Abstract
Hereditary angioedema resulting from the deficiency of the C1 inhibitor (HAE-C1-INH) is a rare, but potentially life-threatening disorder characterized by paroxysmal episodes of subcutaneous or submucosal edema. Early diagnosis is essential. Management is aimed at the prompt elimination of full-fledged attacks, as well as at the prevention of edematous episodes. The most straightforward means for therapy is supplementation with the deficient C1-INH protein. Placebo-controlled and open clinical studies have established that nanofiltered, human C1-INH concentrate, Cinryze® (ViroPharma Inc., Exton, PA, USA) (C1-INHCi), administered in 1,000 U doses is an effective and safe remedy for edematous episodes of HAE-C1-INH, regardless of the localization of the attack. Clinical manifestations rapidly improve and then resolve completely following treatment with this medicinal product. Additionally, C1-INHCi is also appropriate for pre-procedural or for routine prophylaxis. The administration of 1,000 U C1-INHCi before the (dental, surgical, or interventional diagnostic) procedure reduced the incidence of edematous episodes compared with placebo, and this reduction proved significant during routine prophylaxis with the administration of this dose every 3–4 days. Relapses did not occur, and repeated dosing had no influence on the efficacy of the preparation. Patients also tolerated treatment with C1-INHCi well. The safety of this preparation was confirmed by the absence of viral transmission as well as by the lack of antibody formation against C1-INH during treatment. Nowadays, C1-INHCi for intravenous use is the only medicinal product indicated both for the prevention and management of edematous attacks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hereditary angioedema due to C1-inhibitor deficiency (HAE-C1-INH) is a rare, autosomaldominant, potentially life-threatening disorder with a clinical picture characterized by recurrent, nonpruritic, self-limiting edema formation in the subcutaneous and/or submucosal tissues [1].
Mutation of the gene of the C1-INH protein may lead to two different types of the disease. In type I HAE, low levels of C1-INH protein can be due to intracellular degradation or lack of secretion from the cells synthesizing the protein, as well as defective transcription. In type II HAE, a nonfunctional inhibitor protein is transcribed; the serum level of which is normal or even appears elevated. The two types of HAE are phenotypically indistinguishable in the manner of clinical presentation [2, 3]. New mutations can also occur, as observed in approximately 15–25% of cases [4, 5].
The concentrate of human-plasma derived C1-INH, the protein missing in patients, has been in use for the management of HAE, and particularly for the acute therapy of edematous attacks, for decades. Recently, multicenter, randomized clinical trials have demonstrated, for the first time, the effectiveness and safety of C1-INH concentrate, also in the prevention of these attacks. The studies evaluated the nanofiltered human C1-INHCi concentrate (Cinryze®, ViroPharma Inc., Exton, PA, USA). The following is a concise overview of HAE, the results of the clinical trials conducted with C1-INHCi, and the application fields for this preparation.
METHODS
The authors have briefly reviewed the pathomechanism, clinical manifestations, and diagnostics of HAE in light of relevant current knowledge, by relying on important, pertinent publications. As regards to the complex management of HAE, the authors examined the options of drug therapy, both in emergencies and in prevention, in observance of international guidelines. Next, the authors summarized the information pertaining to C1-INHCi, which may be of use to physicians and nurses prescribing and administering the product, as well as to patients wishing to learn a little more about their treatment. The section on C1-INHCi focuses on reports of clinical trials, conference abstracts, the documents published on the Viropharma website, and data available from web pages of the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
HEREDITARY ANGIOEDEMA TYPE I AND TYPE II
Pathomechanism
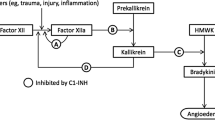
C1-INH is a serine protease inhibitor (serpin) that inactivates several different proteases: C1r, C1s, and mannose-binding-lectin-associated serine proteases in the complement system; factor XII and kallikrein in the contact system; factor XI and thrombin in the coagulation system; and tissue plasminogen activator and plasmin in the fibrinolytic system. C1-INH deficiency may result in activation of these four closely interrelated cascade systems, potentially leading to the release of bradykinin, resulting in edema formation [6, 7].
