrs.2019.104367.
Espin JC, Gonzales-Sarrias A, Tomas-Barberan FA. The gut microbiome: a key factor in the therapeutic effects of (ploy)phenols. Biochem Pharma. 2017;139:82–93.
Langa S, Landete JM. Strategies to achieve significant physiological concentrations of bioactive phytoestrogens in plasma. Critical Rev Food Sci Nutr. 2021;2:1–13. https://doi.org/10.1080/10408398.2021.1971946.
Anna Addor FA. Beyond photoaging: additional factors involved in the process of skin aging. Clin Cosmet Invest Dermatol. 2018;11:437–43.
Grant-Kels JM. Nutrition and the skin: part 1. Clin Dermatol. 2021;39:743–4.
Cao C, Xiao Z, Wu Y, et al. Diet and skin aging-from perspective of food nutrition. Nutrients. 2020;12(3):870. https://doi.org/10.3390/nu12030870.
DoGuilio KM, Rybakovsky E, Abdavies R, et al. Micronutrient improvement of epithelial barrier function in various disease states: a case for adjuvant therapy. Internat J Mol Sci. 2022;23:2995. https://doi.org/10.3390/ijms23062995.
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiome in nutrition and health. BMJ. 2018;361:k2179. https://doi.org/10.1136/bmj.k2179.
Centers for Disease Control and Prevention (CDC), November 16th, 2017. 2017. https://www.cdc.gov/media/releases/2017/p1116-fruit-vegetable-consumption.html. Accessed 16 Feb 2022.
Khmaladze I, Leonardi M, Fabre S, et al. The skin interactome: a holistic “Genome-Microbiome-Exposome” approach to understanding and modulate skin health and aging. Clin Cosmet Invest Dermatol. 2020;13:102–1040. https://doi.org/10.2147/CCID.S239367.
Tumsutti P, Maiprasert M, Sugkraroek P, et al. Effects of combination of botanical actives on skin health and antioxidant status in post-menopausal women: a randomized, double-blind, placebo-controlled clinical trial. J Cosmet Dermatol. 2022. https://doi.org/10.1111/jocd.14345.
Michalak M, Pierzak M, Krecisz B, et al. Bioactive compounds for skin health: a review. Nutrients. 2021;13(1):203. https://doi.org/10.3390/nu13010203.
Muzmdar S, Ferenczi K. Nutrition and youthful skin. Clin Dermatol. 2021;39:796–808.
Lephart ED. Phytoestrogens (resveratrol and equol) for estrogen-deficient skin- controversies/misinformation versus anti-aging in vitro and clinical evidence via nutraceutical-cosmetics. Internat J Mol Sci. 2021;22:11218. https://doi.org/10.3390/ijms222011218.
Rowe IJ, Baber RJ. The effects of phytoestrogens on postmenopausal health. Climacteric. 2021;24:57–63.
Messina M, Mejia SB, Cassidy A, et al. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: a technical review of the observational and clinical data. Crit Rev Food Sci Nutr. 2021. https://doi.org/10.1080/10408398.2021.1895054.
Naftolin F, Friedenthal J, Nachtigall R, Nachtigall L. Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment. Faculty of 1000 Res. F1000Res. 2019;8:F1000.
Acknowledgements
Funding
This review was supported, in part, by the Life Science/TTO funding, 19-2215 at Brigham Young University (BYU).
Editorial Assistance
We thank Janet Faye for her assistance in providing helpful comments and text editing.
Authorship
Both named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the reliability of this work cited, and have given their approval for this version to be published.
Authors’ Contributions
Edwin D. Lephart was involved in the concept/design, drafting/writing editing the manuscript and providing funding (literature journal report searches) for this review. Frederick Naftolin was involved in drafting/writing editing the manuscript.
Disclosures
Edwin D. Lephart has no funding or sponsor conflicts of interest in the decision of the data/research presented in this report and regarding the publication of this manuscript. Edwin D. Lephart is an inventor on equol patents (US and worldwide) on various human health applications. Frederick Naftolin has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed other than previously disclosed information in the public domain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lephart, E.D., Naftolin, F. Estrogen Action and Gut Microbiome Metabolism in Dermal Health. Dermatol Ther (Heidelb) 12, 1535–1550 (2022). https://doi.org/10.1007/s13555-022-00759-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00759-1