Abstract
Introduction
Due to the high prevalence of actinic keratosis (AK) and potential for lesions to become cancerous, clinical guidelines recommend that all are treated. The objective of this study was to evaluate the efficacy and safety of 5-fluorouracil (5-FU) 0.5%/salicylic acid 10% as field-directed treatment of AK lesions.
Methods
This multicenter, double-blind, vehicle-controlled study (NCT02289768) randomized adults, with a 25 cm2 area of skin on their face, bald scalp, or forehead covering 4–10 clinically confirmed AK lesions (grade I/II), 2:1 to treatment or vehicle applied topically once daily for 12 weeks. The primary endpoint was the proportion of patients with complete clinical clearance (CCC) of lesions in the treatment field 8 weeks after the end of treatment. Secondary endpoints included partial clearance (PC; ≥75% reduction) of lesions. Safety outcomes were assessed.
Results
Of 166 patients randomized, 111 received 5-FU 0.5%/salicylic acid 10% and 55 received vehicle. At 8 weeks after the end of treatment, CCC was significantly higher with 5-FU 0.5%/salicylic acid 10% than with vehicle [49.5% vs. 18.2%, respectively; odds ratio (OR) 3.9 (95% CI) 1.7, 8.7; P = 0.0006]. Significantly more patients achieved PC of lesions with treatment than with vehicle [69.5% vs. 34.6%, respectively; OR 4.9 (95% CI 2.3, 10.5); P < 0.0001]. Treatment-emergent adverse events, predominantly related to application- and administration-site reactions, were more common with 5-FU 0.5%/salicylic acid 10% than with vehicle (99.1% vs. 83.6%).
Conclusions
Compared with vehicle, field-directed treatment of AK lesions with 5-FU 0.5%/salicylic acid 10% was effective in terms of CCC. Safety outcomes were consistent with the known and predictable safety profile.
Trial registration
NCT02289768.
Funding
Almirall S.A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinic keratosis (AK) is a common skin condition characterized by dysplastic lesions of keratinocytes that have the potential to become malignant [1, 2]. Infiltrative transformation of AK grade III lesions to invasive squamous cell carcinoma (SCC) was believed to occur via a classical pathway involving sequential progression from grade I through to grade III AK. However, recent findings indicate that invasive SCC can expand directly from a grade I AK lesion [3]. Owing to the high prevalence of AK, the risk of lesions becoming cancerous, and the inability to predict which lesions will progress to SCC, this justifies the treatment of all AK lesions regardless of grade [4, 5].
Current options for the topical treatment of AK lesions include diclofenac, hyaluronic acid, 5-fluorouracil (5-FU), imiquimod, and ingenol mebutate [6,7,8]. Lesion- or field-directed therapy is indicated subject to the specific characteristics of the lesions to be treated (e.g., their number, localization, extent, and clinical course) and patient features (e.g., age, comorbidities, and other risk factors) [9, 10]. Historically, lesion-directed treatments have been the most common approach for treating a single lesion, whereas field-directed therapies, which aim to treat areas with multiple AK lesions of varying degrees of severity, including sub-clinical (non-visible) lesions [9], are now preferred if lesion and patient characteristics permit [11].
5-FU 0.5%/salicylic acid 10% (Actikerall®, Almirall S.A.) is indicated for the topical treatment of slightly palpable and/or moderately thick hyperkeratotic AK lesions (grade I/II according to Olsen et al. 1991 [12]) in immunocompetent adults, with an option to simultaneously treat multiple AK lesions, up to a maximum of 10 lesions in a total skin area of 25 cm2 [13]. However, only a maximum rim of 0.5 cm of healthy skin surrounding the lesions can come into contact with 5-FU 0.5%/salicylic acid 10%.
Here we report new efficacy and safety data from the first randomized controlled trial investigating the efficacy and safety of 5-FU 0.5%/salicylic acid 10% when applied as field-directed treatment to a contiguous area of 25 cm2 in a field cancerization area on the face, bald scalp, or forehead of patients with 4–10 clinically confirmed AK lesions (grade I and II).
