Abstract
Total hip and knee replacement surgery using metal alloy devices is common. Type IV allergic reactions to these implants occur, though infrequently. While uncommon, peri-implant metal allergic reactions may cause significant morbidity for the affected individual—including aseptic loosening, pseudotumor formation and frank device failure. It is challenging to predict who will have these reactions, even in those with established pre-implant metal allergy. At this time, the scientific literature clearly supports few conclusions. Despite this, we believe several conclusions can be made: routine pre-implant testing in asymptomatic individuals is not indicated; listen to patient’s concerns about metal allergy if the concern arises; patch testing is probably the best pre- and post-implant screening test; post-implantation testing is controversial and even positive LTT or patch test does not definitively diagnose morbidity from a metal allergy; and complete recovery following revision placement of an immunologically inert device is diagnostic. More research is needed to scientifically approach this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of metals in orthopedics is widespread, and there has been increasing concern with regards to the possibility of developing cutaneous and systemic hypersensitivity reactions to constituent metals in implant devices. Although hypersensitivity reactions to metals are not common, they require evaluation and management when they do occur. Regrettably, there is an ostensible lack of accord in the field on the appropriate steps to evaluate, diagnose and manage patients with suspected metal hypersensitivity reactions. This review aims to explore the existing literature on hypersensitivity reactions to metallic implants in orthopedic surgery and, in particular, highlight the recent debate surrounding appropriate pre- and post-implantation testing.
In the United States, approximately 5.2 million total knee replacements were performed from 2000 to 2010 [1] and these may double by 2020 [2]. For patients over 45 years old, total hip replacements more than doubled, with 310,800 procedures being performed in 2010 [1]. The total incidence of total shoulder arthroplasty has also been steadily increasing, to 27,000 in 2008 [3].
Orthopedic implants are composed of nickel, cobalt, chromium, molybdenum, zirconium and/or titanium alloys, while stainless steel is used in fixed orthopedic devices such as screws/plates [4, 5, 30]. As a cause of complication after joint replacement, metal allergy was first reported in 1966, with slowly increasing awareness and reported incidence [6,7,8,9,10]. While the association between metal implant failure and allergy is well documented, it remains a phenomenon that is relatively unpredictable, poorly understood and highly debated [11,12,13].
Skin reactions caused by MHR include dermatitis reactions adjacent to and regionally adjacent to the implant site, generalized dermatitis, as well as erythema, generalized urticaria and cutaneous vasculitis. Reactions occur following implantation of static implants as well as dynamic prostheses [14,15,16,17,18,19]. Other adverse reactions including device failure, chronic inflammation, pain, loosening of joint prostheses or re-stenosis of cardiac stents can also occur [20]. In some cases, metallosis (metallic staining of the surrounding tissue), excessive periprosthetic fibrosis and muscular necrosis have also been reported [21,22,23].
With an aging population, clarifying the association between metal hypersensitivity reactions and implant failures bears enormous repercussions for health care costs, and avoids unnecessary morbidity in patients [24]. The lack of clear evidence-based clinical guidance in this area creates a potential breeding ground for unwarranted lawsuits, particularly when patients with self–reported metal allergies pre-implantation allege inadequate pre-operative allergy assessment [25]. Consequently, the possibility of being entangled in needless litigation provides a strong driving force for seeking clarification and consensus in the field. It is worth noting that the following discussion is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
The Association Between Orthopedic Implants and Metal Hypersensitivity
The literature regarding reactions following hip arthroplasty shows conflicting research, and the extent to which metal sensitivity affects implant lifespan and longevity remains debated, without clear evidence-based guidelines. On the one hand, a case–control study (356 cases/712 controls) reported no increase in the risk of total hip arthroplasty (THA) revision in patients with cases with metal allergy, and metal allergy risk was not elevated after THA [26]. Unfortunately, this is not definitive. On the other hand, there are multiple authors reporting opposite data, though the patient groups are smaller. In one series examining 165 patients following orthopedic implant, patients with osteolysis adjacent to the implant had cobalt allergy at a significantly higher rate when compared to controls [27]. Other studies also report increased metal reactions in cases with device loosening/prosthetic failure as well as those undergoing surgery for revision of a failed implant [28, 29]. Hallab’s literature review in 2001 found a metal allergy prevalence of ~25% in patients with well-functioning THA and 60% in those with poorly functioning or failed implants [30]. Histopathological examination of periprosthetic tissue supports the correlation between wear particles leading to metal allergy and subsequent implant failure [31,32,33,34,35,36,37].
