Abstract
The gastrointestinal “hunger” hormone ghrelin is the only known circulating peripheral molecule with the ability to decrease body fat utilization and to increase body weight gain. Accordingly, due to ghrelin’s effects to promote food intake while decreasing energy expenditure ghrelin may offer potential as a drug for treatment of eating/wasting disorders and cachexia. Therapeutic potential of ghrelin and ghrelin analogues to promote food intake and body weight gain was recently indicated in several clinical studies. The recent discovery of the ghrelin O-acyltransferase as the key enzyme responsible for ghrelin acylation has further deepened our understanding of ghrelin activation, thereby paving the way for more efficient targeting of the ghrelin pathway. Here, we summarize the current knowledge pertaining to the potential of the endogenous ghrelin system as a drug target for the treatment of eating/wasting disorders and cachexia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cachexia (Greek: kakós—bad; hexis—condition) is a multifactorial syndrome characterized by substantial loss of body weight due to an involuntarily wasting of skeletal muscle and adipose tissue mass as a result of an imbalance between catabolic and anabolic processes (Fig. 1) [1, 2]. Cachexia frequently develops in advanced stages of various chronic diseases such as chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), sepsis, acquired immune deficiency syndrome and various kinds of cancer [1, 3]. Dependent on the type of tumor, cancer-cachexia is observed in 30–80% of cancer patients with patients suffering from pancreatic or gastric cancer having the highest frequency of weight loss while patients with breast cancer, non-lymphocytic leukemia, and sarcomas having the lowest [4]. Irrespective of the underlying disease, however, cachexia is associated with a low response to drug treatment, a poor quality of life, a poor prognosis, and an increased mortality rate compared to non-cachexia patients [1, 5]. Accordingly, cachexia is believed to be the immediate cause of 10–20% of all deaths in cancer patients [5].
Molecular mechanisms of cachexia. Typical metabolic changes associated with the development of cachexia are an increased release of pro-inflammatory cytokines as well as an overactivity of the sympathetic nervous system, as indicated by increased plasma concentrations of catecholamines. Both, pro-inflammatory cytokines and catecholamines promote catabolic processes leading to skeletal muscle and fat mass wasting, such as stimulation of lipid utilization and skeletal muscle protein breakdown while decreasing energy intake and increasing energy expenditure. ESRD end-stage renal disease, CKD chronic kidney disease, CHF chronic heart failure, COPD chronic obstructive pulmonary disease, IL6 Interleukin 6, IL8 Interleukin 8, IL1β Interleukin 1 beta, TNFα tumor necrosis factor alpha
Cachexia is frequently, but not necessarily, accompanied by anorexia, defined as the loss of the desire to eat [4, 6]. Decreased energy intake due to anorexia contributes to increased weight loss but, however, cannot solely explain the typical metabolic changes associated with cachexia, such as an excess release of pro-inflammatory cytokines and an increased activity of the sympathetic nervous system [1, 3, 4].
Cachexia is typically associated with an increased release of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα), Interleukin-1ß (IL-1ß), IL-6, and IL-8 [5]. Pro-inflammatory cytokines, especially TNFα and IL6, act as catabolic factors in the pathogenesis of cachexia by stimulating proteolytic pathways leading to muscle atrophy and increased adipose tissue breakdown (Fig. 1) [5]. In particular, TNFα stimulates muscle protein breakdown, causes contractile dysfunction and inhibits myogenesis and myogenic differentiation through activation of the nuclear factor-kappa B pathway [5, 7, 8]. TNFα further promotes wasting of adipose tissue through stimulation of lipolysis, inhibition of adipocyte differentiation and by increasing apoptosis in adipocytes [3, 5, 9].
Increased activity of the sympathetic nervous system is frequently described in patients with cachexia [4, 10, 11]. In particular, increased plasma concentrations of catecholamines as a result of sympathetic overactivity contribute to body weight loss and tissue wasting by increasing energy expenditure, stimulation of lipolysis, and stimulation of apoptosis in skeletal muscle [3, 12].