Methods
Study Design and Ethical Conduct
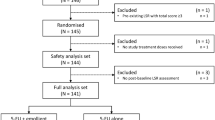
This phase III, multicenter, randomized, double-blind, vehicle-controlled study (ClinicalTrials.gov identifier: NCT02289768) was conducted at 14 sites in Germany and the UK. There were five treatment visits and a follow-up visit 8 weeks after the final treatment application, regardless of whether a patient had completed 12 weeks of treatment or prematurely discontinued from treatment (Fig. 1). Findings from a sub-study that examined the effect of 5-FU 0.5%/salicylic acid 10% on sub-clinical AK lesions using reflectance confocal microscopy (RCM) in a group of 30 patients will be published separately.
The study was conducted in accordance with the recommendations of the Helsinki Declaration of 1964, as revised in 2008, complied with International Conference on Harmonisation Good Clinical Practice guidelines and local regulations and was approved by an independent ethics committee. Informed consent was obtained from all patients in writing prior to inclusion in the study.
Patients
Male and female (non-pregnant, non-lactating in the last 3 months) patients were enrolled in the study if they were aged 18–85 years and had 4–10 clinically confirmed AK lesions (grade I/II [12]) within a field cancerization area of 25 cm2 located on the face, bald scalp, or forehead. Patients had Fitzpatrick skin type I–IV, were in good health and were free of physical and mental conditions that could interfere with the examination or evaluation of the potential treatment area. During the study, patients had to refrain from sunbathing and avoid intense ultraviolet-light exposure/solarium. They also had to avoid the use of moisturizers and topical treatments with anti-aging products, ointments containing vitamins A, C, and/or E, and gels and green-tea preparations in the treatment area. Patients also had to be physically able (or have a supportive person) to apply the study preparations correctly and to follow the study procedure and restrictions.
Key exclusion criteria included patients who ≤3 months before screening had received treatment for AK within the treatment area or if they had dermatological diseases in the treatment area or surrounding area that could be exacerbated by study treatment or could interfere with the study assessments (e.g., psoriasis, eczema). Additionally, patients were excluded if they had received topical treatment with certain pharmacological and non-pharmacological products (e.g., retinoids, steroids, diclofenac or 5-FU preparations, curettage, photodynamic therapy, and chemical peel) in the treatment area 4–8 weeks (depending on the product) prior to randomization. Patients were also excluded if they had received systemic medication (varying between 4 and 12 weeks, depending on the medication), including: interferons; immunomodulators or immunosuppressive drugs; diclofenac or 5-FU preparations; and cytotoxic drugs. The use of phenytoin, methotrexate, or sulfonylurea was also not allowed.
Treatment
At visit 2, patients were randomized 2:1 in ascendant chronological order to receive topical 5-FU 0.5%/salicylic acid 10% or colour/consistency/appearance-matched vehicle, which was self-administered once daily for 12 weeks using a brush enclosed in the cap of the medication container. This was a double-blind study so neither the patient nor the research personnel knew the treatment assigned to each patient.
Study medication was applied at the same time each day according to the instructions in the product patient leaflet, except that the area of application was a contiguous 25 cm2. The dose regimen could be decreased by the physician to three doses per week in the case of severe local skin reactions. Treated areas were left uncovered, and study medication was allowed to dry to enable a film to form over the area. Prior to daily re-application of the medication, the film coating was peeled off and the skin washed with water and a damp cloth. Patients were instructed to not apply medication to bleeding lesions.
The location of the treated area was chosen according to the ability of the patient to conveniently apply study medication on a daily basis. A plastic template was used to mark and later identify the same 25 cm2 treated area. The first and final treatment applications were performed at the study center by study staff.