Unsurprisingly, studies for total knee arthroplasty have also not been wholly consistent. A prospective examination in patients following total knee arthroplasty (TKA) showed metal allergy by patch test was often seen in those with aseptic loosening (59%) versus stable prosthesis (48%) versus controls without prior orthopedic device implantation (20%) [38]. Individuals reporting a prior history of metal reactions before device implantation were four times more likely to develop implant failure [39]. A lymphocyte stimulation test before implantation of a chromium-containing device in those positive for chromium increased the risk of post-implant eczema [39].
Having said that, metal hypersensitivity reactions following TKA are rare—the exact prevalence of MHR is unknown but estimates ranges from 0 to 5% of implanted devices [20]. Common sources of morbidity must thus be ruled out before a diagnosis of metal allergy is made. Pain and other symptoms such as instability, implant loosening or malrotation and referred or chronic regional pain are more likely caused by infection [40]. A correlation with metal allergy and device failure is not certain. A cohort study of 127 patients with 161 TKA compared to 161 control knee arthroplasty revealed that those with patch test positives to metal had similar complication, reoperation or revision rates when compared to those without allergy/matched controls [41]. Rates of post-operative pain were similar in those with metal allergy determined by patch testing, compared to control patients. In a separate study, patients receiving a metal TKA showed no increase in joint loosening in those with metal allergy prior to implant as determined by patch testing [42]. Another author concluded that there was no evidence of implant failure due to metal allergy [43]. However, patient-reported allergy was associated with decreased functional outcomes after TKA and poorer scoring of mental health after THA [44].
Although there are multiple studies for total hip and knee replacements, unfortunately there is no definitive research that reports a link between metal allergy and morbidity following shoulder arthroplasty [4].
There is thus extensive literature on both sides that asserts or renounces a correlation between metal hypersensitivity reaction and metallic implant failure, which only serves to add to the existing confusion. What is clear, however, is that even if a correlation is purported to exist, none of the authors are able to conclusively report the direction of causation. It remains unknown whether implants fail or function poorly due to a pre-existing metal hypersensitivity, or that secondary sensitization happened due to excessive metal release from failing implants [24].
The Debate Surrounding Appropriate Testing
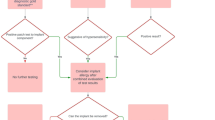
Given that there is no clear conclusion on the link between metal allergy and implant failure, it logically follows that there is a similar lack of consensus on the approach to the testing and management of patients. The crucial question at hand is whether there is a need to carry out screening prior to implanting metal devices. If screening is needed, what is the most effective determination of metal allergy: epicutaneous patch testing, a lymphocyte transformation test or a self-reported history of metal reactions? In addition, how should we tailor the pre-implantation management plan for those who test positive? Should hypoallergenic alloys, with which surgeons may be less familiar and are more expensive, be used? What about the management plan for patients with suspected metal hypersensitivity post-implantation? This paper aims to answer each question in turn.
Is there a need for pre-implant testing?
Routine pre-implant screening or testing prior to surgery is not indicated, and opinions regarding the appropriate patients to test prior to surgery are controversial. There are no scientific or expert agreements on whether metal hypersensitivity reactions cause joint morbidity or failure following implant, and thus there is also no agreement on which patients require pre-surgical allergy evaluation.
