Abstract
Introduction
Patients with mild-to-moderate plaque psoriasis often experience reduced quality of life and increased disease burden due to itch or involvement of psoriasis in special areas such as the scalp and nails. Systemic therapy may be used concurrently with topical therapy in patients with active disease not controlled by topical therapy alone. The objective of PROMINENT was to evaluate the efficacy and safety of apremilast in combination with topical therapy in patients with mild-to-moderate psoriasis in Japan.
Methods
PROMINENT, a phase 3b, open-label, single-arm study in Japan, enrolled adults ≥ 20 years of age with plaque psoriasis [static Physician Global Assessment (sPGA) 2 (mild) or 3 (moderate)] not adequately controlled by topical therapy. Patients received apremilast 30 mg twice daily for 16 weeks in addition to their existing topical therapy, with the option of topical therapy reduction at the discretion of their physician while continuing apremilast treatment from weeks 16 to 32.
Results
Of the 152 patients enrolled in the study, 136 completed week 32. The primary endpoint of sPGA response [score 0 (clear) or 1 (almost clear)] was achieved by 43.7% of patients at week 16, and 40.8% maintained response at week 32. Clinically meaningful improvements in skin, scalp, and nails were observed in > 40% of patients at weeks 16 and 32. Similarly, improvements in pruritus, quality of life, and treatment satisfaction were observed at week 16 and maintained at week 32. Common treatment-emergent adverse events through week 32 included gastrointestinal events, nasopharyngitis, and headache.
Conclusions
Apremilast in combination with topical therapy resulted in clinically meaningful and sustained efficacy in physician- and patient-reported outcomes at weeks 16 and 32 in Japanese patients with mild-to-moderate psoriasis. Tolerability was consistent with available prior safety data for apremilast.
Trial Registration
ClinicalTrials.gov identifier, NCT03930186.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There have been limited studies of apremilast in combination with topical therapies, which is closer to the real-world treatment paradigm in Japan, where apremilast is approved for patients with an inadequate response to topical therapy; |
Patients with mild-to-moderate plaque psoriasis often experience reduced quality of life and increased disease burden due to itch or involvement of psoriasis in special areas such as the scalp and nails; |
Despite treatment with topical therapies, patients in Japan with mild-to-moderate psoriasis have limited systemic treatment options. |
What was learned from this study? |
Apremilast was effective in Japanese patients with mild-to-moderate psoriasis receiving concurrent topical therapy; |
Apremilast treatment improved overall disease severity, involvement of psoriasis in the scalp and nails, pruritus, and quality of life after 16 weeks; |
Improvements were sustained through 32 weeks of treatment, even with an optional topical therapy reduction phase during the last 16 weeks. |
Introduction
Psoriasis is a chronic, systemic inflammatory disease present in 0.34% of the population in Japan [1]. Even with limited skin involvement, mild-to-moderate psoriasis can impact quality of life (QoL) due to symptoms like itch and involvement in special areas such as the scalp and nails [2, 3]. Despite disease burden and impaired QoL, patients with mild-to-moderate psoriasis are often untreated or inadequately treated [2, 4]. Topical treatments are often recommended as a first treatment for patients with mild and moderate psoriasis [5]. However, some patients with mild or moderate psoriasis have active disease despite treatment with topical therapies, requiring additional treatment [6].
Patients with mild-to-moderate psoriasis in Japan have limited systemic treatment options, as systemic biologic therapies are reserved for patients with more severe psoriasis [≥ 10% body surface area (BSA) involvement], and inadequate response to conventional therapies, including phototherapy [7]. Apremilast is a phosphodiesterase 4 inhibitor that was effective in treating moderate-to-severe psoriasis [8,9,10,11] and mild-to-moderate psoriasis in phase 3 clinical trials [12]. A pharmacokinetic modeling and exposure–response analysis study in patients with moderate-to-severe psoriasis treated with apremilast demonstrated similar maximal effects and response rates in a Japanese population versus a predominantly Caucasian population [9]. However, clinical data in Japanese patients with mild-to-moderate psoriasis treated with apremilast are lacking.