Study Assessments and Endpoints
The primary endpoint was the proportion of patients with complete clinical clearance (CCC) of AK lesions in the treatment field at 8 weeks after the end of treatment. CCC of AK lesions at each treatment visit (i.e., after 2, 4, 6, and 12 weeks of treatment) was measured as a secondary endpoint. Additional secondary endpoints reporting the effect of treatment on lesions included: partial clearance (PC; defined as ≥75% reduction in clinically visible AK lesions) at each treatment visit and 8 weeks after the end of treatment; proportional change from baseline in the total number of AK lesions at each treatment visit and 8 weeks after the end of treatment; the number of lesions by AK grade severity at baseline and 8 weeks after the end of treatment; and proportional change from baseline in the total number of lesions at each treatment visit and 8 weeks after the end of treatment.
A physician-reported outcome was global assessment of efficacy [Physician Global Assessment (PGA)] at each treatment visit and 8 weeks after the end of treatment. Patient-reported outcomes included patient satisfaction with treatment by recording individual domain scores (i.e., effectiveness, side effects, convenience, and overall satisfaction) of the Treatment Satisfaction Questionnaire for Medication (TSQM, version 1.4) at 8 weeks after the end of treatment and quality-of-life assessment by recording the change from baseline in total and individual domain scores (i.e., daily activities, leisure, personal relationships, symptoms and feelings, treatment, work, and school) of the Dermatology Life Quality Index (DLQI) after 12 weeks of treatment and at 8 weeks after the end of treatment. DLQI was calculated by summing the score of each question resulting in a maximum of 30 and a minimum of 0, and the higher the score, the more quality of life is impaired.
Adverse events (AEs) were collected from the time of informed consent to the follow-up visit; this was always performed 8 weeks post last treatment, regardless of whether the patient had completed the 12-week treatment period or had prematurely discontinued from the study. The reporting of AEs was elicited by asking patients non-leading questions and by collecting information regarding the AEs spontaneously reported by the patients to study staff.
Statistical Analysis
It was determined that a total of 146 patients (97 receiving active treatment, 49 receiving vehicle) was required to complete the study to provide 80% power to detect as “significant” a difference of 25% between 5-FU 0.5%/salicylic acid 10% and vehicle in the proportion of patients with CCC, assuming a clearance rate in the active group of 50% and a clearance rate in the vehicle group of 25%. To allow for an estimated 10% dropout rate, approximately 162 patients (108 receiving treatment, 54 receiving vehicle) were to be randomized.
The primary endpoint (CCC) comparison of 5-FU 0.5%/salicylic acid 10% versus vehicle was analyzed using the Cochran-Mantel-Haenszel test statistic adjusting for anatomical site (face/scalp) and baseline (number of AK lesions). The number and proportion of responders for each treatment group, odds ratio (OR), corresponding 95% confidence interval (CI), and the two-sided P value associated with the Cochran-Mantel-Haenszel test were calculated. The 95% CI for the proportion of patients with CCC was calculated using the exact binomial test.
The secondary endpoint of the proportion of patients with CCC of AK lesions in the treatment field at each treatment visit was analyzed in the same way as the primary efficacy variable. Other secondary endpoints and safety data were analyzed using descriptive statistics. Missing efficacy data were handled using the last observation carried forward (LOCF). An analysis of covariance model with treatment arm and anatomical site as factors and baseline as covariate was used for the analysis of total and individual domain scores of the TSQI and DLQI. All secondary endpoints were analyzed using the intent-to-treat (ITT) population only.
Results
Patients
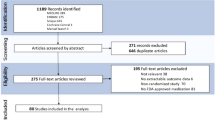
This study was conducted between 17 October 2014 and 10 August 2015. Of the 175 patients screened, 166 were randomized. Of these, 111 patients received 5-FU 0.5%/salicylic acid 10% (108 patients in the safety and ITT populations) and 55 patients received vehicle (Fig. 2).