A cohort study of 127 patients with 161 TKA (56 patients with patch test positives) versus 161 matched control TKAs without known metal allergy history or positive patch testing were followed over a period of 5.3 years [41]. Most interestingly, those with patch test positives had similar reoperation, revision or complication rates in comparison to those with a normal skin patch testing as well as matched controls. Post-operative pain was not different between any of the groups. In view of the findings of the study, skin patch testing showed little value for predicting the clinical outcomes and was not recommended as a guide for implant alloy choice. A recent review lends support to the stance that pre-implantation testing is not routinely needed: the review acknowledged the presence of an association between implant failure and metal hypersensitivity, but concludes that the absence of a casual relationship means that the use of “hypoallergenic” implants cannot be justified [43]. Other studies advocate the view that pre-implantation testing is unhelpful and of minimal benefit. For instance, Lachiewicz et al. proposed that pre-implantation screening prior to TKA is not necessary and that metal allergy post-TKA should only be diagnosed after all other possibilities are excluded [40].
A group of 18 patients with pre-implant confirmed nickel allergy were followed for 6.3 years following implantation of a nickel-containing device. None of these patients developed cutaneous or systemic signs of metal hypersensitivity [45]. In another study of 50 patients following TKA, 32% had positive skin patch tests to the metal constituents of the device (n = 16), but there was no correlation between allergy and loosening or other prosthesis morbidity [42]. At this time, there are no definitive studies supporting any diagnostic test for routine pre-implant screening.
While these studies are not supportive of pre-implant metal allergy evaluation, it is also still important to consider patient history of possible metal allergy when making an implant choice prior to surgery. A patient’s psychological status has strong influences on their clinical outcomes [46]. Patient reports of metal allergy prior to implantation were associated with poorer functional outcomes (TKA) and mental health scores (THA) [44]. Similar findings have been reported, finding that patient-reported allergies are a surrogate for mental health factors that lead to increased postoperative morbidity, and poorer functional/psychosocial outcomes [47,48,49]. In a study of 459 THA or TKA patients reporting ≥4 allergies, they had decreased improvement and functional outcomes following surgery when compared to those with fewer allergies [50]. A strong predictor of post-operative satisfaction following TKA is whether or not the surgeon met the patient’s pre-operative expectations [51]. Thus, clear communication and making an effort to define patient expectations is important. Defining metal allergy status is one of many factors necessary to building a unique management plan for the individual. In some cases, it may be beneficial and indicated to use an appropriate allergen-free implant to eliminate patient worry as a potential source of post-operative pain in those reporting clinical metal reactions [44].
The Danish experience as reported by Thyssen advises against routine pre-surgery patch testing unless there is a patient or clinical history of metal reactions “of a magnitude sufficient to cause concern to the patient or the doctor” [52]. In Sweden, “virtually no such patients are evaluated” [53]. In the United Kingdom, a Delphi Analysis of orthopedic surgeons reported that standard cobalt chromium/stainless steel devices should be implanted regardless of the patient’s metal allergy status [54]. In Germany, a consensus group pragmatically suggests using titanium alloys for any patient self-reporting metal allergy. No pre-implant testing was recommended [55]. Earlier perspectives from the United States were from Granchi and Reed, both suggesting patch testing prior to surgery in patients reporting a clinical history or metal sensitivity [56, 57]. Recently, the American Contact Dermatitis Society (ACDS) published a consensus opinion regarding metal hypersensitivity reactions to implanted devices. Routine pre-implant testing is not recommended. In those rare patients self-reporting metal reactions on the skin, evaluation is suggested but not mandatory [58].
In a survey performed at the European Society of Contact Dermatitis (ESCD) and subsequently the ACDS meetings, 54% of respondents considered patch testing prior to surgery indicated for those individuals reporting moderate or severe rashes after metal contact. For those not agreeing with preoperative testing, 38% considered a titanium-based alloy an acceptable alternative [59]. Schalock and colleagues recommend a thoughtful and custom approach to pre-implant metal allergy: when the patch test is positive, other factors must still be taken into account, such as choosing the device that will be the best functional and durable implant [60]. Ultimately, it is up to the patient and surgeon to decide the ‘best’ and most appropriate device.
Which Test is Preferred Pre-Implantation?
