Abstract
Schnitzler syndrome is a rare acquired systemic disease with a chronic evolution and difficult treatment. We report a 50-year-old woman with Schnitzler syndrome for 10 years, with major impact on her quality of life and refractory to conventional therapies. The patient was started on anakinra, an IL-1 receptor antagonist, with a rapid and sustained remission of the syndrome manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schnitzler syndrome (SS) is a rare and acquired systemic disease with many common features of the group of inherited diseases referred to as auto-inflammatory syndromes, presenting with chronic urticaria and monoclonal gammopathy [1]. Conventional therapies including anti-histamines, anti-inflammatory drugs, steroids and immunosuppressive drugs, are usually ineffective. However, the IL-1 receptor antagonist anakinra was found to rapidly control all the symptoms of this syndrome [1].
Case Report
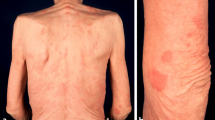
We report a 50-year-old Caucasian woman with SS for 10 years. The leading symptoms were chronic urticarial rash (nonpruritic) (Fig. 1), recurrent fever up to 39 °C, fatigue, general malaise and bone pain. Laboratory analyzes conducted regularly showed permanent leukocytosis with neutrophilia, elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and a monoclonal gammopathy IgM kappa. Bone marrow biopsy was never consistent with malignancy. Bone scintigraphy was performed and hyper-fixation was observed on distal left femur, proximal right femur and skull. CT scan revealed hepatomegaly and cervical lymphadenopathy. A lymph node biopsy excluded malignant infiltration. Histologic examination from an urticarial lesion showed a mixed inflammatory infiltrate (predominantly of neutrophils) in the dermis with no evidence of vasculitis (Fig. 2). All these findings were consistent with the diagnosis of SS according to the Lipsker et al. [2] criteria. The disease had a major impact on quality of life of our patient and the manifestations were refractory to treatment with hydroxyzine, prednisolone, hydroxychloroquine, naproxen and colchicine. As the disease was not controlled, the patient was started on anakinra (100 mg/day subcutaneously). Two days after initiating the treatment, the patient noticed a dramatic clinical improvement. The urticarial lesions, bone pain and fatigue completely disappeared, allowing the patient to perform activities she had not been able to carry out for years. Within 2 weeks, inflammatory parameters also showed a major improvement. Laboratory assessment before starting treatment showed a leukocyte count of 25.420 cells/mm3 [normal range (NR) 4000–11,000 cells/mm3], neutrophil level of 84.1% (NR 40–60%), ESR of 120 mm (NR ≤ 10 mm) and CRP of 19.2 mg/dL (NR < 0.5 mg/dl); after 2 weeks, all values were normal, except for ESR (78 mm). Monoclonal gammopathy remained unchanged. Treatment was well tolerated with no side effects, the only complaint reported was transient myalgia (with no concomitant laboratory abnormalities) assumed to be secondary to increased physical activity of the patient. After 1 year of therapy, the patient maintains complete remission. Informed consent was obtained from all patients for being included in the study.
Discussion
The evolution of SS is usually chronic and, before the introduction of anakinra, most treatment options were ineffective. To our knowledge, only one case of spontaneous and persistent remission has been reported in the literature [3]. Its origin is still unknown, but it is likely to be a link between the systemic manifestations and a deregulation of the interleukin 1 (IL-1) pathway [3]. However, the role of this pathway in paraproteinemia is unclear [4]. Anakinra is a recombinant form of human IL-1ra which competitively inhibits the binding of IL-1α and IL-1β to the IL-1 receptor type 1 [5]. IL-1 inhibition is the only regularly and completely efficient treatment for patients with SS, therefore Simon et al. recommend that patients with an alteration in their quality of life or persistent elevation of markers on inflammation should be treated with anakinra [4]. SS patients have a higher risk of developing a lymphoproliferative disorder, similar to other patients with monoclonal IgM gammopathies of undetermined significance [4]. However the effect of IL-1 inhibition on the risk of the development of malignancy is still unknown [6].
Conclusion
We highlight the efficacy of anakinra in SS. In our patient, this treatment was well tolerated and resulted in a rapid, complete and sustained remission of the manifestations of the syndrome, and more important led to a major improvement in the patient’s quality of life.
References
Lipsker D. The Schnitzler syndrome. Orphanet J Rare Dis. 2010;5:38.
Lipsker D, Veran Y, Grunenberger F, Cribier B, Heid E, Grosshans E. The Schnitzler syndrome. Four new cases and review of the literature. Medicine (Baltimore). 2001;80:37–44.
Asli B, Brouet JC, Fermand JP. Spontaneous remission of Schnitzler syndrome. Ann Allergy Asthma Immunol. 2011;107:87–8.
Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562–8.
Eiling E, Möller M, Kreiselmaier I, Brasch J, Schwarz T. Schnitzler syndrome: treatment failure to rituximab but response to anakinra. J Am Acad Dermatol. 2007;57:344–61.
Schuster C, Kränke B, Aberer E, Arbab E, Sturm G, Aberer W. Schnitzler syndrome: response to anakinra in two cases and a review of the literature. Int J Dermatol. 2009;48:1190–4.
Acknowledgments
We thank Prof. Dr. Luis Soares de Almeida for his contribution in the histological examination. No funding or sponsorship was received for this study or the publication of this article. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
A.I. Gouveia, M. Micaelo, F. Pierdomenico and J.P. Freitas declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Content
To view enhanced content for this article go to www.medengine.com/Redeem/2A84F06046897D5D.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gouveia, A.I., Micaelo, M., Pierdomenico, F. et al. Schnitzler Syndrome: A Dramatic Response to Anakinra. Dermatol Ther (Heidelb) 6, 299–302 (2016). https://doi.org/10.1007/s13555-016-0108-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-016-0108-7