Various previous studies have, with only limited success, focused on the evaluation of potential drug targets for the treatment of eating/wasting disorders and cachexia. One of the endogenous peptides that, due to its beneficial effects on e.g. food intake, energy expenditure, and inflammation has recently reached scientific interest is the gastrointestinal “hunger” hormone ghrelin. The ability of ghrelin and ghrelin analogues to promote food intake and body weight gain in patients with eating disorders and cachexia was recently demonstrated in several clinical studies. The importance of the ghrelin system in the neuroendocrine control of energy balance has recently been highlighted in several excellent review articles [13, 14]. The aim of this review is to summarize the current knowledge pertaining to the endogenous ghrelin system as a potential target for the treatment of pathological reduced body mass, the key clinical feature of cachexia.
1.1 Ghrelin synthesis and activation
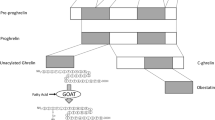
The gastrointestinal peptide hormone ghrelin was discovered in 1999 as an endogenous ligand for the growth hormone secretagogue 1a receptor (GHS-R1a) [15]. Ghrelin is synthesized as a 117 amino acid pre-prohormone, which is post-translational cleaved into a 28 amino acid peptide [16]. Ghrelin is predominantly synthesized and secreted by X/A-like cells in the oxyntic glands of the mucosa of the gastric fundus [17]. However, lower levels of ghrelin expression can also be found in, e.g., the intestine, pancreas, kidney, lung, ovaries and the brain [14]. Since its discovery in 1999, a tremendous amount of research efforts have focused on revealing ghrelin’s mechanisms of action. To promote its biological action, ghrelin is acylated on its serine 3 residue by the recently discovered membrane-bound O-acyltransferase 4 (MBOAT4), which was later accordingly renamed to ghrelin O-acyl-transferase (GOAT; Fig. 2) [18, 19]. The observation that acyl-ghrelin is absent in mice lacking Goat indicates that Goat is the only enzyme capable to activate ghrelin in vivo [18].
Ghrelin-mediated neuroendocrine alterations of energy metabolism. Ghrelin is secreted from the stomach and is acylated at its serine 3 residues by the ghrelin O-acyltransferase (GOAT). Central-mediated effects of ghrelin include (besides others) the stimulation of food intake and the decrease of energy expenditure through stimulation of hypothalamic neurons expressing neuropeptide Y (NPY) and agouti-related peptide (AgRP). In the anterior pituitary ghrelin stimulates the release of growth hormone (GH), which in turn stimulates the release of hepatic insulin-like growth factor-1 (IGF-1). Both, GH and IGF-1 increase lean body mass by inhibition of skeletal muscle protein breakdown. In adipose tissue, ghrelin stimulates the expression of genes coding for fat-storage promoting enzymes, such as lipoprotein lipase (LPL), fatty acid synthase (FAS), acetyl-CoA carboxylase α, and steaoryl-CoA desaturase-1 (SCD1). Either through central or peripheral mechanisms ghrelin further inhibits the release of pro-inflammatory cytokines, such as Interleukin (IL) 6, IL 8, IL1β and the tumor necrosis factor α. Dashed lines indicate potential signal pathways. ARC arcuate nucleus
1.2 Ghrelin-mediated regulation food intake and energy balance
Ghrelin is secreted from the stomach into the bloodstream under conditions of fasting, thus serving as a “hunger” hormone that signals the gastrointestinal fuel status from the periphery to the central nervous system in order to stimulate food intake and to adjust energy balance through a decrease in energy expenditure. Plasma levels of ghrelin typically follow a circadian rhythm with a preprandial rise which peaks directly at meal initiation followed by a postprandial decrease to baseline levels within the first hour after a meal [20–22]. In accordance to its role as a meal initiation hormone, ghrelin stimulates food intake and adiposity through stimulation of hypothalamic orexigenic neuropeptides [23]. In the arcuate nucleus (ARC), a hypothalamic key center in the control of energy metabolism, GHS-R1a is co-expressed with the agouti-related peptide (AgRP) and the neuropeptide Y (NPY), both prototypic anabolic neuropeptides that promote a positive energy balance through stimulation of food intake and by decreasing energy expenditure [23–25]. Accordingly, ghrelin-mediated activation of hypothalamic GHS-R1a entails an increased expression and release of NPY and AgRP in the ARC, thus entailing an activation of anabolic downstream pathways that lead to a stimulation of food intake and a decrease of energy expenditure [26–28]. Recent evidence further indicates that the ability of GOAT-mediated ghrelin acylation depends on specific dietary medium chain triglycerides as acylation sustrates [29]. These findings indicate that ghrelin, in contrast to its commonly accepted role as a hunger hormone, might rather serve as a nutrient sensor that signals the gastrointestinal nutrient availability to the central nervous system.