Clinical Symptoms
Time of onset, frequency, duration, and severity of individual attacks varies. In approximately 50% of cases, clinical manifestations may appear as early as during childhood. Establishing the diagnosis early, preferably before onset of clinical symptoms, is essential in cases with a positive family history. The time course of the swelling episode typically worsens over 24 hours and gradually self-limits over the next 2–3 days. Subcutaneous edema is not accompanied by pruritus or urticaria [1]. Cutaneous manifestations usually regress spontaneously over several days. Edema involving the submucosa of the upper airways can cause airways obstruction and, therefore, may rapidly lead to suffocation [1, 8, 9].
Edema localized to the gastrointestinal mucosa may mimic clinical manifestations of the “acute abdomen” (including colicky abdominal pain, vomiting, watery post-attack diarrhea), which often lead to unnecessary surgery during the abdominal edematous attack [10]. In a proportion of patients, exploration reveals the etiological role of certain factors in the evolution of edematous episodes. The most common triggering factors include mechanical trauma, surgical procedures performed in the head and neck region, mental stress, hormonal effects (menstruation, pregnancy), drug effects (e.g., of estrogen-containing oral contraceptives, angiotensin converting enzyme inhibitors), and certain infections [11, 12].
Diagnostics
HAE-C1-INH can be diagnosed by exploring the patient’s family history, as well as by evaluating clinical symptoms and laboratory signs. The occurrence of edematous symptoms on additional family members may aid in establishing the diagnosis. Types I and II HAE may be diagnosed by performing complement studies. The disorder can be diagnosed in this manner when a family history is absent (Table 1) [13].
Although genetic testing is not necessary in most patients, it may aid in the diagnosis of cases where biochemical measurements are inconclusive. However, the mutation responsible for C1-INH deficiency is only identified in 90–92% of patients with HAE-C1-INH [5].
Complex Management
The management of patients with HAE requires a complex therapeutic approach that consists of the therapy and prevention of manifest edematous attacks [13–15].
Management of Edematous Attacks
All attacks, irrespective of their location, are eligible for treatment as soon as they are clearly recognized by the patient [14]. The medicinal products appropriate for the treatment of edematous attacks differ according to their mechanisms of action (Fig. 1), processes of manufacture, or methods of dosage.
Deficient or dysfunctional C1-INH can be substituted by administering human plasma-derived C1-INH concentrate. Currently, three products of this type are available commercially: Cinryze® (ViroPharma Inc.) (C1-INHCi), Cetor® (Sanquin, Amsterdam, The Netherlands), and Berinert® (CSL Behring, Marburg, Germany) [16–18]. An alternate replacement for the deficient protein is recombinant human C1-INH concentrate, Rhucin® (Pharming NV, Leiden, The Netherlands) [19]. A novel therapeutic option is ecallantide (Kalbitor®, Dyax, Cambridge, MA, USA), an inhibitor of human kallikrein produced by the yeast, Pichia pastoris [20]. The action of bradykinin, released during the edematous episode, can be blocked by administering the bradykinin B2 receptor antagonist, icatibant (Firazyr®, Shire, Jersey, JE, USA) [21].
If none of the aforementioned drugs are available, fresh frozen plasma may be administered to relieve severe attacks of patients in a critical condition. Antifibrinolytics, however, may be considered for add-on treatment only [12, 22]. Conventionally used glucocorticosteroids and antihistamines are ineffective in bradykinin-mediated edema. Epinephrine may be administered as add-on therapy in upper airways involvement [23].
Prophylaxis
The initial step of prophylaxis should be the elimination of the aforementioned triggering factors. The second step is the introduction of pharmacotherapy. The goal of prophylactic treatment is either to decrease the number and severity of angioedema attacks (longterm prophylaxis) or to reduce the likelihood of swelling in a patient undergoing a stress or procedure likely to precipitate an attack (short-term prophylaxis) [14, 24].
Introducing long-term prophylaxis becomes necessary if: the attacks recur frequently and in a severe form; the patient fails to benefit from on-demand therapy; HAE leads to significant anxiety and poor quality of life; or the patient has limited access to emergency medical care [13–15, 25].
C1-INHCi has been approved for routine prophylaxis. Its mechanism of action is known; increasing the plasma levels of C1-INH activity, and suppressing contact system activation; thus, preventing the generation of bradykinin. The properties of C1-INHCi will be detailed later.