Some patients with inadequate response to topicals start systemic treatment concurrently [5, 6]. PROMINENT was a 32-week, phase 3b, open-label, single-arm study conducted in Japan to evaluate apremilast in combination with topical therapy in patients with mild-to-moderate psoriasis who had not achieved an adequate response with topicals alone. The objective was to assess efficacy and safety of apremilast with stable concomitant topical therapy, including efficacy in treating special areas of psoriasis and itch. PROMINENT is the first study of patients with mild-to-moderate psoriasis treated concurrently with topicals and apremilast. This is closer to the real-world treatment paradigm in Japan, where apremilast is approved for patients with an inadequate response to topical therapy.
Methods
Study Design
PROMINENT (NCT03930186) was a phase 3b, open-label, single-arm study conducted at 28 sites in Japan in patients with mild-to-moderate psoriasis not adequately controlled by topical therapy alone. After a 5-day dose titration, patients received apremilast 30 mg orally twice daily (BID) in addition to existing stable topical therapy for 16 weeks. Patients were required to continue on their existing topical therapy at the same quantity or frequency through week 16; patients were not permitted to change topical therapies. From weeks 16–32, patients could reduce topical therapy at the physician’s discretion while maintaining apremilast 30 mg BID throughout. The study was approved by the institutional review board/ethics committee before commencement and conducted in compliance with Good Clinical Practice, the International Council for Harmonisation Guideline E6, the Declaration of Helsinki, and applicable regulatory requirements. Patients provided written informed consent before study-related procedures.
Patients
PROMINENT enrolled biologic-naive adults aged ≥ 20 years with chronic plaque psoriasis for ≥ 6 months before baseline and currently treated with topicals for ≥ 4 weeks before baseline. Patients had mild-to-moderate psoriasis [static Physician Global Assessment (sPGA) score of 2 (mild) or 3 (moderate)] that was inadequately controlled by topical therapy. Patients with a condition that might confound interpretation of study data, including other types of psoriasis, were excluded. Other key exclusion criteria included use of phototherapy or conventional systemic therapy for psoriasis within 8 weeks before baseline and during the study.
Efficacy Assessments
The primary efficacy endpoint was the proportion of patients achieving sPGA response [score of 0 (clear) or 1 (almost clear)] at week 16. Secondary endpoints included the proportions of patients achieving ≥ 75% reduction from baseline in BSA (BSA-75) and ≥ 50% or ≥ 75% reduction from baseline in Psoriasis Area and Severity Index (PASI) score (PASI-50 and PASI-75) at weeks 16 and 32. A post hoc analysis assessed how many sPGA responders at week 16 were also responders at weeks 2 and 4.
Change from baseline in itch scores at weeks 16 and 32 was evaluated using the pruritus visual analog scale (VAS; 0–100 mm) and Shiratori’s pruritus severity score [13, 14], a pruritus severity assessment tool used in Japan with scores ranging from 0 (no symptoms) to 4 (severe). Psoriasis in special areas was assessed by proportions of patients achieving a Scalp Physician’s Global Assessment (ScPGA) score of 0 or 1 and proportions of patients with a ≥ 50% reduction from baseline in Nail Psoriasis Severity Index (NAPSI-50). Change from baseline in NAPSI score was evaluated.
Patient-reported outcomes of the proportion of patients achieving Dermatology Life Quality Index (DLQI) score 0 or 1, the proportion of patients achieving Patient Benefit Index (PBI) ≥ 1, and mean Treatment Satisfaction Questionnaire for Medication (TSQM) version II overall and subdomain scores are reported. The DLQI is a 10-item questionnaire with a score range of 0 (best QoL) to 30 (worst QoL); a score of 0 or 1 indicates no effect of the disease on the patient’s life [15]. The PBI evaluates patient-perceived benefit of treatment on a scale ranging from 0 (no benefit) to 4 (maximum benefit) [16]. The TSQM version II is an 11-question, self-administered patient questionnaire that assesses patient satisfaction with treatment; scores range from 0 to 100, with higher TSQM scores depicting greater satisfaction.
