Abstract
Introduction
The lifetime incidence of nail psoriasis in patients with psoriasis is 80–90%, with 23–27% of patients having nail psoriasis at any given time. Nail psoriasis is even more prevalent in patients with comorbid psoriatic arthritis. Complete psoriasis clearance, an achievable therapeutic goal, should ideally include the resolution of nail psoriasis. Here, we assessed simultaneous skin and nail clearance in patients with psoriasis across five head-to-head trials comparing ixekizumab with other biologics.
Methods
Data were assessed in patients with moderate-to-severe psoriasis (with or without psoriatic arthritis) with nail psoriasis at baseline from the IXORA-R, IXORA-S, UNCOVER-2, UNCOVER-3, and SPIRIT-H2H trials. Ixekizumab patients received IXEQ2W to week 12 and IXEQ4W beyond week 12. PASI 100 depicted complete skin clearance, and PGA-F 0 (IXORA-R) or NAPSI 0 (all other trials) depicted complete nail clearance. Treatment comparisons were evaluated using the Cochran-Mantel-Haenszel test. Non-responder imputation was used for missing data.
Results
Ixekizumab achieved significantly greater simultaneous skin and nail complete clearance than etanercept (UNCOVER-2: p < 0.001 and UNCOVER-3: p < 0.001) at week 12, demonstrating an efficacious and rapid response. Across all five head-to-head trials, ixekizumab achieved a high rate of simultaneous skin and nail clearance (range: 28.6–45.9% of patients) by week 24 that was maintained up to week 52 (range: 40.5–51.4% of patients). Ixekizumab achieved numerically greater simultaneous complete clearance than guselkumab at week 24 (p = 0.079), but statistically significant greater simultaneous clearance compared to ustekinumab (p < 0.001) and adalimumab (p = 0.006) at week 24 and week 52 (p < 0.001 and p = 0.007, respectively).
Conclusion
In five head-to-head trials, ixekizumab-treated patients had higher rates of simultaneous complete skin and nail clearance compared to etanercept, guselkumab, ustekinumab, and adalimumab, thereby reinforcing ixekizumab’s ability to achieve high levels of efficacy in multiple domains of psoriatic disease.
Trial registration
NCT01474512, NCT01597245, NCT01646177, NCT03573323, NCT02561806, and NCT03151551.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Psoriasis in challenging body areas, such as nails, may lead to larger impacts on patients’ quality of life; it is clinically important to obtain resolution in both skin and nails in this patient population, and comparisons of treatments can help guide dermatologists to prescribe the most appropriate treatment |
This study compared the efficacy of ixekizumab in simultaneous clearance of skin and nail psoriasis over time with other biologics |
What was learned from this study? |
Ixekizumab has greater efficacy at clearing psoriasis of the skin and nails compared to four other biologics with three different mechanisms of action |
Introduction
Plaque psoriasis is a common chronic inflammatory skin disease characterized by red scaly plaques that manifest on the skin, often alongside various debilitating comorbidities such as psoriatic arthritis, diabetes, cardiovascular disease, and depression [1]. Combined with the classic itching and overt nature of the disease, plaque psoriasis can have a significant impact on patients’ mental health and quality of life [2].
Ixekizumab is a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A, blocking the binding of IL-17A to its cognate receptor and neutralizing its activity [3]. The rapid onset of action and the long-term efficacy and safety of ixekizumab for up to 5 years have been demonstrated in pivotal phase 3 clinical trials (UNCOVER-1, UNCOVER-2, and UNCOVER-3) and head-to-head studies (UNCOVER-2, UNCOVER-3, IXORA-R, IXORA-S, and SPIRIT-H2H) [4,5,6,7,8,9]. Ixekizumab is approved for use in patients with moderate-to-severe plaque psoriasis, genital psoriasis, and psoriatic arthritis.