If pre-implantation testing is needed, the question that follows is which test would be most appropriate? Determining delayed-type hypersensitivity to metals can be done via two routes: by skin patch testing or through a blood test such as the lymphocyte transformation test (LTT) or leukocyte migration inhibition test.
The patch test is performed on the skin and is simple to perform, widely available and offers a wide variety of possible testing when compared to the LTT [61]. Intradermal testing is rarely used due to false positive reactions with metal allergens [59, 62,63,64,65,66]. The LTT is a measurement of lymphocyte proliferation in the presence and absence of a potential allergen. The patient’s lymphocytes are taken from peripheral blood and incubated for 7 days, with and without the allergen presence. The result is reported as a stimulation index, comparing the reactions. In the leukocyte migration inhibition test, mixed population leukocyte migration activity is measured in the presence of antigen. If the result is positive, migration is faster in non-allergic individuals [30].
The patch test is considered the gold standard for detecting systemic type IV hypersensitivity reactions in the opinion of dermatologists. In a survey of the ACDS and EACD members, 83% of respondents considered the patch test to be the diagnostic test of choice for evaluation of metal allergy. Only 12% of dermatologist commonly used the LTT [59, 67]. Orthopedic surgeons have different views of metal allergy and the necessity of testing. Their general opinion is that there is not a relevant correlation between patch testing on the skin and the immunologic responses in and around the bone–implant interface [68]. This reluctance may in fact be correct, since the relationship between actual skin reactions in response to implanted metal allergy as well as peri-implant morbidity continues to be unclear [69]. Skin exposure is not the same as the constant exposure experienced in the closed subcutaneous environment adjacent to the metallic implant. The dendritic cells present similar, but not the same. It is possible that the patch test only partially reproduces this peri-implant environment [70, 71]. In the skin, the Langerhans cells are the primary antigen-presenting cell, while other similar dendritic cells and macrophages take on this role adjacent to the bone–implant interface. Langerhans cells seem to have a greater antigen-presenting ability when compared to macrophages in the blood [30, 72]. Due to this, some believe that the LTT is more useful for prognosis and diagnosis of metal reactions when compared to patch testing [73, 74].
Despite this, it is unlikely that the LTT will replace the patch test as the gold standard and most commonly clinically used test. Unfortunately, the LTT is not widely available for clinical use, is not standardized, has inter-laboratory variability and is often not covered by insurance (leading to higher patient costs). Also, the LTT may produce false negative results if the test is not transported and processed in a timely manner. Due to rapid T cell decay, even short delays can lead to false negative results [60].
At this time, the scientific literature and these authors thus favor the skin patch test as the best available test to evaluate potential metal hypersensitivity reactions, both prior to and following implantation. The role of the LTT remains unclear, but seems to be gaining support for use in conjunction with the patch test and potentially coupled with peri-implant histopathology [60, 67, 75, 76]. Protocols for patch testing have been proposed based on implant type and surgical location [60, 77, 78].
One suggested use for the LTT is for further evaluation of those patients with negative patch testing and a residual strong clinical suspicion for metal allergy. In an evaluation of 56 patients with titanium alloy implants with systemic symptoms and negative skin patch testing, 54/56 had positive LTT. These 54 had complete symptom resolution after implant replacement with a non-titanium device [79]. Another study combined three in vitro assays, measuring different aspects of lymphocyte activation in the hope of improving diagnosis [80]. At this time, more research is needed to definitively determine the validity and appropriate clinical use of the LTT [81].
Post-Implantation Testing
Surprisingly, there is unanimous consensus on how patients with asymptomatic, well-functioning devices should be managed: there is no indication for metal allergy testing.