Topical use, as approximated by tube weight, was investigated as an exploratory analysis. Because tubes were dispensed at each visit (weeks 0, 2, 4, and 8) and returned at each visit (weeks 2, 4, 8, and 16) from weeks 0–16, total tube weight reduction during this period was calculated as the sum of dispensed tube weight at weeks 0, 2, 4, and 8 minus the sum of returned tube weight at weeks 2, 4, 8, and 16.
Likewise, total tube weight reduction during weeks 16–32 was calculated as the sum of dispensed tube weight at weeks 16, 24, and 32 minus the sum of returned tube weight at weeks 24 and 32.
Safety Assessments
Safety was evaluated throughout the study on the basis of adverse events (AEs), discontinuations due to AEs, and clinically significant changes in physical examinations, vital signs, or laboratory findings. AEs were recorded from informed consent up to 28 days after the last dose of study drug; any time thereafter, reported serious AEs (SAEs) with a suspected relationship to study drug were recorded.
Statistical Analysis
Sample size was calculated assuming a 15% response rate (on the basis of data from phase 3 trials of apremilast in psoriasis). Efficacy analyses were conducted in the enrolled population; the safety population included all enrolled patients who received ≥ 1 dose of study drug. The primary endpoint was evaluated using multiple imputations (MIs) for missing values, with a sensitivity analysis using nonresponder imputation (NRI). Key categorical endpoints were assessed using NRI; continuous endpoints were analyzed using descriptive statistics.
Results
Patients
Of 152 patients enrolled in the study who received ≥ 1 dose of apremilast, 66.4% were men; mean age was 48.0 years. Baseline clinical characteristics are presented in Table 1. Mean psoriasis duration was 11.9 years. At baseline, 33.6% of patients had an sPGA score of 2 (mild) and 66.4% had an sPGA score of 3 (moderate). Mean PASI score was 8.9 and mean BSA was 13.4%. Topical antipsoriatic medications were the most frequently used topical therapy at baseline (92.1%; Table 1). A total of 136 patients completed week 32. Common reasons for discontinuation included patient withdrawal (5.9%) and AEs (4.6%).
Efficacy
Primary Endpoint
The primary endpoint of sPGA 0/1 response at week 16 was achieved by 43.7% of patients; this response was maintained at week 32 (Fig. 1). In a sensitivity analysis using NRI, sPGA 0/1 response was achieved by 41.4% of patients at week 16.
Achievement of physician-reported efficacy outcomes, enrolled population. NRI used for missing data. Error bars represent 95% confidence intervals. *The primary endpoint (sPGA 0 or 1) response rate with apremilast was 43.7% when multiple imputation was used for missing data. BSA psoriasis-involved body surface area, BSA-75 a ≥ 75% reduction from baseline in BSA, NRI nonresponder imputation, PASI Psoriasis Area and Severity Index, PASI-50 a ≥ 50% reduction from baseline in PASI score, PASI-75 a ≥ 75% reduction from baseline in PASI score, sPGA static Physician Global Assessment
Skin Involvement
Improvements were observed in skin involvement at week 16 and sustained at week 32 as measured by decreases from baseline in PASI score (mean change, −6.1 and −6.2, respectively) and BSA (mean change, −7.9% and −8.3%, respectively). PASI-50 and PASI-75 responses were achieved by 79.6% (121/152) and 43.4% (66/152) of patients, respectively, at week 16 with apremilast treatment; improvements were sustained at week 32 (Fig. 1). BSA-75 response was achieved by 40.8% (62/152) of patients at weeks 16 and 32 (Fig. 1).