Early clinical trials investigating the efficacy of first-generation biologics, such as tumor necrosis factor inhibitors, used endpoints such as ≥ 50% and ≥ 75% improvement from baseline in the Psoriasis Area Severity Index (PASI) (PASI 50 and PASI 75, respectively). Since the advent of newer biologic treatments, including ixekizumab, the benchmark for treatment has been raised to nearly complete (PASI 90) or complete skin clearance (PASI 100) [10]. Patients achieving PASI 100 are more likely to report higher quality of life outcomes than patients who achieve skin clearance just below the threshold (PASI 90 to < 100) [11].
As treatments improve and high clearance scores of PASI 90 and 100 become the goal/expectation of psoriasis therapy, simultaneous skin clearance and resolution in special challenging body areas such as scalp, genitals, and nails may be the next ideal therapeutic goal. Despite the small surface area affected by psoriasis in most challenging body areas, patients can experience disproportionate levels of physical impairment, emotional distress, and quality of life reduction [12,13,14]. The presence of disease in the above-mentioned special areas has been identified as a criterion for psoriasis patients to be classified as being eligible for systemic therapy based on the Delphi consensus from the International Psoriasis Council [15]. Nail and hand psoriasis in particular can reduce workplace productivity, leading to financial burdens from disease impairment [12, 14]. Common signs of nail psoriasis include pitting and onycholysis with subungual hyperkeratosis, nail plate abnormalities, and nail bed discoloration [16]. Using PASI alone does not capture the impact of psoriasis in challenging body areas as it does not consider the specific location of the disease.
Evaluation scales such as the Nail Psoriasis and Severity Index (NAPSI) or Physician’s Global Assessment-Fingernails (PGA-F) have been developed to specifically measure the severity of nail psoriasis. New goals for current and emergent therapies for psoriasis should aim for resolution beyond PASI 100, aiming to achieve simultaneous clearance of skin and nail psoriasis for a more holistic and complete resolution of disease. Here, we assessed the simultaneous improvement in skin and nail clearance in patients with psoriasis and psoriatic arthritis with ixekizumab over time and compared ixekizumab with other biologics.
Methods
Study Design
The UNCOVER-1 (NCT01474512), UNCOVER-2 (NCT01597245), UNCOVER-3 (NCT01646177), IXORA-R (NCT03573323), IXORA-S (NCT02561806), and SPIRIT-H2H (NCT03151551) trials were randomized, double-blinded, (SPIRIT-H2H was assessor blinded only) phase 3 studies (IXORA-R was a phase 4 study) conducted in patients with moderate-to-severe plaque psoriasis or patients with psoriatic arthritis (SPIRIT-H2H only). The full study design and the efficacy and safety data from these studies have been published previously [4,5,6,7,8,9]. For this post hoc analysis, data from clinical trials were analyzed individually except for UNCOVER-1 and UNCOVER-2 data, which were similar in design and therefore combined for time points after week 12.
Efficacy Assessments
Psoriasis severity was assessed by PASI in the intent-to-treat population for all trials. Complete resolution of psoriasis was determined by PASI 100 (defined as 100% improvement from baseline PASI). Fingernail psoriasis was assessed by PGA-F (in IXORA-R) or NAPSI (in all other trials) for all patients who presented with fingernail psoriasis at baseline. Complete resolution of nail psoriasis was defined as either PGA-F 0 or NAPSI 0.
Patient and Public Involvement Statement
Patients were not involved in the research process.
Statistical Analyses
For this post hoc analysis, data were assessed in patients with NAPSI > 0 (or PGA-F > 0) at baseline from each comparative trial. Treatment comparisons were evaluated using the Cochran-Mantel-Haenszel test. Non-responder imputation was used for missing data in the comparative trials, and observed results were used for the integrated UNCOVER-1-UNCOVER-2 analysis (Fig. 5). All data are presented as response rates unless stated otherwise.
Compliance with Ethics Guidelines
All studies were approved by the applicable ethical review boards at each participating study site and were conducted in accordance with the principles expressed in the Declaration of Helsinki of 1964 and its subsequent amendments. Written informed consent was obtained from each patient at study entry before any study procedures took place.