Management of patients who suffer from residual post-implantation pain is not as well defined. It is difficult to ascertain, using patch testing alone, if a patient truly does suffer from metal hypersensitivity, and idetermining which patient would benefit from implant removal/revision is also challenging. Granchi et al. concluded that testing is indicated in failed metal-on-metal temporomandibular joint replacements with unclear diagnosis [82]. The assumption is that there exist numerous more common causes for pain, loosening and/or failure and that these should be explored prior to considering metal hypersensitivity as the cause. These include component malalignment, complex regional pain syndrome, crepitation, early aseptic loosening, infection, instability, patellofemoral symptoms or patellar clunk syndrome [83]. For patients who experience residual pain after TKA, metal hypersensitivity should only be suspected if the patient had a normal physical exam and radiographs/CT scans or MARS MRI, and normal laboratory work-up [83]. An alternative approach uses clinical findings to identify those with a high suspicion of metal allergy who may benefit from metal allergy evaluation [77].
Major diagnostic criteria for post-implantation metal hypersensitivity reactions include [78]:
-
Eruption overlying the metal implant.
-
Positive patch test reaction to a metal used in the implant.
-
Complete recovery after removal of the offending implant.
-
Chronic dermatitis beginning weeks to months after metallic implantation.
While reactions considered to be less important are:
-
Dermatitis is therapy-resistant.
-
Morphology consistent with dermatitis (erythema, induration, papules, vesicles).
-
Systemic allergic dermatitis reaction.
-
Histology consistent with allergic contact dermatitis.
-
Positive in vitro test to metals, e.g., the lymphocyte transformation test.
Paradoxically, to arrive at a definitive diagnosis of metal allergy, it is necessary for the patient to undergo complete resolution of symptoms after device replacement with a non-allergenic implant. In a similar vein, Middleton suggests that reaching a definitive diagnosis of allergy-related implant is near-impossible, as not only does it require a show of improvement of clinical symptoms after implant replacement with an immunologically inert device but there should also technically be evidence of typical T-lymphocyte-rich immunohistopathology and a positive implant-relevant epicutaneous patch test [44].
While it is promising that the academic discussion surrounding metal hypersensitivity is thriving, the conflicting conclusions in the literature shed insufficient light on pertinent issues, including but not limited to how patients who suffer from chronic post-surgical pain should be managed and the extent to which symptoms may be caused by metal allergy. More studies are needed before a definitive, evidence-based algorithm for diagnosis and management can be generated to tackle the existing dilemma.
Conclusion
If an evidence-based approach is desired, there is only one consensus regarding the morbidity of metal allergy from implanted devices—there is no agreement. In clinical practice, it is a luxury to have guidelines which are clearly supported by a body of evidence. Since this is not the case, patch testing dermatologists, allergists and the surgeons using the metal devices need to understand the possible risks of using an “allergenic” device and appropriately consent each individual based on their own history and concerns. A stronger body of research is needed to clarify the relationship between metal allergy and reactions to implanted metal devices. Once a clear understanding of this relationship is defined, if it exists, appropriate guidelines can be drafted in the attempt to clarify management of or completely avoid allergic reactions to metal implants.
Some simple conclusions:
-
Reactions to metal orthopedic implants do occur, though rarely, even in those with metal allergy.
-
Routine pre-implant testing in asymptomatic individuals is not indicated.
-
Listen to patient’s concerns about metal allergy if the concern arises.
-
Patch testing is probably the best pre- and post-implant screening test.
-
Post-implantation testing is controversial and even positive LTT or patch test does not definitively diagnose morbidity from a metal allergy. Complete recovery following revision with an immunologically inert device is diagnostic.
-
More research is needed to scientifically approach this issue.
References
Williams SN, Wolford ML, Bercovitz A. Hospitalization for total knee replacement among inpatients aged 45 and over: United States, 2000–2010. NCHS Data Brief. 2015;210:1–8.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780.
Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–54.
Morwood MP, Garrigues GE. Shoulder arthroplasty in the patient with metal hypersensitivity. J Shoulder Elbow Surg. 2015;24(7):1156–64.
Disegi JA, Eschbach L. Stainless steel in bone surgery. Injury. 2000;31(Suppl 4):2–6.
Foussereau J, Langier P. Allergic eczemas from metallic foreign bodies. Trans St Johns Hosp Dermatol Soc. 1966;52:220–5.
Benson MK, Goodwin PG, Brostoff J. Metal sensitivity in patients with joint replacement arthroplasties. Br Med J. 1975;15:374–5.