Patient disposition. a Includes 3 patients who were counted only as randomized; b overall, 12 patients prematurely discontinued treatment but completed follow-up: 5-FU/SA, n = 9; vehicle, n = 3. c All patients who discontinued follow-up also discontinued treatment: 5-FU/SA, n = 6; vehicle, n = 2. 5-FU/SA 5-fluorouracil 0.5%/salicylic acid 10%
Baseline demographics were similar between the groups, with slightly more female patients in the active treatment arm (14.8%) versus vehicle (7.3%; Table 1). At baseline, 56.6% of lesions were classified as grade I and 43.4% were classified as grade II; similar ratios of grade I and grade II AK lesions were present in each treatment arm.
Efficacy
The percentage of patients with CCC 8 weeks after the end of treatment (primary endpoint) was significantly higher in the 5-FU 0.5%/salicylic acid 10% arm compared with vehicle in both the ITT and per protocol (PP) populations [ITT LOCF: 49.5% vs. 18.2%; OR 3.9 (95% CI 1.7, 8.7) P = 0.0006 (Fig. 3a); PP LOCF: 55.1% vs. 19.6%; OR 5.1 (95% CI 2.1, 12.2) P = 0.0002]. During treatment, there were no significant differences between 5-FU 0.5%/salicylic acid 10% and vehicle (Fig. 3b); however, it should be noted that it can be difficult to assess lesion counts during treatment because of irritation at the site of administration.
Proportion of patients with complete clinical clearancea of AK lesions in the treatment field a at 8 weeks after the end of treatment (intent-to-treat population) and b during treatment (after 2, 4, 6, and 12 weeks) and 8 weeks after the end of treatment (intent-to-treat population). *P = 0.0006. Analysis was performed using the Cochran-Mantel-Haenszel test, adjusting for anatomical site and baseline. The last observation carried forward was used for missing data; however, for 5-FU/SA, three patients had only baseline data available so it was not possible to replace the missing data. a Complete clinical clearance defined as all lesions cleared and lesion count of zero at each visit. 5-FU/SA 5-fluorouracil 0.5%/salicylic acid 10%, AK actinic keratosis
Eight weeks after the end of treatment, the proportion of patients who achieved PC of AK lesions was significantly greater in the 5-FU 0.5%/salicylic acid 10% arm than in the vehicle arm [69.5% vs. 34.6%; OR 4.9 (95% CI 2.3, 10.5) P < 0.0001 (Fig. 4)]. The proportional reduction from baseline in the total number of AK lesions per patient was significantly greater with 5-FU 0.5%/salicylic acid 10% than with vehicle: 78.0% versus 46.9%, respectively; P < 0.0001 (Fig. 5). For both PC and reduction in lesion count, there were no significant differences between the treatment groups at each visit of the 12-week treatment period. At 8 weeks after the end of treatment, a higher proportion of AK lesions in the active treatment arm had transitioned from grade I/II to grade 0 compared with the vehicle arm (Fig. 6).
Proportion of patients with partial clearancea of AK lesions in the treatment field during treatment (after 2, 4, 6, and 12 weeks) and 8 weeks after the end of treatment (intent-to-treat population). *P < 0.0001. a Partial clearance defined as ≤75% reduction in the number of clinically visible AK lesions. 5-FU/SA 5-fluorouracil 0.5%/salicylic acid 10%, AK actinic keratosis
Proportion of AK lesions by severity a(according to Olsen et al. [12]) at a baseline and b 8 weeks after the end of treatment (intent-to-treat population). a 5-FU/SA 5-fluorouracil 0.5%/salicylic acid 10%, AK actinic keratosis
Assessment of treatment efficacy according to PGA found that for 5-FU 0.5%/salicylic acid 10%, those with a PGA score of “good” or “very good” increased from 45.2% at week 2 to 90.2% at follow-up (Fig. 7). In contrast, this increased from 61.1% at week 2 to no more than 75.5% at follow-up for vehicle (P < 0.0001).