Additionally, antifibrinolytics (epsilonaminocaproic acid, tranexamic acid) and attenuated androgens (danazol, stanozolol, oxandrolone) may be administered for prophylaxis. Antifibrinolytics are used primarily in women and in pediatric patients. Their safety profile is superior to that of attenuated androgens. Nevertheless, their use may be associated with hypotension, cardiac arrhythmias, as well as rhabdomyolysis or thromboembolism, and their prophylactic efficacy is adequate only in a small proportion of patients [26].
Nowadays, a 17-alpha-alkylated anabolic androgen steroid, known as danazol, is the most commonly used prophylactic drug; however, the exact mode of action has not yet been elucidated. In some patients, a variety of undesirable effects should be expected during treatment with 17-alpha-alkylated anabolic androgen steroid, which are highly dose-dependent, such as weight gain, acne, virilization, altered libido, menstrual irregularities, headaches, depression, fatigue, pro-atherogenic changes in lipid profile [27], hepatotoxicity, elevated liver enzyme activity, cholestatic jaundice, peliosis hepatis, and various neoplastic lesions [28–30].
Short-term prophylaxis is recommended for patients undergoing surgical or diagnostic procedures undertaken in the head and neck region, or for those to undergo an operation performed in general anesthesia with endotracheal intubation.
The most appropriate strategy is to administer C1-INH concentrate 1 hour before surgery, or as close to the procedure as is feasible, but less than 6 hours before the intervention. Alternatively, attenuated androgens used for long-term prophylaxis may be administered in higher doses before surgery and for 4–5 days thereafter, to prevent an acute episode [13, 31, 32].
C1-INHCi
The C1-INH protein derived from human plasma is highly purified and equivalent to the endogenous C1-INH. C1-INHCi is a sterile, stable, lyophilized preparation of the C1-INH, prepared by Sanquin in the Netherlands with the use of plasma obtained from healthy blood donors.
Its manufacturing process combines various methods for purification, such as cryoprecipitation, anion-exchange chromatography, polyethylene glycol precipitation 4,000, pasteurization (heat treatment at 60°C for 10 hours in solution with stabilizers), and nanofiltration through two sequential 15 nm filters. The use of these three viral inactivation steps effectively reduces the load of enveloped and nonenveloped viruses (the overall virus reducing capacities are > 8.7 and > 19.1 log10, respectively), and prions (reduction of prion load is estimated > 9 log10). The purity is ≥ 90% human C1-INH. One unit of C1-INHCi corresponds to the mean quantity of C1-INH present in 1 mL of normal fresh plasma [33, 34].
Pharmacodynamics
In clinical studies, the intravenous (i.v.) administration of C1-INHCi demonstrated an increase in plasma levels of the C1-INH within approximately 1 hour or less of administration.
Both antigenic and functional levels of C1-INH increased significantly in patients treated with C1-INH, but not in the placebo group [16]. The mean increases in functional C1-INH activity from before to after treatment were generally similar for varying numbers of attacks. The C4 values after treatment did not change significantly compared with preinjection values [35].
Pharmacokinetics
A randomized, parallel-group, open-label pharmacokinetics study of C1-INHCi was performed in patients with nonsymptomatic HAE-C1-INH. The patients received either a single dose of 1,000 U, or 1,000 U followed by a second 1,000 U 60 minutes later. The maximum plasma concentration (C max) of functional C1-INH and the area under the plasma concentration-time curve (AUC) increased from the single to the repeated dose, although the increase was not dose proportional. C max was 0.68 ± 0.08 (n = 12) versus 0.33 ± 0.20 (n = 12), and AUC was 74.5 ± 30.3 (n = 12) versus 95.9 ± 19.6 (n = 13), respectively. The mean half-life of C1-INHCi was 56 hours (range: 11–108 hours) for a single dose and 62 hours (range: 16–152 hours) for the repeated dose [36, 37].
Acute- and repeated-dose toxicity studies were performed in Sprague-Dawley rats, with i.v. administration of C1-INHCi at dose levels of 1, 7, and 28 times the normal dose. No signs of toxicity were observed in the single dose study. In vitro and in vivo thrombogenicity studies showed a potential for clot formation when C1-INHCi was administered in doses 14 times higher than the recommended clinical dose (over 200 U/kg). It is not known whether C1-INHCi passes into the milk. Drug interaction studies have not been conducted with C1-INHCi [34, 36].