Christiansen K, Holmes K, Zilko PJ. Metal sensitivity causing loosened joint prostheses. Ann Rheum Dis. 1980;39(5):476.
Lalor PA, Revell PA, Gray AB, et al. Sensitivity to titanium. A cause of implant failure? J Bone Joint Surg Br. 1991;73(1):25.
Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implantsda prospective study. Int J Occup Med Environ Health. 2012;25(4):463.
Basketter DA, Briatico-Vangosa G, Kaestner W, Lally C, Bontinck WJ. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis. Contact Dermatitis. 1993;28:15–25.
Cramers M, Lucht U. Metal sensitivity in patients treated for tibial fractures with plates of stainless steel. Acta Orthop Scand. 1977;48:245–9.
Fisher AA. Allergic dermatitis presumably due to metallic foreign bodies containing nickel or cobalt. Cutis. 1977;19:285–6.
Rostoker G, Robin MD, Binet MD, et al. Dermatitis due to orthopaedic implants: a review of literature and report of three cases. J Bone Joint Surg. 1987;69:1408–12.
Merle C, Vigan M, Devred D, et al. Generalized eczema from Vitallium osteosynthesis material. Contact Dermatitis. 1992;27:257–8.
Ridley CM. How relevant is cobalt sensitivity in a patient with unsatisfactory total knee replacement? Clin Exp Dermatol. 1977;2:401–4.
Symeonides PP, Paschaloglu C, Papageorgiou S. An allergic reaction after fixation of a fracture using a Vitallium plate. J Allergy Clin Immunol. 1993;51:251–2.
Munro-Ashman D, Miller AJ. Rejection of metal to metal prosthesis and skin sensitivity to cobalt. Contact Dermatitis. 1976;2:65–7.
Verma SB, Mody B, Gawkrodger DJ. Dermatitis on the knee following knee replacement: a minority of cases show contact allergy to chromate, cobalt or nickel but a causal association is unproven. Contact Dermatitis. 2006;54:228–9.
Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22(2):65–79.
Halpin DS. An unusual reaction in muscle in association with a Vitallium plate: a report of possible metal hypersensitivity. J Bone Joint Surg Br. 1975;57:451–3.
Black J, Sherk H, Bonini J, Rostoker WR, Schajowicz F, Galante JO. Metallosis associated with a stable titanium-alloy femoral component in total hip replacement: a case report. J Bone Joint Surg Am. 1990;72:126–30.
Nakamura S, Yasunaga Y, Ikuta Y, Shimogaki K, Hamada N, Takata N. Autoantibodies to red cells associated with metallosis—a case report. Acta Orthop Scand. 1997;68:495–6.
Thyssen JP, Johansen JD, Menné T, Lidén C, Bruze M, White IR. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010; 162:235–6.
Zamzow H. Allergic reactions to knee prostheses from the viewpoint of a trauma surgeon of the “Medizinischer Dienst der Krankenversicherung”. Orthopade. 2008;37(2):1190–4.
Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Soballe K, Menne T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646–52.
Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–21.
Antony FC, Holden CA. Metal allergy resurfaces in failed hip endoprostheses. Contact Dermatitis. 2003;48:49–50.
Milavec-Puretic V, Orlic D, Marusic A. Sensitivity to metals in 40 patients with failed hip endoprosthesis. Arch Orthop Trauma Surg. 1998;117:383–6.
Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83A:428–36.
Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80:653–9.
Fang CS, Harvie P, Gibbons CL, Whitwell D, Athanasou NA, Ostlere S. The imaging spectrum of peri-articular inflammatory masses following metal-on-metal hip resurfacing. Skeletal Radiol. 2008;37:715–22.
Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36.
Mikhael MM, Hanssen AD, Sierra RJ. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J Bone Joint Surg Am. 2009;91:443–6.
Campbell P, Shimmin LW, Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008;23:1080–5.
Jensen P, Thyssen JP, Retpen JB, Menne T. Cobalt allergy and suspected aseptic lymphocyte-dominated vascular-associated lesion following total hip arthroplasty. Contact Dermatitis. 2009;61:238–9.