At 8 weeks after the end of treatment, 5-FU 0.5%/salicylic acid 10% was associated with significant improvements in overall treatment satisfaction and effectiveness domain mean scores in the TSQM compared with vehicle [69.2 vs. 56.1 (P = 0.0019); 70.8 vs. 59.2 (P = 0.0064), respectively]. No statistically significant differences were observed between the study arms for the TSQM convenience (70.7 and 71.2, respectively) and side effect (92.4 and 96.4, respectively) domain scores.
The clinical impact of AK lesions on the DLQI individual domains at baseline was low, with symptoms and feelings being the most affected. As shown in Table 2, improvement in DLQI total score was statistically greater for vehicle versus 5-FU 0.5%/salicylic acid 10% during the treatment phase; this was attributed to local skin reactions associated with 5-FU 0.5%/salicylic acid 10%. However, the improvement in DLQI total score switched in favor of 5-FU 0.5%/salicylic acid 10% 8 weeks after the end of treatment, although this was not statistically significant (P = 0.0725; Table 2; Supplementary Fig. S1).
Safety
The incidence of treatment-emergent AEs (TEAEs) was slightly higher in the 5-FU 0.5%/salicylic acid 10% study arm compared with vehicle (99.1% vs. 83.6%, respectively) (Table 3). This was predominantly a consequence of application- and administration-site reactions, occurring in 99.1% and 76.4% of respective patients in the active treatment and vehicle arms; the majority of these were of mild or moderate intensity. Nine (5.4%) patients had 11 treatment-emergent serious AEs (TESAEs): in the 5-FU 0.5%/salicylic acid 10% arm, 6 (5.6%) patients had a total of 8 TESAEs, and, in the vehicle arm, 3 (5.5%) patients had a total of 3 TESAEs.
Treatment was discontinued as a result of a TEAE in two (1.9%) patients in the 5-FU 0.5%/salicylic acid 10% study arm; no patients in the vehicle arm discontinued treatment because of TEAEs. One discontinuation was because of application-site bleeding, which was deemed to be related to treatment, and one discontinuation was because of bladder neoplasm, which was not deemed to be treatment related. There were no deaths during the study.
Discussion
Field-directed treatment of AK may offer the most effective means of eliminating both clinical and subclinical lesions, thereby preventing progression to invasive SCC [14]. Our study, the first randomized controlled trial of field-directed therapy with 5-FU/SA, showed that field-directed treatment of AK lesions with 5-FU 0.5%/salicylic acid 10% applied once daily via topical administration for 12 weeks was associated with significantly higher rates of CCC at 8 weeks after the end of treatment compared with vehicle. PC of AK lesions and the proportional reduction from baseline in total number of AK lesions followed similar trends. In addition, at 8 weeks after the end of treatment, the severity of AK lesions was reduced to a greater extent with 5-FU 0.5%/salicylic acid 10% than with vehicle.
The efficacy of 5-FU 0.5%/salicylic acid 10% applied as lesion-directed therapy for a maximum of 12 weeks with 8-week follow-up was explored previously in a phase III study in which it was used for the treatment of patients with AK lesions of similar grade and location to those in the present study [15]. In the previous study, histological clearance at 8 weeks, the primary study objective, was achieved in 72.0% of patients, a significantly higher rate than that achieved following treatment with diclofenac 3% in hyaluronic acid (59.1%; P < 0.01) and vehicle (44.8%; P < 0.0001). Low-dose 5-FU 0.5%/salicylic acid 10% was also superior to diclofenac 3% in hyaluronic acid and vehicle in terms of CCC (55.4% vs. 32.0% and 15.1% for diclofenac 3% in hyaluronic acid and vehicle, respectively; P < 0.001 for both comparisons). In a 12-month follow-up study [16], the percentage of sustained clearance of AK lesions was higher for 5-FU 0.5%/salicylic acid 10% (85.8%) versus diclofenac 3% in hyaluronic acid (81.0%; P = 0.02) and vehicle (79.8%; P = 0.04).