No animal studies have been completed to evaluate the effects of C1-INHCi on carcinogenesis, mutagenesis, and impairment of fertility.
Clinical Trials With C1-INHCi
Four phase 3 clinical studies (two placebo-controlled, two open-label extension) have been performed to date.
A Double-Blind, Placebo-Controlled, Clinical Study to Investigate the Efficacy and Safety of Purified Nanofiltered C1-INH (Human) for the Treatment of Hereditary Angioedema in Acute Attacks
In this study, the median time to onset of unequivocal relief from an attack was 2.41 hours in subjects treated with C1-INH concentrate, but longer than 4 hours in those given placebo (P = 0.02) [16].
A Double-Blind, Placebo-Controlled, Clinical Study to Investigate the Efficacy and Safety of Purified Nanofiltered C1-INH (Human) as Prophylactic Treatment to Prevent Hereditary Angioedema Attacks
In this crossover study, the number of attacks per 12-week period was 6.26 with the administration of 1,000 U C1-INHCi every 3–4 days given as prophylaxis, compared with 12.73 with placebo (P < 0.001). Subjects who received the C1-INH concentrate also had significant reductions in both severity and duration of attacks, open-label rescue therapy, and total days with swelling compared with placebo [16].
Open-Label Safety/Efficacy Repeat Exposure Study of Nanofiltered C1-INH (Human) in the Treatment of Acute Hereditary Angioedema Attacks
Subjects were treated for a total of 609 acute HAE attacks (n = 101; median: three attacks per subject; range: 1–57). Within 4 hours after C1-INHCi dosing, 87% of attacks achieved unequivocal relief of the defining symptom. For 95% of attacks, clinical relief was observed and/or subjects were discharged home within 4 hours. For subjects with > 1 attack, the proportion of attacks responding within 4 hours after C1-INHCi dosing and the time to response was comparable regardless of the number of attacks treated. Among 84 separate laryngeal HAE attacks, none required intubation following treatment with C1-INHCi [38].
Open-Label Use of Nanofiltered C1INH (Human) for the Prophylactic Treatment to Prevent Hereditary Angioedema Attacks
A total of 146 subjects received C1-INHCi as HAE prophylaxis for periods ranging from 8 days to approximately 32 months (median: 8 months). Before enrollment, subjects reported a median monthly HAE attack rate of 3.0 (range: 0.08.28.00). During therapy with prophylactic C1-INHCi, this rate was 0.21 (range: 0–4.56), and 86% of subjects experienced an average of ≤ 1 attack per month. For subjects receiving C1-INHCi prophylaxis for at least 1 year, the monthly attack rate per subject remained consistently low (0.34 attacks per month) relative to pre-study rates [39].
The authors summarized the objectives, design, and primary and secondary endpoints, as well as the key findings of these studies in Table 2 [16, 37, 39].
Tolerability and Safety
C1-INHCi has been proven to be well tolerated and safe, with an adverse event profile indifferent to that of placebo. The most common adverse reactions are listed in Table 3. Hypersensitivity reactions, viral transmission of hepatitis B and C virus, and HIV, or the development of clinically relevant anti-C1-INH antibodies related to C1-INHCi did not occur [16, 35, 38].
The data accumulated in clinical studies have been subjected to detailed analyses according to various considerations. Reviewing these might be of importance for clinical practice and, accordingly, a brief summary of additional subset analyses follows.
The safety and efficacy of C1-INHCi in attacks of diverse localizations are presented below.
Laryngeal Attacks
Eighty-five subjects (74 adults, 11 children) received C1-INHCi for 267 laryngeal attacks. Only a single subject required intubation after treatment with C1-INHCi. The median time to the onset of relief during the first treated laryngeal attacks was 60 minutes, comparable with the overall median response time for attacks, at all anatomic locations (45 minutes). The efficacy of C1-INHCi for the treatment of HAE in subjects with more than one laryngeal attack did not diminish with subsequent, repeated administrations [35].
Gastrointestinal Attack
Gastrointestinal attacks represented 59% (351 of 598) of the HAE attacks recorded in this study. Seventy-seven subjects experienced 351 gastrointestinal attacks, with 97% (339 of 351) achieving relief within 4 hours after C1-INHCi administration. The median time to onset of relief was 30 minutes. The efficacy of C1-INHCi did not diminish with subsequent, repeated administrations [40].