Counsell A, Heasley R, Arumilli B, Paul A. A groin mass caused by metal particle debris after hip resurfacing. Acta Orthop Belg. 2008;74:870–4.
Granchi D, Cenni E, Tigani D, Trisolino G, Baldini N, Giunti A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494–500.
Niki Y, Matsumoto H, Otani T, Yatabe T, Kondo M, Yoshimine F, Toyama Y. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005;26:1019–26.
Lachiewicz PF, Watters TS, Jacobs JJ. Metal hypersensitivity and total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):106–12.
Bravo D, Wagner ER, Larson DR, Davis MP, Pagnano MW, Sierra RJ. No increased risk of knee arthroplasty failure in patients with positive skin patch testing for metal hypersensitivity: a matched cohort study. J Arthroplasty. 2016;31(8):1717–21.
Webley M, Kates A, Snaith ML. Metal sensitivity in patients with a hinge arthroplasty of the knee. Ann Rheum Dis. 1978;37:373–5.
Middleton S, Toms A. Allergy in total knee arthroplasty: a review of the facts. Bone Joint J. 2016; 98-B:437–41.
Nam D, Li K, Riegler B, Barrack RL. Patient-reported metal allergy: a risk factor for poor outcomes after total joint arthroplasty. J Arthroplasty. 2016;31(9):1910–5.
Carlsson A, Möller H. Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69:62–6.
Giesinger JM, Kuster MS, Behrend H, et al. Association of psychological status and patient-reported physical outcome measures in joint arthroplasty: a lack of divergent validity. Health Qual Life Outcomes. 2013;11:64.
Perruccio AV, Davis AM, Hogg-Johnson S, et al. Importance of self-rated health and mental well-being in predicting health outcomes following total joint replacement surgery for osteoarthritis. Arthritis Care Res (Hoboken). 2011;63(7):973.
Lavernia CJ, Alcerro JC, Brooks LG, et al. Mental health and outcomes in primary total joint arthroplasty. J Arthroplasty. 2012;27(7):1276.
Browne JA, Sandberg BF, D’Apuzzo MR, et al. Depression is associated with early postoperative outcomes following total joint arthroplasty: a nationwide data- base study. J Arthroplasty. 2014;29(3):481.
Graves CM, Otero JE, Gao Y, et al. Patient reported allergies are a risk factor for poor outcomes in total hip and knee arthroplasty. J Arthroplasty. 2014;29(9 Suppl):147.
Bourne RB, Chesworth BM, Davis AM, et al. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010; 468(1):57.
Thyssen JP, Menné T, Schalock PC, Taylor JS, Maibach HI. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473–478.
Bruze M. Thoughts on implants and contact allergy. Arch Dermatol. 2008;144(8):1042–4.
Razak A, Ebinesan AD, Charalambous CP. Metal allergy screening prior to joint arthroplasty and its influence on implant choice: a delphi consensus study amongst orthopaedic arthroplasty surgeons. Knee Surg Relat Res. 2013;25:186–93.
Thomas P, Schuh A, Ring J, Thomsen M. Orthopedic surgical implants and allergies: joint statement by the implant allergy working group (AK 20) of the DGOOC (German Association of Orthopedics and Orthopedic Surgery), DKG (German Contact Dermatitis Research Group) and DGAKI (German Society for Allergology and Clinical Immunology). Orthopade. 2008;37:75–88.
Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94:1126–34.
Reed KB, Davis MD, Nakamura K, Hanson L, Richardson DM. Retrospective evaluation of patch testing before or after metal device implantation. Arch Dermatol. 2008;144:999–1007.
Schalock PC, Crawford G, Nedorost S, Scheinman PL, Atwater AR, Mowad C, Brod B, Ehrlich A, Watsky KL, Sasseville D, Silvestri D, Worobec SM, Elliott JF, Honari G, Powell DL, Taylor J, Dekoven J. American Contact Dermatitis Society: patch testing for evaluation of hypersensitivity to implanted metal devices. Dermatitis. 2016;27:241–7.