Results
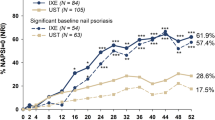
Baseline demographics were generally similar across the five head-to-head trials in the population of patients with skin and nail psoriasis at baseline (Table 1). There were some notable differences in baseline characteristics between the trial populations such as a lower psoriasis duration in SPIRIT-H2H and a lower proportion of males in IXORA-R and SPIRIT-H2H compared to the other trial populations. In the UNCOVER-2 and UNCOVER-3 trials, ixekizumab achieved significantly greater simultaneous skin and nail clearance than etanercept (UNCOVER-2: p < 0.001 and UNCOVER-3: p < 0.001, Fig. 1a, b) at 12 weeks, demonstrating ixekizumab’s rapidity of onset. In the IXORA-R, IXORA-S, and SPIRIT-H2H trials, ixekizumab achieved numerically greater simultaneous skin and nail clearance than guselkumab (IXORA-R: p = 0.079, Fig. 2a) at 24 weeks and significantly greater simultaneous skin and nail clearance than ustekinumab (IXORA-S: p < 0.001, Fig. 2b) and adalimumab (SPIRIT-H2H: p = 0.006, Fig. 2c) at 24 weeks and ustekinumab (IXORA-S: p < 0.001, Fig. 3a) and adalimumab (SPIRIT-H2H: p = 0.007, Fig. 3b) at 52 weeks, reinforcing previous findings that ixekizumab’s efficacy on different domains of psoriasis is maintained up to 1 year of treatment [9]. Furthermore, the combined results from the four psoriasis head-to-head trials (UNCOVER-2, UNCOVER-3, IXORA-R, and IXORA-S) show that treatment with ixekizumab continues to improve simultaneous skin and nail clearance up to week 52 (Fig. 4). Additionally, in an analysis of observed PASI 100 responders at week 52 in the pooled population from UNCOVER-1 and UNCOVER-2 trials, ixekizumab demonstrated high efficacy in complete clearance of nail psoriasis (69.4% of PASI 100 responders, Fig. 5).
Simultaneous skin and nail clearance at Week 12. Ixekizumab achieved significantly greater simultaneous PASI 100 and NAPSI 0 clearance vs. etanercept (a, b) at week 12. All data are presented as % response. IXE ixekizumab, ETA etanercept, PASI Psoriasis Area Severity Index, n number of patients, NAPSI Nail Psoriasis and Severity Index
Simultaneous skin and nail clearance at Week 24. Ixekizumab achieved numerically greater simultaneous PASI 100 and PGA-F 0 clearance vs. guselkumab (a) and significantly greater simultaneous PASI 100 and NAPSI 0 clearance vs. ustekinumab (b) and adalimumab (c) by week 24. All data are presented as % response. IXE ixekizumab, ADA adalimumab, UST ustekinumab, GUS guselkumab, PASI Psoriasis Area Severity Index, n number of patients, NAPSI Nail Psoriasis and Severity Index, PGA-F Physician’s Global Assessment-Fingernails
Simultaneous skin and nail clearance at Week 52. Ixekizumab achieved significantly greater simultaneous PASI 100 and NAPSI 0 clearance vs. ustekinumab (a) and adalimumab (b) at Week 52. All data are presented as % response. IXE ixekizumab, ADA adalimumab, UST ustekinumab, PASI Psoriasis Area Severity Index, n number of patients, NAPSI Nail Psoriasis and Severity Index
Simultaneous skin and nail clearance with ixekizumab up to Week 52. Simultaneous skin and nails clearance in psoriasis patients with ixekizumab treatment at Weeks 12 (UNCOVER-2 and -3), 24 (IXORA-R and -S), and 52 (IXORA-S). All data are presented as % response. PASI Psoriasis Area Severity Index, n number of patients, NAPSI Nail Psoriasis and Severity Index, PGA-F Physician’s Global Assessment-Fingernails
Ixekizumab complete skin response (PASI 100) with no residual disease in nails. Integrated analysis of UNCOVER-1 and UNCOVER-2 in patients with moderate-to-severe plaque psoriasis. Fifty patients out of 72 (69.4%) PASI 100 Week 52 responders had no residual disease in nails. PASI Psoriasis Area Severity Index, n number of patients, NAPSI Nail Psoriasis and Severity Index
Discussion
The aims of this study were to investigate the efficacy of ixekizumab in simultaneous skin and nail clearance over time and to compare its efficacy to that of other biologics using direct head-to-head data. While improvement in skin and nail clearance was observed in all treatment groups across the head-to-head trials, simultaneous complete resolution of skin and nails was numerically greater with ixekizumab treatment at all time points measured versus all other biologics and was significantly greater against ustekinumab and adalimumab at weeks 24 and 52. It should also be noted that ixekizumab has been reported to achieve greater complete clearance of skin and nails when measured separately at the timepoints presented here against other biologics, except for PASI100 at week 24 vs. guselkumab, where both ixekizumab and guselkumab achieved similar levels of complete skin clearance [7]. These results reinforce ixekizumab’s ability to achieve high levels of efficacy even in special areas such as nails.