Subcutaneous Attacks
Subcutaneous attacks on the extremities (n = 86) and face (n = 70) represented the second largest proportion (156 of 598, 26%) of HAE attacks in this study. Fifty-one subjects experienced a total of 156 cutaneous attacks, with 96% (149 of 156) achieving relief within 4 hours after C1-INHCi administration. The median time to onset of relief was 30 minutes. The efficacy of C1-INHCi did not diminish with subsequent, repeated administrations [41].
The Safety and Efficacy of C1-INHCi in Various Patient Populations
Pediatric Patients
The management of acute episodes
Overall, this open-label, multicenter study evaluated subjects aged ≥ 1 year, with a diagnosis of HAE; this subset analysis presents data on patients aged < 18 years. Twenty-two pediatric subjects [aged 2–5 years old (n = 1), 6–11 years old (n = 9), and 12–17 years old (n = 12)] experienced 121 HAE attacks in total, with 89% (108 of 121) achieving relief within 4 hours of C1-INHCi administration. A 2-year-old subject was given two 500 U doses for a facial attack, and reported symptom relief within 3 hours. Gastrointestinal attacks were the most common manifestations of HAE. Of the 64 gastrointestinal attacks seen in 6–11-year-old and in 12–17-year-old subjects, 97% (35 of 36) and 89% (25 of 28), respectively, relief ensued within 4 hours. No subjects with a laryngeal attack required intubation [42].
Prophylaxis
This subset analysis from the open-label extension study evaluated the use of C1-INHCi for routine prophylaxis in pediatric subjects < 18 years of age with HAE. Before enrollment, the 23 children (aged 2–5 years old [n = 2], 6–11 years old [n = 9], and 12–17 years old [n = 12]) in this study reported a mean HAE attack rate of 4–4 ± 5.7 per month. C1-INHCi therapy reduced HAE attacks to ≤ 1 per month in the majority of the pediatric subjects. The only treatment-emergent AEs considered drug-related were headache, nausea, and infusion-site erythema; none of which were severe [43].
Pregnant Women
Unlike placebo-controlled trials, pregnancy was not an exclusion criterion in two open-label studies investigating the use of C1-INHCi for the treatment of acute attacks and for prophylaxis of HAE attacks. In these studies, women were successfully treated with C1-INHCi during pregnancy.
Fourteen pregnant women were treated with C1-INHCi in the studies; one subject who was treated in both studies delivered a healthy neonate. Of the 13 remaining subjects, three subjects enrolled in the acute treatment study. One patient received eight doses and two subjects received a single dose of C1-INHCi at delivery only. All three subjects delivered healthy neonates. Ten subjects in the prophylaxis study received a median of 34 doses (range: 2–85) during their pregnancy and reported the following outcomes: seven subjects delivered eight healthy neonates (one set of twins), one subject (45 years old) with a history of miscarriage and ectopic pregnancy had a spontaneous abortion (reported as possible ectopic pregnancy), and one subject delivered a stillborn neonate with multiple congenital anomalies. This subject was first exposed to C1-INHCi in the second trimester. One subject had an unknown outcome [44].
Pre-procedure Administration of C1-INHCi
C1-INHCi 1,000 U i.v. was administered within 24 hours before elective medical, dental, or surgical procedures that may trigger HAE. Forty-one patients (eight children, 33 adults) received C1-INHCi before 91 procedures (40 in children, 51 in adults). Approximately 56% of the procedures were dental, and 44% involved surgery or diagnostic intervention. A single 1,000 U dose was administered before 96% of the procedures; two separate 1,000 U doses were used before two coronary artery bypass surgeries, one gastrointestinal endoscopy, and during labor/delivery of one pregnancy. HAE attacks did not occur after 72 hours of C1-INHCi administration in 98% of the procedures. A genitourinary attack occurring after a dental procedure, and a laryngeal attack seen after laparoscopy, were reported within 72 hours after the administration of C1-INHCi. Both resolved after treatment with an additional dose of C1-INHCi [45].