Itch
At baseline, mean pruritus VAS score was 37.7 mm, and mean Shiratori’s pruritus severity scores were 1.7 (day) and 1.6 (night). The mean change from baseline in pruritus VAS score was −17.3 mm at week 16 and −18.4 mm at week 32. Improvements observed in Shiratori’s pruritus severity scores at week 16 (mean change from baseline, −0.7 for both day and night) were also sustained at week 32 (Fig. 2).
Psoriasis in Special Areas
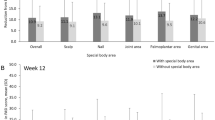
At baseline, 128 (84.2%) patients had an ScPGA score ≥ 2 (Table 1). Of these, 67 (52.3%) achieved an ScPGA score of 0 or 1 at week 16 (Fig. 3). Of 76 (50.0%) patients with a NAPSI score ≥ 1 at baseline (Table 1), 34 (44.7%) achieved NAPSI-50 at week 16 (Fig. 3). NAPSI scores decreased by a mean of 0.5 points from baseline to week 16 (Fig. 2). Improvements in scalp and nail involvement were maintained with continued apremilast treatment at week 32 (Figs. 2 and 3).
Achievement of treatment responses in special areas. NRI used for missing data. Error bars represent 95% confidence intervals. *In patients with baseline ScPGA score ≥ 2. †In patients with baseline NAPSI score ≥ 1. NAPSI Nail Psoriasis Severity Index, NAPSI-50 a ≥ 50% reduction from baseline in NAPSI score, NRI nonresponder imputation, ScPGA Scalp Physician’s Global Assessment
Patient-Reported Outcomes
Improvements in patient-perceived benefit from treatment and QoL as measured by the PBI and DLQI were observed with apremilast. A PBI score ≥ 1, considered a clinically meaningful benefit [17], was achieved by 91.4% (139/152) of patients at week 16 and 88.2% (134/152) at week 32 (Fig. 4). Among patients with a DLQI score ≥ 2 at baseline, almost half [41.2% (49/119)] achieved a DLQI score of 0 or 1 at week 16; response was maintained at week 32 (Fig. 4).
Onset of Efficacy
Although time to response was not a prespecified endpoint, a post hoc analysis found that among patients with sPGA 0/1 response at week 16, 15.9% (10/63) had maintained response from week 2 and 54.0% (34/63) had maintained response from week 4. Improvements were seen with apremilast treatment as early as week 4 with substantial mean reductions from baseline in itch (pruritus VAS: −18.3, Shiratori’s pruritus: −0.6). Early patient-reported treatment benefit also occurred, with 94.1% of patients achieving a PBI score ≥ 1 by week 4. Response for several additional outcomes was observed as early as week 8: 38.2% of patients achieved sPGA 0/1 response, 77.6% achieved PASI-50 response, and 42.1% achieved PASI-75 response. ScPGA score of 0 or 1 was achieved by 51.6% of patients at week 8. Improvements in QoL were also seen at week 8, with 42.0% of patients achieving DLQI score 0 or 1.
Treatment Satisfaction
Mean TSQM effectiveness, convenience, and global satisfaction scores improved by ~15 from baseline at weeks 16 and 32 with continued apremilast treatment (Table 2). Side effect scores at weeks 16 and 32 did not improve from baseline; however, scores (range 0–100) remained above 90 at all time points.
Topical Use
Topical use, as measured by a difference in dispensed tube weight versus returned tube weight, was reduced during the topical reduction phase (weeks 16–32) versus the combination therapy phase (weeks 0–16). A mean [standard deviation (SD)] decrease in tube weight of 292.4 (287.7) g was observed during weeks 0–16, whereas a mean decrease of 240.6 (264.0) g was observed during weeks 16–32 (on the basis of the patients who completed the study without losing their topical treatment tube). We also assessed the amounts of topical treatment dispensed and returned from all patients during each phase. The average amount of topical treatment dispensed was reduced when patients had the option to stop or reduce their topical treatment use during weeks 16–32 (531.7 g) compared with weeks 0–16 (752.3 g). The average amount of topical returned was 473.9 g during weeks 0–16 and 294.0 g during weeks 16–32. These results suggest an overall reduction in the use of topical treatments after the initial 16 weeks of apremilast treatment.