Ghrelin further stimulates the expression and release of growth hormone (GH) from the anterior pituitary gland and thus indirectly triggers expression and secretion of hepatic insulin-like growth factor-1 (IGF-1) [15, 30, 31]. Both, GH and IGF-1 are anabolic hormones known to increase lean body mass by stimulation of skeletal muscle growth and inhibition of skeletal muscle protein breakdown [32–34]. During catabolic states, such as cachexia, GH further stimulates lipolysis through increased release and oxidation of free fatty acids which leads to decreased glucose and protein oxidation and preservation of lean body mass [33]. The fundamental importance of the endogenous GOAT/ghrelin system was very recently demonstrated by Zhao and colleagues, who showed in Goat −/− mice that ghrelin-mediated regulation of GH release prevents death by preserving blood glucose levels under conditions of severe caloric restriction [35]. Other beneficial effects of ghrelin include a decrease of body fat utilization [36] and a decrease of sympathetic nerve activity [37–39]. In white adipocytes, ghrelin further stimulates the expression of fat-storage promoting enzymes, such as lipoprotein lipase, acetyl-CoA carboxylase α, fatty acid synthase, and steaoryl-CoA desaturase-1 [40]. In brown adipocytes, ghrelin dose-dependently lowers the expression of the thermogenesis-related mitochondrial uncoupling proteins 1 and 3, presumably through ghrelin’s ability to decrease sympathetic nerve activity [40]. Ghrelin further has been reported to attenuate skeletal muscle and adipose tissue wasting by decreasing the release of pro-inflammatory cytokines, such as TNFα, IL-1ß, IL-6 and IL-8 [39–43] while increasing the release of anti-inflammatory cytokines, such as IL-10 [43]. In summary, these data indicate that the endogenous GOAT/ghrelin system plays a fundamental role in the neuroendocrine adaptation to starvation and that modulation of the ghrelin system might be an interesting target for the treatment of pathological reduced body weight and tissue wasting.
2 Ghrelin levels in patients with eating disorders and cachexia
2.1 Anorexia nervosa
Anorexia nervosa (AN) is an eating disorder of unknown etiology, typically characterized by an abnormal eating behavior with disturbances of attitudes towards body weight and shape [44, 45].
Several forms of ghrelin (octanoyl-, desacyl, (non-octanoyl) acyl-ghrelin) can be found in circulation. However, for most available immunoassays it is either not well known or not sufficiently disclosed which of those ghrelin analogues they are binding and to which extent they are cross reacting with other related peptides such as motilin. Most assays reported in the literature likely measure total ghrelin-like immunoreactivity including large amounts of presumably inactive ghrelin peptide. Nevertheless, several studies have shown a negative correlation between the body mass index (BMI) and plasma levels of ghrelin in AN [46–48]. Accordingly, plasma levels of ghrelin are elevated in the acute phase of AN [48–52] and decline to normal values upon weight restoration [49, 53, 54]. Compared to normal weight healthy controls, also plasma levels of acyl-ghrelin are elevated in patients with AN, even during all phases of an oral glucose tolerance test [46, 48]. Some studies further report higher plasma levels of acyl-ghrelin when compared to BMI-matched lean women [47, 51–56] thus indicating that impaired ghrelin sensitivity due to persistent hyperghrelinemia might play a role in the pathogenesis of AN, comparable to leptin resistance in persistently hyperleptinemic obese individuals. Persistent hyperghrelinemia might further contribute to the frequently described impairment of the GH/IGF-1 axis in AN, as plasma levels of GH are typically elevated while IGF-1 levels are paradoxically decreased in patients with AN [57–60].