A recent review of topical treatments for AK lesions suggested that 5-FU 0.5%/salicylic acid 10% was associated with a higher incidence of CCC than imiquimod 2.5% and 3.75% and ingenol mebutate [17]. It should be noted that the 5-FU 0.5%/salicylic acid 10% treatment group also included patients with hyperkeratotic lesions, which are more severe and have a potentially higher rate of malignant transformation [18, 19]; these were not included in the imiquimod and ingenol mebutate studies [17]. Longer-term clinical trials need to be carried out to validate these findings.
At the end of the active treatment period, lesion counts were similar between 5-FU 0.5%/salicylic acid 10% and vehicle arms, a finding that may be attributable to ongoing difficulties in lesion assessment caused by irritation at the site of administration of both 5-FU/SA and vehicle. However, it is also possible that vehicle itself may have had a mild therapeutic effect mediated via an unknown mechanism; DMSO has a known irritant effect [13] and may thus activate the skin’s defense mechanisms [15]. Eight weeks following the end of treatment, 5-FU 0.5%/salicylic acid 10% was associated with a significant 78% decrease in the number of AK lesions. This is comparable to the corresponding decrease in lesion count of approximately 70% observed in a non-interventional study of 1051 patients [20]. It should be borne in mind that 48.6% (498/1025) of patients in the study by Szeimies and colleagues received 5-FU 0.5%/salicylic acid 10% treatment for less than 6 weeks, which may account for the slight difference in lesion count reduction.
Given that treatment with 5-FU 0.5%/salicylic acid 10% was associated with a greater number of lesions transitioning from grade I/II to grade 0 than with vehicle, this suggests that active treatment affects the histopathology of the AK lesions. Two small (case series) studies using RCM recently determined that field-directed 5-FU 0.5%/salicylic acid 10% for up to 6 weeks of treatment was effective not only for investigating the transitioning of clinically visible lesions from a higher to lower grade, but also for clearing sub-clinical lesions after field-directed treatment [21, 22]. The results from the RCM sub-study in a group of 30 patients from the current study will be published separately.
At 8 weeks after the end of treatment, PGA of treatment efficacy was rated as “very good” or “good” in 90.2% of patients receiving 5-FU 0.5%/salicylic acid 10%. This was similar to the 89% reported in the non-interventional study by Szeimies and colleagues [20]. Overall treatment satisfaction, as measured using the TSQM questionnaire, was greater among patients in the 5-FU 0.5%/salicylic acid 10% arm than in the vehicle arm (P = 0.0019), as was satisfaction in relation to treatment effectiveness (P = 0.0064). In the 12-month follow-up study by Stockfleth et al., it was reported that 93.2% of patients receiving 5-FU 0.5%/salicylic acid 10% assessed treatment efficacy as “very good” or “good” compared with 81.6% in the diclofenac 3% in hyaluronic acid group and 66.7% in the vehicle group [16]. Although improvement in DLQI total score was statistically higher for vehicle versus 5-FU 0.5%/salicylic acid 10% during the treatment phase, this switched in favor of 5-FU 0.5%/salicylic acid 10% 8 weeks after the end of treatment and was attributed to local skin reactions associated with 5-FU 0.5%/salicylic acid 10%.
Safety data in our study were consistent with the known and predictable tolerability profile of 5-FU 0.5%/salicylic acid 10% [15, 16]. As with other topical treatments for AK [23, 24], 5-FU 0.5%/salicylic acid 10% caused administration-site reactions, such as erythema, inflammation, and scabbing, prior to demonstrating clear evidence of efficacy. Based on our clinical experience and with the vast majority of topical treatments for AK, local adverse effects are expected and proportionally correlate with treatment duration and efficacy. For some patients who withdrew from treatment, administration-site local skins reactions were mentioned but not cited as the main reason for drug withdrawal; six patients (5.6%) in the 5-FU 0.5%/salicylic acid 10% group and two patients (3.6%) in the vehicle group. The number of patients for whom the investigator considered that administration-site local skin reactions justified treatment discontinuation was low (5-FU 0.5%/salicylic acid 10% arm: n = 1; vehicle arm: n = 0). Conservatively taking these numbers into account, treatment discontinuation rates due to safety/tolerability issues were low in this study.