Site of Care Analysis on Administration of C1-INHCi
In June 2010, and before implementation of an infusion training program, 516 patients were on commercial C1-INHCi and were analyzed regarding their site of care status (note: 11 patients had no data). Of these 516 patients, 243 patients administered C1-INHCi at home. A total of 42% reported self-administration (overall, self-administration was chosen by 20% of patients). In 16% of patients, the drug was administered by a family member, and in 23%, by a home healthcare worker. A total of 120 patients received treatment at an infusion center, and 142 in the physician’s office. The age of the study population ranged from 5 to 84 years. Thus, home infusion and self-administration is a viable option for patients with HAE [46, 47].
There are programs in place that offer convenience and support to patients with HAE. An infusion training program in the United States and European Union is a benefit designed to develop the skills of patients or caregivers in reconstitution and administration technique, as well as to provide a resource to patients and caregivers after initial training has been completed. Other programs include the home delivery of medications. In the United States, there is a program designed to offer assistance with insurance coverage and financial support for insurance claims and co-payments.
Approved Indications
United States
C1-INHCi is the first and only C1-INH approved by the FDA for the routine prophylaxis of HAE attacks in adults and adolescent patients (12 years of age and older) with hereditary C1-INH deficiency. It also gained approval for self-administration in May 2009 [37, 48].
Europe
The European Union approved C1-INHCi in 2011 for the treatment and pre-procedure prevention of angioedema attacks in adults and adolescents with HAE. Additionally, it is indicated for the routine prevention of severe and recurrent HAE attacks in adults and adolescents. In addition, the EMA granted approval for self-administration and the medicine is available on medical prescription only [36].
Administration and Dosage
C1-INHCi is for i.v. use only. The freeze-dried C1-INHCi powder should be stored between 2 and 25°C. Patients should not attempt to self-administer unless trained appropriately by a clinician.
A dose of 1,000 U C1-INHCi can be administered every 3–4 days for routine prophylaxis to prevent angioedema attacks in patients with HAE. For pre-procedure prophylaxis before medical, dental, or surgical interventions, 1,000 U C1-INHCi is given within 24 hours before the procedure.
For the acute treatment of HAE attacks, 1,000 U should be administered at the first sign of an attack. Regardless of the patient’s body weight, a second dose of 1,000 U may be given if the patient has not responded adequately after 1 hour, or sooner for severe attacks, or if the start of treatment had been delayed.
Treatment with C1-INHCi is contraindicated if the patient has known, life-threatening hypersensitivity (e.g., anaphylaxis) to C1-INH (human), or to any ingredient of the preparation [37].
CONCLUSION
The management of HAE-C1-INH has changed significantly during recent years. Therapeutic options have increased, and new medicinal products have been introduced. The latter provide the means for developing individualized treatment strategies on one hand, as well as widen patient access to therapy on the other. C1-INHCi is approved for long-term prophylaxis both in Europe, and in the United States. Clinical studies have clearly confirmed the efficacy and safety of this drug in HAE attacks (regardless of the localization of edema), during pre-procedure prophylaxis before elective medical, dental, or surgical interventions, or for long-term prophylaxis. C1-INHCi was quick and efficient in relieving clinical manifestations. Relapses did not occur and repeated administration did not reduce therapeutic efficacy. Neither viral transmission nor the development of anti-C1-INH antibodies has been observed. Pre-procedure prophylaxis proved effective for the prevention of edematous attacks. Longterm prophylaxis reduced the number, severity, and duration of edematous episodes. Although i.v. drug delivery is relatively problem-free and feasible, subcutaneous administration would be more convenient and more suitable for self-administration.
The clinical evaluation of the newly developed C1-INHCi formulation for subcutaneous dosing is currently in phase 2 studies. The dose appropriate for long-term prophylaxis may be individually adjusted, determining the lowest effective dose and dosing frequency best suited for the given patient. It must be stressed that no treatment modality exists for preventing attacks with certainty and, hence, severe upper airways edema can occur despite the therapy being administered. In such a case, emergency therapy should be introduced promptly, especially in airways edema. In addition to using stateof- the art therapeutic modalities, the regular monitoring and uninterrupted education of patients, establishing the means for home-based treatment, and defense of the patients’ interests are all integral elements of efficient, complex management.
References
Agostoni A, Aygoren-Pursun E, Binkley KE, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004; 114(Suppl.): S51–131.
Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C′ 1-esterase. Am J Med. 1963; 35: 37–44.
Rosen FS, Pensky J, Donaldson V, Charache P. Hereditary angioneurotic edema: two genetic variants. Science. 1965; 148: 957–8.
Tosi M. Molecular genetics of C1 inhibitor. Immunobiology. 1998; 199: 358–65.
Kalmar L, Hegedus T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive, locus-specific mutation database for the C1 inhibitor gene. Hum Mutat. 2005; 25: 1–5.
Cugno M, Zanichelli A, Foieni F, Caccia S, Cicardi M. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med. 2009; 15: 69–78.
Kaplan AP, Ghebrehiwet B. The plasma bradykininforming pathways and its interrelationships with complement. Mol Immunol. 2010; 47: 2161–9.
Bork K. Recurrent angioedema and the threat of asphyxiation. Dtsch Arztebl Int. 2010;107: 408–14.
Farkas H. Management of upper airway edema caused by hereditary angioedema. Allergy Asthma Clin Immunol. 2010; 6: 19.
Farkas H, Harmat G, Kaposi PN, et al. Ultrasonography in the diagnosis and monitoring of ascites in acute abdominal attacks of hereditary angioneurotic oedema. Eur J Gastroenterol Hepatol. 2001; 13: 1225–30.
Bouillet L, Longhurst H, Boccon-Gibod I, et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol. 2008; 199: 4841–4.
Agostoni A, Cicardi M. Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore). 1992; 71: 206–15.
Bowen T, Cicardi M, Farkas H, et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol. 2010; 6: 24.
Cicardi M, Bork K, Caballero T, et al. Evidencebased recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012; 67: 147–57.
Gompels MM, Lock RJ, Abinun M, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005; 139: 379–94.
Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010; 363: 513–22.
Krassilnikova S, Craig ET, Craig TJ. Summary of the International Multicenter Prospective Angioedema C1-inhibitor Trials 1 and 2 (IMPACT1 and 2). Expert Rev Clin Immunol. 2010; 6: 327–34.
Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009; 124: 801–8.
Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010; 126: 821–7, e14.
Cicardi M, Levy RJ, McNeil DL. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010; 363: 523–31.
Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010; 363: 532–41.
Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001; 161: 2417–29.
Bas M, Adams V, Suvorava T, et al. Nonallergic angioedema: role of bradykinin. Allergy. 2007; 62: 842–56.
Gower RG. Hereditary angioedema caused by c1esterase inhibitor deficiency: a literature-based analysis and clinical commentary on prophylaxis treatment strategies WAO J. 2011; 4: S9–21.
Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009; 102: 366–72.
Bowen T. Hereditary angioedema consensus 2010. Allergy Asthma Clin Immunol. 2010; 6: 13.
Szeplaki G, Varga L, Valentin S, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. J Allergy Clin Immunol. 2005; 115: 864–9.
Bork K, Pitton M, Harten P, Koch P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet. 1999; 353: 1066–7.
Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol. 1997; 99: 194–6.
Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008; 100: 153–61.
Farkas H, Gyeney L, Gidofalvy E, Fust G, Varga L. The efficacy of short-term danazol prophylaxis in hereditary angioedema patients undergoing maxillofacial and dental procedures. J Oral Maxillofac Surg. 1999; 57: 404–8.
Frank MM. Hereditary angioedema: the clinical syndrome and its management in the United States. Immunol Allergy Clin North Am. 2006; 26: 653–68.
European Medicines Agency. Guideline on plasma derived medicinal products. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109627.pdf. Accessed Jul 21 2011.
European Medicines Agency. Cinryze European public assesment report. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001207/WC500108898.pdf. Accessed Jul 15 2011.
Riedl M, Baker J, Hurewitz D, et al. Safety and efficacy of nanofiltered C1 esterase inhibitor (human) (C1-INH NF®) for the treatment of laryngeal attacks in subjects with hereditary angioedema (HAE). J Allergy Clin Immunol. 2011; 127(Suppl.): AB233.
European Medicines Agency. Cinryze 500 units powder and solvent for solutions for injection: summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001207/WC500108895.pdf. Accessed Jul 15 2011.
Cinryze (C1 inhibitor, [human]) freeze dried powder US prescribing information; Exton (PA): ViroPharma Inc. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM129918.pdf. Accessed Jan 28 2011.