The orexigenic effects of ghrelin and ghrelin analogues have been assessed in several human studies revealing that ghrelin promotes food intake in both healthy individuals [61–63] and patients with AN [64]. Ghrelin treatment of anorectic individuals for 14 days (3 μg/kg twice daily) increased energy intake by 12–36% compared to baseline values in one study [64], although in another study, where ghrelin was continuously infused for 300 min at rates of 5 pmol/kg/min, ghrelin failed to detect an effect on appetite, as assessed by a visual analog scale [65]. Potential pitfalls of these studies are, however, the limited amount of study samples and the short duration of ghrelin treatment. Notably, however, both studies report no adverse side effects of ghrelin treatment [64, 65]. A further beneficial effect of ghrelin treatment in AN include an increase of blood glucose levels [66] which is in accordance with recent findings indicating that ghrelin prevents death by preservation of normoglycemia in Goat −/− mice under conditions of starvation [35].
3 Cancer-cachexia
Several animal studies have recently assessed the potential of ghrelin in the treatment of cancer-cachexia [67–70]. Plasma concentrations of ghrelin rise with the progression of cachexia in mice inoculated with human melanoma cells [68] and ghrelin treatment of these mice attenuates cachexia by stimulation of food intake and suppression of body weight loss [67]. Similar improvements on food intake and body weight gain were reported in tumor-implanted rats treated with either ghrelin or the ghrelin analog BIM-28131 [70]. In the latter study, ghrelin increased the hypothalamic expression of AgRP and NPY whereas, interestingly, plasma levels of GH were unchanged [70]. However, not all studies were able to replicate this finding [68]. It is further noteworthy that no differences in tumor size have been observed between ghrelin and saline treated animals [67, 70].
In accordance to the animal studies, plasma concentrations of ghrelin are elevated in patients with cancer-cachexia when compared to those without cachexia [71, 72]. Continuous infusion of ghrelin (5 pmol/kg/min for 180 min) in patients with breast and colon cancer increased energy intake by 31% compared to saline treated controls [73]. However, in a 2-week randomized, double blind, placebo-controlled trial, where ghrelin was given intravenously at two time points at doses of 2 or 8 μg/kg, ghrelin failed to affect nutritional intake despite elevated plasma levels of GH indicated an increased ghrelin activity [74]. Notably, however, both studies report no adverse side effects of ghrelin treatment [73, 74].
Noteworthy is that not all studies report elevated levels of ghrelin in patients with advanced cancer and weight loss. In one study, where ghrelin was measured in 30 patients with different malignancies and 27 healthy controls, ghrelin levels were reported to be lower in patients with advanced cancer as compared to the healthy controls [75]. Further studies in other study samples are required to clarify this observation but the reduction of ghrelin levels might be attributed to the severity and progression of the disease.
Even though substantial evidence indicates the safety and tolerability of ghrelin at doses up to 10 μg/kg [62, 73, 74, 76], no long-term studies are available pertaining to the potential implication of the ghrelin/GH/IGF-1 axis on tumor growth and carcinogenesis. Both, ghrelin and/or the ghrelin receptor are expressed in various tumorous tissues, especially in tumors of the gastrointestinal tract, such as in gastric endocrine tumors [77, 78], intestinal endocrine tumors [77, 78], pancreatic endocrine tumors [79, 80] but also in, e.g., pituitary tumors [81, 82], bronchial endocrine tumors [83] and testicular tumors [84]. It remains unknown as and to what extend ghrelin secretion from these tumors affects tumor growth and/or energy balance. Increased ghrelin secretion from tumorous tissues might be implicated in either promoting or inhibiting tumor growth via autocrine/paracrine pathways [13]. On the other hand, ghrelin released from tumors might counteract skeletal muscle and fat mass wasting by stimulation of food intake and activation of anabolic pathways. Tumor-related alterations of ghrelin secretion might thus contribute to the different rates of weight loss, which are typically observed in different kinds of cancers.