One potential limitation of our study was the higher incidence of local skin reactions in the 5-FU 0.5%/salicylic acid 10% arm, which may have compromised the blinding of the study. However, the incidence of local skin reactions in the vehicle group, which are similar to those reported previously in a pivotal clinical trial using a lesion-directed approach [15] and may have been linked to the irritant effects of dimethyl sulfoxide that are part of the known safety profile of 5-FU 0.5%/salicylic acid 10% [13], minimized the unblinding risk. Also, during the 8-week period after the end of treatment, patients were expected not to use further treatments for AK lesions, which may have proven difficult for some patients. However, the specification that patients had grade I/II lesions, rather than more severe AK, may have helped avoid the situation where additional treatment was required. While it is clearly important to evaluate clinical endpoints, selecting endpoints that only included clinical evaluation of lesions could be regarded as a potential limitation of the study. Good correlation between routine histology and the results of RCM, a novel non-invasive imaging technique that allows the in vivo evaluation of skin at near-histological resolution, has been reported previously [25]. Results from the RCM sub-study in a group of 30 patients from the current study will be published separately.
A strength of the present study was the inclusion of patients with hyperkeratotic lesions, which enables demonstration of the efficacy of 5-FU 0.5%/salicylic acid 10% against these more clinically severe lesions. In addition, the findings from this first randomized study with field-directed therapy with 5-FU/SA are robust, with consistency across all reported variables, both objectively measured and patient-reported.
Conclusion
In this randomized, double-blind, vehicle-controlled study, once-daily topical treatment with field-directed 5-FU 0.5%/salicylic acid 10% applied over a 12-week period was an effective treatment for grade I and II AK lesions. Furthermore, treatment was well accepted by both physicians and patients in terms of overall treatment satisfaction and clinical outcomes. In addition, safety outcomes were consistent with the known and predictable safety profile of 5-FU 0.5%/salicylic acid 10%.
References
Ulrich M, Pellacani G, Ferrandiz C, Lear JT. Evidence for field cancerisation treatment of actinic keratoses with topical diclofenac in hyaluronic acid. Eur J Dermatol. 2014;24:158–67.
Kirby JS, Scharnitz T, Seiverling EV, Ahrns H, Ferguson S. Actinic keratosis clinical practice guidelines: an appraisal of quality. Dermatol Res Pract. 2015;2015:456071.
Fernández-Figueras MT, Carrato C, Sáenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29:991–7.
Stockfleth E. The paradigm shift in treating actinic keratosis: a comprehensive strategy. J Drugs Dermatol. 2012;11:1462–7.
Rosen T, Lebwohl MG. Prevalence and awareness of actinic keratosis: barriers and opportunities. J Am Acad Dermatol. 2013;68(1 Suppl 1):S2–9.
Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
Nashan D, Meiss F, Muller M. Therapeutic strategies for actinic keratoses–a systematic review. Eur J Dermatol. 2013;23(1):14–32.
Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interv Aging. 2013;8:29–35.
Werner RN, Stockfleth E, Connolly SM, et al. Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis—international league of dermatological societies in cooperation with the European Dermatology Forum - Short version. J Eur Acad Dermatol Venereol. 2015;29:2069–79.
Bower C, Keohane S, Kownacki S, et al. Primary care dermatology society clinical guidance. Actinic (solar) keratosis—primary care treatment pathway. http://www.pcds.org.uk/a-z-clinical-guidance/clinical-a-z-list. Accessed July 20, 2016.
Stockfleth E, Ferrandiz C, Grob JJ, Leigh I, Pehamberger H, Kerl H. Development of a treatment algorithm for actinic keratoses: a European consensus. Eur J Dermatol. 2008;18:651–9.
Olsen EA, Abernethy ML, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24:738–43.