Limitations of the findings presented here include that it is a post hoc analysis of results from six separate clinical trials, across two indications, and at multiple time points. Comorbid psoriatic arthritis is known to have a higher association with nail disease than skin psoriasis without psoriatic arthritis, and nail psoriasis is one of the most predictive clinical indicators of the development of psoriatic arthritis [17]. It has been suggested that nail involvement not only predicts, but also can be a causative element of the distal interphalangeal (DIP) joint inflammation seen in psoriatic arthritis [18]. Of the clinical trials discussed here, ixekizumab had the highest simultaneous skin and nail clearance in the population of patients with psoriatic arthritis (SPIRIT H2H, Figs. 2 and 3). From this, and because of the relationship of the nails and DIP joint, it could be posited that ixekizumab is particularly effective in patients with psoriatic arthritis, leading to higher simultaneous skin and nail clearance rates.
The debilitating nature and severity of psoriasis in challenging body areas, such as in the nails, is often underestimated when using traditional scoring systems due to the relatively low area affected. In fact, nail psoriasis is not included in the PASI score at all. The patient’s quality of life is often impacted by psoriasis in challenging body areas disproportionately to the affected area [12,13,14]. Nail involvement is found in 23–27% of patients with psoriasis [19] and is associated with physical impairment, pain, anxiety and/or depression, and substantial impairments in quality of life [20]. As treatments for psoriasis advance, therapeutic goals for treatments should look beyond PASI 100 and consider their efficacy in treating challenging body areas such as nails.
The analysis presented here highlights the efficacy of ixekizumab compared to other biologics in achieving beyond PASI 100 resolution of disease, successfully resolving skin and nail disease in 28.6–45.9% of patients by week 24 and maintaining high levels of resolution up to and beyond week 52 (40.5–51.4%). Considering the broad selection of available effective treatments for psoriasis in skin (21), there is a need for dermatologists to personalize or tailor treatments for individual patients by considering the impact of disease in special challenging body areas such as nails and the efficacy of these treatments in such areas. The post hoc analysis presented here highlights the need to consider the efficacy of treatments not only in clearing skin psoriasis, but also in clearing nail psoriasis.
Conclusion
In summary, across five head-to-head trials, ixekizumab-treated patients had higher rates of simultaneous complete skin and nail clearance compared to etanercept, guselkumab, ustekinumab, and adalimumab, with statistical significance achieved for comparisons with etanercept, ustekinumab, and adalimumab. This reinforces ixekizumab’s high sustained levels of efficacy in multiple domains of psoriatic disease.
References
Kimball AB, Gladman D, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58(6):1031–42.
Sampogna F, Tabolli S, Abeni D. Investigators IMPRoVEI. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol. 2012;92(3):299–303.
Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50.
Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51.
Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(21):2102.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70-9.e3.
Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184(6):1047–58.
Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31.
Leonardi C, Reich K, Foley P, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther (Heidelb). 2020;10(3):431–47.
Blauvelt A, Wu JJ, Armstrong A, Menter A, Liu C, Jacobson A. Importance of complete skin clearance in psoriasis as a treatment goal: implications for patient-reported outcomes. J Drugs Dermatol. 2020;19(5):487–92.
Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to psoriasis area severity index 90 and 100. J Drugs Dermatol. 2015;14(10):1086–8.
Augustin M, Reich K, Blome C, Schäfer I, Laass A, Radtke MA. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol. 2010;163(3):580–5.
Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Derm Venereol. 2014;94(4):411–4.
Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: Nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589.
Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–22.
Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(Suppl 1):1–5.
Langenbruch A, Radtke MA, Krensel M, Jacobi A, Reich K, Augustin M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol. 2014;171(5):1123–8.
Scarpa R, Soscia E, Peluso R, et al. Nail and distal interphalangeal joint in psoriatic arthritis. J Rheumatol. 2006;33(7):1315–9.
Merola JF, Li T, Li WQ, Cho E, Qureshi AA. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41(5):486–9.
Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease. J Eur Acad Dermatol Venereol. 2014;28(12):1690–5.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Acknowledgements
We thank the patients and investigators who participated in the study. Eli Lilly or its representatives provided data, laboratory, and site monitoring services.
Funding
This study was sponsored by Eli Lilly and Company. Dermatology and Therapy’s Rapid Service Fee was paid for by Eli Lilly and Company. The funder of the study had a role in study design, data analysis, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Medical Writing and/or Editorial Assistance
Writing assistance was provided by Conor McVeigh, PhD, of Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Data analysis was performed by Missy McKean-Matthews. All authors contributed to critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Prior Presentation
Part of the analysis presented here has been submitted as an abstract to the American Academy of Dermatology Annual Meeting 2022 taking place March 25–29, 2022, in Boston MA.
Disclosures
Boni E. Elewski receives research funding from AbbVie, AnaptysBio, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Incyte, Leo, Lilly, Merck, Menlo, Novartis, Pfizer, Regeneron, Janssen, Sun Pharma, UCB, Valeant (Ortho Dermatology), and Vanda; and receives honoraria from Arcutis, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Leo, Eli Lilly and Company, Menlo, Novartis, Pfizer, Sun Pharma, UCB, Valeant (Ortho Dermatology), and Verrica. Andrew Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, EcoR1, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Landos, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Vibliome. Alice B. Gottlieb is a consultant and/or investigator for Boehringer Ingelheim, Incyte, Janssen, Novartis, Sun Pharma, UCB, Xbiotech, AnaptsysBio, Avotres Therapeutics, Bristol-Myers Squibb, Dermavant GSK, Eli Lilly and Company, Novartis, Pfizer; an advisor for AnaptsysBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, GSK, Janssen, Eli Lilly and Company, Novartis, Pfizer, Sun Pharma, UCB, Xbiotech. Joseph F. Merola is a consultant and/or investigator for Amgen, Bristol-Myers Squibb, AbbVie, Dermavant, Eli Lilly and Company, Novartis, Janssen, UCB, Sanofi, Regeneron, Sun Pharma, Biogen, Pfizer and Leo Pharma. Lyn C. Guenther has been a consultant, investigator, and speaker for AbbVie, Allergan, Amgen, Bausch, Celgene, Eli Lilly and Company, Galderma, Janssen, La Roche-Posay, Leo Pharma, Merck Frosst, Novartis, Pfizer, Sun Pharmaceutical Industry Ltd and UCB Pharma, and has received research grants from Bristol-Myers Squibb and Boehringer Ingelheim. Eric Wolf, Gaia Gallo, and Russel Burge are employees and stockholders of Eli Lilly and Company. Missy Mckean-Matthews is an employee of Syneos Health, on behalf of Eli Lilly and Company.
Compliance with Ethics Guidelines
All studies were approved by the applicable ethical review boards at each participating study site and was conducted in accordance with the principles expressed in the Declaration of Helsinki of 1964 and its subsequent amendments. Written, informed consent was obtained from each patient at study entry before any study procedures took place.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Elewski, B.E., Blauvelt, A., Gallo, G. et al. Simultaneous Nail and Skin Clearance in Ixekizumab Head-to-Head Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis. Dermatol Ther (Heidelb) 12, 911–920 (2022). https://doi.org/10.1007/s13555-022-00704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00704-2