Schalock PC, Thyssen JP. Metal hypersensitivity reactions to implants—opinions and practices of patch testing dermatologists. Dermatitis. 2013;24:313–20.
Schalock PC, Menné T, Johansen J, Taylor JS, Maibach HI, Lidén C, Bruze M, Thyssen JP. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66(1):4–19.
Thyssen JP, Menné T, Schalock PC, Taylor JS, Maibach HI. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011; 164:473–8.
Baik JJ, Yoon YB, Park HS. Cobalt-induced occupational asthma associated with systemic illness. J Korean Med Sci. 1995;10:200–4.
Leguy-Seguin V, Jolimoy G, Coudert B, Pernot C, et al. Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol. 2007;119:726–30.
Morris DL. Intradermal testing and sublingual desensitization for nickel. Cutis. 1998;61:129–32.
Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from titanium. Contact Dermatitis. 2000;42:52–3.
Herbst RA, Lauerma AI, Maibach HI. Intradermal testing in the diagnosis of allergic contact dermatitis. A reappraisal. Contact Dermatitis. 1993;29(1):1–5.
Mowad CM. Practice gap: the role of patch testing in the selection and management of metal device implants. Arch Dermatol. 2012;148(6):693–4.
Cousen PJ, Gawkrodger DJ. Metal allergy and second generation metal on metal arthroplasties. Contact Dermatitis. 2012;66(2):55–62.
Jacobs JJ. Clinical manifestations of metal allergy. Adverse reactions to byproducts of joint replacements (AAOS/ORSI). Presented at the American academy of Orthopaedic Surgery 2012 Annual Meeting. Feb. 7–11. San Francisco.
Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19(2):213–24.
Münch HJ, Jacobsen SS, Olesen JT, Menné T, Søballe K, Johansen JD, Thyssen JP. The association between metal allergy, total knee arthroplasty, and revision. Acta Orthop. 2015;13:1–6.
Everness KM, Gawkrodger DJ, Botham PA, Hunter JA. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol. 1990;122:293–8.
Luque I, Leyva L, Jose Torres M, Rosal M, Mayorga C, Segura JM, Blanca M, Juarez C. In vitro T-cell responses to beta-lactam drugs in immediate and nonimmediate allergic reactions. Allergy. 2001;56:611–8.
Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997;27:175–81.
Atanaskova Mesinkovska N, Tellez A, Molina L, Honari G, Sood A, Barsoum W, Taylor JS. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148(6):687–93.
Shah B, Cohee A, Deyerle A, Kelly CS, Frantz F, Kelly RE, Kuhn MA, Lombardo M, Obermeyer R, Goretsky MJ. High rates of metal allergy amongst Nuss procedure patients dictate broader pre- operative testing. J Pediatr Surg. 2014;49(3):451–4.
Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013; 24:183–5.
Mitchelson AJ, Wilson CJ, Mihalko WM, Grupp TM, Manning BT, Dennis DA, Goodman SB, Tzeng TH, Vasdev S, Saleh KJ. Biomaterial hypersensitivity: is it real? Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. Biomed Res Int. 2015;2015:137287.
Muller KE, Valentine-Thon E. Hypersensitivity to titanium: clinical and laboratory evidence. Neuro Endocrinol Lett. 2006;27:311–3.
Hallab NJ, Mikecz K, Jacobs JJ. A triple assay technique for the evaluation of metal-induced, delayed-type hypersensitivity responses in patients with or receiving total joint arthroplasty. J Biomed Mater Res. 2000;53:480–9.
Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252–62.
Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94:1126–34.
Park CN, White PB, Meftah M, Ranawat AS, Ranawat CS. Diagnostic Algorithm for residual pain after total knee arthroplasty. Orthopedics. 2016;39:246–52.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Disclosures
Wendy Z. W. Teo and Peter C. Schalock have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/2A47F0604291DE8E.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Teo, W.Z.W., Schalock, P.C. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol Ther (Heidelb) 7, 53–64 (2017). https://doi.org/10.1007/s13555-016-0162-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-016-0162-1