Actikerall summary of product characteristics. www.medicines.org.uk/emc/medicine/24614/SPC/Actikerall+5mg+g+%2B+100mg+g+Cutaneous+Solution/. Accessed July 20, 2016.
Chetty P, Choi F, Mitchell T. Primary care review of actinic keratosis and its therapeutic options: a global perspective. Dermatol Ther (Heidelb). 2015;5(1):19–35.
Stockfleth E, Kerl H, Zwingers T, Willers C. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101–8.
Stockfleth E, Zwingers T, Willers C. Recurrence rates and patient assessed outcomes of 0.5% 5-fluorouracil in combination with salicylic acid treating actinic keratoses. Eur J Dermatol. 2012;22:370–4.
Stockfleth E, Sibbring GC, Alarcon I. New topical treatment options for actinic keratosis: a systematic review. Acta Derm Venereol. 2016;96:17–22.
Quaedvlieg PJ, Tirsi E, Thissen MR, Krekels GA. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16(4):335–9.
Suchniak JM, Baer S, Goldberg LH. High rate of malignant transformation in hyperkeratotic actinic keratoses. J Am Acad Dermatol. 1997;37(3 Pt 1):392–4.
Szeimies RM, Dirschka T, Prechtl A, Melzer A. Efficacy of low-dose 5-fluorouracil/salicylic acid in actinic keratoses in relation to treatment duration. J Dtsch Dermatol Ges. 2015;13:430–8.
Ulrich M, Alarcon I, Malvehy J, Puig S. In vivo reflectance confocal microscopy characterization of field-directed 5-fluorouracil 0.5%/salicylic acid 10% in actinic keratosis. Dermatology. 2015;230:193–8.
Malvehy J, Alarcon I, Montoya J, Rodriguez-Azeredo R, Puig S. Treatment monitoring of 0.5% 5-fluorouracil and 10% salicylic acid in clinical and subclinical actinic keratoses with the combination of optical coherence tomography and reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2016;30:258–65.
Hanke CW, Beer KR, Stockfleth E, Wu J, Rosen T, Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J Am Acad Dermatol. 2010;62:573–81.
Berman B. Safety and tolerability of ingenol mebutate in the treatment of actinic keratosis. Expert Opin Drug Saf. 2015;14(12):1969–78.
Ulrich M, Lange-Asschenfeldt S, Gonzalez S. In vivo reflectance confocal microscopy for early diagnosis of nonmelanoma skin cancer. Actas Dermosifiliogr. 2012;103:784–9.
Acknowledgements
Sponsorship and article processing charges for this study were funded by Almirall S.A. Medical. Writing support was provided by Rhian Harper Owen on behalf of Complete Medical Communications Ltd., funded by Almirall S.A.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
This work was previously presented as an abstract and oral presentation at the European Association of Dermato Oncology Congress, Vienna, Austria, 31 August–3 September 2016 and as an abstract and poster presentation at the European Academy of Dermatology and Venereology Congress, Vienna, Austria, 28 September–2 October 2016.
Data Availability Statement
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosures
Eggert Stockfleth is a consultant for Almirall S.A. and has been a Principal Investigator in a number of Almirall-funded studies.
Ralph von Kiedrowski is a consultant and speaker for Almirall S.A.
Meritxell Falqués is an employee of Almirall S.A.
Nathalie Ivanoff is an employee of Almirall S.A.
Rosario Rodriguez Azeredo is an employee of Almirall S.A.
John Ryan has nothing to disclose apart from being a paid clinical researcher on this project.
Adam Ellery and Rolf Dominicus have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D947F06075886CCC.
An erratum to this article is available at http://dx.doi.org/10.1007/s13555-017-0179-0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stockfleth, E., von Kiedrowski, R., Dominicus, R. et al. Efficacy and Safety of 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Actinic Keratosis: A Phase III, Randomized, Double-Blind, Vehicle-Controlled Trial. Dermatol Ther (Heidelb) 7, 81–96 (2017). https://doi.org/10.1007/s13555-016-0161-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-016-0161-2