Safety
Most treatment-emergent AEs (TEAEs) reported by 75.7% (115/152) of patients were mild to moderate. Few patients reported TEAEs that were severe (n = 4), serious (n = 4), or led to drug withdrawal (n = 7) (Table 3). Most common TEAEs (≥ 5% of patients) through week 32 included gastrointestinal events, nasopharyngitis, and headache (Table 3). No clinically significant changes in physical examinations, vital signs, or laboratory findings were observed.
Discussion
In the PROMINENT study, the addition of apremilast to topical therapy resulted in clinically meaningful and sustained improvements in physician- and patient-reported outcomes in patients whose psoriasis was not adequately controlled by topical therapy alone. Improvements in most measures occurred rapidly, and benefits were maintained through week 32. In the second phase of the study (weeks 16–32), the dose of topical therapy could be reduced at the physician’s discretion. Although difficult to formally assess, there was a reduction of approximately 30% in the amount of topical treatments dispensed to patients, and a 20% decrease in change in tube weight in the second study phase (a raw proxy measure of overall topical use). The AE profile of apremilast was consistent with prior clinical trials in mild-to-moderate psoriasis and moderate-to-severe psoriasis, including a trial in Japanese patients [8, 10,11,12, 18, 19].
Approximately 40% of patients in PROMINENT achieved the primary endpoint, sPGA score of 0 or 1 at week 16. This response occurred rapidly (38.2% at week 8) and before the prespecified time of analysis of the primary endpoint and was maintained at week 32. This is notable, as sPGA score of 0 or 1 is a clinically meaningful benefit for patients with sPGA of 2 or 3 at baseline with limited treatment options. Although direct comparisons with other studies are not feasible owing to differences in study designs, populations, statistical power, and endpoints, rates of achieving sPGA response in PROMINENT were higher than rates observed at week 16 in placebo-controlled studies of apremilast in patients with mild-to-moderate psoriasis [12, 19]. Efficacy and Safety in Patients With Moderate Plaque Psoriasis (UNVEIL) was a phase 4, placebo-controlled study of patients with moderate psoriasis who were naive to conventional systemic and biologic therapies. Among patients in UNVEIL [19], 30.4% achieved sPGA response (score of 0 or 1, secondary endpoint) at week 16 with apremilast treatment versus 9.6% receiving placebo. In the placebo-controlled Apremilast as a Direct Treatment for Mild-to-Moderate Plaque Psoriasis Versus Placebo (ADVANCE) trial, 12 patients were biologic naive and inadequately controlled by 1 or more topical therapies. The primary endpoint of sPGA response was defined as a score of 0 or 1 with a ≥ 2-point reduction from baseline at week 16. In ADVANCE, sPGA response was achieved by 21.6% of patients receiving apremilast and 4.1% receiving placebo at week 16. Of note, concomitant topical therapy usage was not allowed in UNVEIL and ADVANCE.
Results at week 32 in PROMINENT were similar to those reported in a phase 2b study of a Japanese population with moderate-to-severe psoriasis (PSOR-011) where the secondary endpoint was sPGA score 0 or 1 in patients with sPGA ≥ 3 at baseline [8]. In the phase 2b study PSOR-011, prolonged sun or ultraviolet exposure, use of biologics within 12–24 weeks, conventional systemic treatments or phototherapy within 4 weeks, or active topical treatments for psoriasis within 2 weeks was prohibited. Greater sPGA response rates were seen at week 16 in patients with mild-to-moderate disease in PROMINENT (43.7%) versus patients with moderate-to-severe disease in PSOR-011 (29.6%), which may be expected owing to differences in disease severity, the open-label design, and concomitant use of topicals in PROMINENT.