4 Chronic obstructive pulmonary disease
Cachexia is frequently described in patients with advanced stages of COPD [11, 85–87] and is considered as an independent risk factor for mortality in these patients [87, 88]. Plasma levels of ghrelin are negatively correlated with BMI in COPD patients [89] and 3-week treatment of COPD patients with ghrelin (2 μg/kg twice a day) significantly increased food intake, body weight, lean body mass, and muscle strength [11]. Intriguingly, ghrelin further increased plasma levels of GH while epinephrine levels are decreased, thus indicating a decrease of sympathetic nerve activity due to ghrelin treatment [11]. Ghrelin-mediated modulation of the GH/IGF-1 axis might be important for pulmonary cachexia as GH treatment has previously been shown to increase muscle mass in patients with COPD [90]. Together, these data support the potential of ghrelin to promote food intake and body weight gain in patients with pulmonary cachexia.
5 Chronic heart failure
Chronic heart failure is a major public health problem affecting approximately 5 million Americans with nearly 500,000 new cases every year [91]. Cardiac cachexia is observed in 10–15% of patients with CHF [85] and cachexia in these patients is associated with a poor prognosis and an increased mortality rate compared to non-cachexia patients [92]. Accordingly, mortality rates in patients with CHF are as high as 50% in patients with cachexia compared to 17% in patients without cachexia [1, 92].
Left ventricular dysfunction and left ventricular remodeling (dilatation and wall thinning) are frequently observed in patients with advanced stages of CHF [86, 93]. Growth hormone and IGF-1 are important physiological regulators of myocardial growth and performance [94, 95] and patients with CHF show typically elevated serum levels of GH and normal to decreased levels of IGF-1 [96, 97], thus indicating that alterations of the GH/IGF-1 axis might be implicated in the myocardial dysfunction and cachexia in these patients. Several studies have assessed the therapeutic potential of GH supplementation in the treatment of CHF, revealing that GH treatment improves left ventricular dysfunction and cardiac performance in both CHF rats [98–100] and patients with CHF [101]. Due to ghrelins beneficial effects on energy metabolism and GH/IGF-1 secretion it is suggested that ghrelin might improve cardiac performance and cachexia in CHF patients through GH-dependent and -independent mechanisms [91, 102]. Accordingly, 3-week treatment of CHF rats with ghrelin (100 μg/kg/day) increased serum GH and IGF-1 levels and promoted body weight gain and improved cardiac performance by increasing the diastolic thickness of the non-infarcted posterior wall and by inhibition of left ventricular enlargement [102]. In line with these observations, twice-daily treatment of CHF patients with ghrelin at doses of 2 μg/kg for 3 weeks improved cardiac performance and attenuated cachexia by increasing muscle strength and lean body mass [10]. Ghrelin further inhibits apoptosis of cardiomyocytes and endothelial cells in vitro [103] and decreases arterial pressure while increasing cardiac output in CHF rats [102] and healthy humans [104]. Together, these data indicate that ghrelin has beneficial effects on cardiovascular performance and cachexia through GH-dependent and independent mechanisms and that modulation of the ghrelin system is an interesting target for the treatment of myocardial dysfunction and cachexia in patients with CHF.
6 Renal failure
Anorexia and cachexia are frequently observed in patients with chronically decreased renal function, such as in patients with ESRD or chronic kidney disease [105, 106]. Renal insufficiency in these patients is often accompanied by increased serum levels of pro-inflammatory cytokines, such as TNFα and IL-6 [105, 106], which promote tissue wasting and cachexia by, e.g., inhibition of myogenesis and stimulation of skeletal muscle protein breakdown [5, 7].
Plasma levels of desacyl- and total ghrelin are elevated in patients with renal failure [107–110] and decline upon dialysis treatment [109, 110]. As the kidney is the primary site of ghrelin clearance [111] it is likely that ghrelin accumulates in these patients as a result of renal insufficiency [13, 112]. Several studies have assessed the therapeutic potential of ghrelin in the treatment of anorexia and cachexia in patients with renal failure [112–114]. Continuous infusion of nephrectomized rats for 14 days with either ghrelin or ghrelin analogues (BIM-28125 and BIM-28131, 150 nmol/kg/day) significantly increased food intake and lean body mass and tended to decrease overall pro-inflammatory cytokines compared to saline treated controls [112]. Accordingly, in a randomized, double blind, placebo-controlled crossover design, in which patients with peritoneal dialysis where treated with a single subcutaneous injection of ghrelin (3.6 nmol/kg), ghrelin increased immediate food intake by 57% compared to saline treated controls [113]. A similar effect on food intake was found in a double-blinded randomized crossover study where malnourished dialysis patients were treated for 1 week with 12 μg/kg/day of ghrelin [114].