Riedl MA, Hurewitz DS, Levy R, et al. Nanofiltered C1 esterase inhibitor (human) for the treatment of acute attacks of hereditary angioedema: an open-label trial. Ann Allergy Asthma Immunol. 2012; 108: 49–53.
Zuraw BL, Baker J, Hurewitz D, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1-INH NF) for the prophylaxis of hereditary angioedema (HAE) attacks [abstract]. Ann Allergy Asthma Immunol. 2010; 105(5 Suppl. 1): 100.Abstract 262.
Lumry W, Baker J, Levy R, et al. Use of nanofiltered C1 esterase inhibitor (human) for the treatment of gastrointestinal (GI) attacks in subjects with hereditary angioedema (HAE) [abstract]. Allergy. 2011; 66(Suppl. 94):418. Abstract 1088.
Riedl M, Lumry W, Baker J, et al. Use of nanofiltered C1 esterase inhibitor (human) for the treatment of extremity and facial attacks in subjects with hereditary angioedema (HAE) [abstract]. Allergy. 2011; 66(Suppl. 94): 421. Abstract 1095.
Lumry W, Baker J, Davis-Lorton M, et al. Openlabel use of nanofiltered C1 esterase inhibitor (human) (C1-INH NF) for treatment of acute attacks of hereditary angioedema (HAE) in pediatric subjects [abstract]. Ann Allergy Asthma Immunol. 2010; 105(5 Suppl. 1): 100–1. Abstract 264.
Hurewitz D, Grant JA, Busse P, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1-INH NF) for the prophylaxis of attacks of hereditary angioedema (HAE) in pediatric subjects [abstract]. Ann Allergy Asthma Immunol. 2010; 105(5 Suppl. 1): 100. Abstract 263.
Baker J, Riedl M, Banerji A, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1-INH NF) for treatment or prophylaxis of acute attacks of hereditary angioedema (HAE) in pregnant subjects [abstract]. Ann Allergy Asthma Immunol. 2010;105(5 Suppl. 1):10. Abstract 27.
Lumry W, Busse P, Baker J, et al. Pre-procedure administration of C1 esterase inhibitor (human) (C1-INH NF) for the prevention of hereditary angioedema (HAE) attacks after medical, dental, or surgical procedures [abstract]. J Allergy Clin Immunol. 2011;127:234. Abstract 903.
Landmesser L, Tillotson G, Mariano D. Site of care of nanofiltered C1 esterase inhibitor [human] (nf-C1INH) in patients with hereditary angioedema (HAE) [abstract]. Pharmacotherapy. 2010; 30: 456c. Abstract 324.
Longhurst HJ, Farkas H, Craig T, et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol. 2010;6:22.
Cinryze® (C1 inhibitor, human) for the prophylactic treatment of HAE: briefing document. Blood Product Advisory Committee Meeting; New York: Lev Pharmceuticals. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=130944&p=irolnewsArticle&ID=1139130&highlight=. Accessed May 2 2008.
ACKNOWLEDGMENTS
Before peer review, Viropharma were offered the opportunity to review this paper for scientific accuracy. No writing assistance, other editorial involvement, or financial support was provided by the manufacturer in the production of this manuscript. This article does not necessarily reflect the opinions, policies, or recommendations of Viropharma or any of its employees. Dr. Farkas is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of Interest. Dr. Farkas has received consultancy/speaker fees from Shire Human Genetic Therapies Inc., Pharming Group NV, Viropharma, and CSL Behring. Dr. Varga has received travel grants from CSL Behring and Shire Human Genetic Therapies Inc.
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.biologicstherapy-open.com
This article is published with open access at Springerlink.com
An erratum to this article is available at http://dx.doi.org/10.1007/s13554-012-0004-3.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Farkas, H., Varga, L. Human Plasma-Derived, Nanofiltered, C1-Inhibitor Concentrate (Cinryze®), a Novel Therapeutic Alternative for the Management of Hereditary Angioedema Resulting from C1-Inhibitor Deficiency. Biol Therapy 2, 2 (2012). https://doi.org/10.1007/s13554-012-0002-5
Received:
Published:
DOI: https://doi.org/10.1007/s13554-012-0002-5