Skin involvement also improved rapidly with apremilast treatment in the current study population, with PASI responses reaching a plateau by week 8. PASI-50 response was achieved by three-quarters of patients, and PASI-75 and BSA-75 responses were each achieved by almost half of patients at week 32.
Itch is one of the most bothersome symptoms for patients with psoriasis [2]. In the PROMINENT study, substantial improvements in itch were observed with apremilast as early as week 4, and were similar to those observed in patients with moderate to severe psoriasis enrolled in the ESTEEM trial [10]. In contrast to atopic dermatitis, which is associated with intense nighttime itching [20], itch severity did not vary much between day and night in patients with psoriasis in the current study, and apremilast showed similar suppression of itch at both times.
Topical therapies are often insufficient for treatment of psoriasis in special areas. Application of topical preparations to the scalp is messy and burdensome [21], and their efficacy for treatment of nail psoriasis is hindered by inadequate penetration of the nail plate [22, 23]. The addition of apremilast to topical therapy improved psoriasis in both of these special areas during PROMINENT. More than 50% of patients with an ScPGA score of ≥ 2 at baseline achieved an ScPGA score of 0 or 1 at week 16, with responses maintained at week 32. Nearly 50% of patients achieved NAPSI-50 at week 16, and 57.9% achieved this reduction at week 32. This response is consistent with the extended duration of treatment generally required for maximal improvement of nail psoriasis [24, 25]. The proportion of patients achieving NAPSI-50 at week 16 in PROMINENT was greater than the proportion with moderate-to-severe disease who achieved NAPSI-50 at week 16 in the ESTEEM 1 trial (33%) [10].
Apremilast showed benefit in patient-reported outcomes. Approximately 90% of patients reported a PBI ≥ 1 and 42.9% achieved a DLQI score of 0 or 1 at week 32. Treatment satisfaction with apremilast was high and increased compared with satisfaction with topical therapies at baseline.
Overall results of the PROMINENT study support the addition of apremilast in patients who do not respond to topical medications.
Limitations
Data should be interpreted within the context of the limitations of a single-arm, open-label study. There are limitations to the assessment of topical use during the two study periods; there were fewer patients in the topical reduction phase than the combination therapy phase (140 versus 152), making the amount of topical dispensed difficult to interpret. Additionally, the method to assess topical use by comparing dispensed versus returned weights of multiple tubes at multiple timepoints is not a direct measure. Time to response was not a prespecified endpoint, limiting conclusions regarding early onset of efficacy. Nail psoriasis may take longer to improve than the current study period allowed; longer observation may be needed to assess the full impact of apremilast on nail psoriasis. Finally, guidance for topical application and preference for topical agent class likely differed between physicians at different study sites, which may have influenced study outcomes.
Conclusions
In this phase 3b, open-label study in Japanese patients with mild-to-moderate psoriasis, apremilast treatment in combination with topical therapy was associated with improvements in psoriasis severity and skin involvement. Furthermore, improvements were observed in scalp and nail psoriasis, itch, QoL, and treatment satisfaction. Tolerability was consistent with the known apremilast safety profile. Results support apremilast as an effective treatment option for Japanese patients with mild-to-moderate psoriasis who are not adequately treated with topical therapy alone.
References
Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1): e006450.
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81.
Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–23; discussion ii24–15.
Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–5.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–70.
Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–22.
Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873–84.
Okubo Y, Ohtsuki M, Komine M, et al. Population pharmacokinetic and exposure-response analysis of apremilast in Japanese subjects with moderate to severe psoriasis. J Dermatol. 2021;48(11):1652–64.
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73(1):37–49.
Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99.
Stein Gold L, Papp K, Leonardi C, et al. Efficacy and safety of apremilast in patients with mild to moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2022;86(1):77–85.
Kinugasa E, Igawa K, Shimada H, et al. Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: a phase II, randomized, double-blind, placebo-controlled clinical study. Clin Exp Nephrol. 2021;25(8):875–84.
Dominick F, van Laarhoven AIM, Evers AWM, Weisshaar E. A systematic review of questionnaires on itch by the Special Interest Group “Questionnaires” of the International Forum for the Study of Itch (IFSI). Itch. 2019;4(3): e26.
Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–64.
Augustin M, Radtke MA, Zschocke I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301(8):561–71.
Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304(6):433–41.
Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–17.
Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16(8):801–8.
Lavery MJ, Stull C, Kinney MO, Yosipovitch G. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17(3):425.
Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3): e12589.
Armstrong AW, Tuong W, Love TJ, et al. Treatments for nail psoriasis: a systematic review by the GRAPPA Nail Psoriasis Work Group. J Rheumatol. 2014;41(11):2306–14.
Thatai P, Khan AB. Management of nail psoriasis by topical drug delivery: a pharmaceutical perspective. Int J Dermatol. 2020;59(8):915–25.
Crowley JJ, Weinberg JM, Wu JJ, Robertson AD, Van Voorhees AS. Treatment of nail psoriasis: best practice recommendations from the medical board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151(1):87–94.
Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–42.
Acknowledgements
The authors gratefully acknowledge Hidehisa Noguchi for contributions to conception and design of the study; patient data collection/acquisition of data; and analysis and interpretation of data.
Funding
This study was sponsored by Amgen Inc. Amgen Inc. is funding the journal’s rapid service fees.
Medical Writing and/or Editorial Assistance
Writing support was funded by Amgen Inc. and provided by Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company, and Dawn Nicewarner, PhD, employee of and stockholder in Amgen Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors participated in drafting the work or revising it critically for important intellectual content and provided final approval. Conceptualization: Satoru Kikuchi, Taichi Nakamura, and Yasushi Ozeki. Formal analysis and investigation [patient data collection/acquisition of data, and/or analysis and interpretation of data]: Masatoshi Abe, Koki Endo, Ryosuke Hino, Yukari Okubo, Maria Paris, Hidetoshi Takahashi, Satoru Kikuchi, Taichi Nakamura, and Yasushi Ozeki.
Disclosures
Yukari Okubo: Eisai, Maruho, Shiseido, and Torii—research grants; AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, Jimro, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe-Mitsubishi, Torii, and UCB Pharma—honoraria; AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen Pharma, LEO Pharma, Maruho, Pfizer, Sun Pharma, and UCB Pharma—clinical trials. Hidetoshi Takahashi: AbbVie, Amgen Inc., Eli Lilly, Janssen, Kaken Pharmaceutical, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, Sanofi, Sato Pharmaceutical, Sun Pharma, Taiho Pharmaceutical, Torii, and UCB—honoraria. Ryosuke Hino: AbbVie, Amgen, Celgene, Eli Lilly, Janssen Pharma, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Sanofi, Sun Pharma, Taiho, Tanabe-Mitsubishi, Torii Pharma, and UCB Pharma—honoraria; Amgen, Celgene, Eli Lilly, LEO Pharma, Maruho, Otsuka Pharma, and Sanofi—clinical trials. Koki Endo: Nothing to disclose. Satoru Kikuchi, Yasushi Ozeki, Taichi Nakamura: Amgen K.K.—employment. Maria Paris: Amgen Inc.—employee and stockholder. Masatoshi Abe: Celgene and Maruho—consultant and/or paid speaker for and/or accepted a research grant from and/or participated in clinical trials.
Compliance with Ethics Guidelines
The study was approved by the institutional review board/ethics committee before commencement and conducted in compliance with Good Clinical Practice, the International Council for Harmonisation Guideline E6, the Declaration of Helsinki, and applicable regulatory requirements. Patients provided written informed consent before study-related procedures.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Okubo, Y., Takahashi, H., Hino, R. et al. Efficacy and Safety of Apremilast in the Treatment of Patients with Mild-to-Moderate Psoriasis in Japan: Results from PROMINENT, A Phase 3b, Open-Label, Single-Arm Study. Dermatol Ther (Heidelb) 12, 1469–1480 (2022). https://doi.org/10.1007/s13555-022-00747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00747-5