7 Conclusion
Total ghrelin-like immunoreactivity in plasma is typically elevated in patients with anorexia nervosa as well as in cachexia associated with chronic heart failure [115, 116], renal failure [107, 117], chronic obstructive pulmonary disease [85, 89], and various forms of cancer [71, 118, 119]. Hyperghrelinemia in these patients may reflect a compensatory response to counteract the weight loss associated with skeletal muscle and fat mass wasting.
Several animal studies support the potential of ghrelin and ghrelin analogues to promote food intake and body weight gain in cachexia associated with heart failure [91, 102, 120, 121], chronic kidney disease [112] and cancer [67, 68, 70]. Accordingly, several human trials report improvements of appetite and body mass upon ghrelin treatment in patients with anorexia nervosa [64] and cachexia associated with renal failure [113, 114], chronic heart failure [10], chronic obstructive pulmonary disease [11], and cancer [73]. Notably, as yet all studies support the safety and tolerability of ghrelin treatment and no serious adverse side effects have so far been reported [73, 74]. Together, these data indicate that the endogenous ghrelin system represents an attractive target for the treatment of pathologically reduced body weight and tissue wasting, the key clinical feature of cachexia. However, further studies in larger populations are necessary to clarify the long-term effects of ghrelin treatment and to assess the possible impact of ghrelin and ghrelin induced growth factor release on tumor growth and carcinogenesis.
References
Von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–52.
Ashby D, Choi P, Bloom S. Gut hormones and the treatment of disease cachexia. Proc Nutr Soc. 2008;67:263–9.
Ashitani J, Matsumoto N, Nakazato M. Ghrelin and its therapeutic potential for cachectic patients. Peptides. 2009;30:1951–6.
Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410.
Langhans W. Peripheral mechanisms involved with catabolism. Curr Opin Clin Nutr Metab Care. 2002;5:419–26.
Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–3.
Karin M, Ben Neriah Y. Phosphorylation meets ubiquitination: the control of NF-kappa B activity. Ann Rev Immunol. 2000;18:621–63.
Li YP, Reid MB. Effect of tumor necrosis factor-alpha on skeletal muscle metabolism. Curr Opin Rheumatol. 2001;13:483–7.
Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol. 1999;10:19–29.
Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–9.
Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–93.
Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316.
Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430–81.
Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70.
Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, et al. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–6.
Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA. 2008;105:10750–5.
Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–5.
Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135:1320–5.
Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600.
Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–16.
Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept. 2005;126:55–9.
Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–800.
Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–43.
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95.
Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–5. Epub 2009 Jun 5. Erratum in: Nat Med. 2009;15:1093.
Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–74.
Peino R, Baldelli R, Rodriguez-Garcia J, Rodriguez-Segade S, Kojima M, Kangawa K, et al. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol. 2000;143:R11–4.
Amato G, Carella C, Fazio S, La Montagna G, Cittadini A, Sabatini D, et al. Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671–6.
Fuller J, Mynett JR, Sugden PH. Stimulation of cardiac protein synthesis by insulin-like growth factors. Biochem J. 1992;282:85–90.
Moller N, Vendelbo MH, Kampmann U, Christensen B, Madsen M, Norrelund H, et al. Growth hormone and protein metabolism. Clin Nutr. 2009;28:597–603.
Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010. In press [Epub ahead of print].
Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Matsumura K, Tsuchihashi T, Fujii K, Abe I, Iida M. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension. 2002;40:694–9.
Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43:977–82.
Wu R, Zhou M, Das P, Dong W, Ji Y, Yang D, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–702.
Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–93.
Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66.
Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–9.
Delgado M, Ganea D. Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–51.
Hebebrand J, Muller TD, Holtkamp K, Herpertz-Dahlmann B. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry. 2007;12:23–35.
Müller TD, Reichwald K, Brönner G, Kirschner J, Nguyen TT, Scherag A, et al. Lack of association of genetic variants in genes of the endocannabinoid system with anorexia nervosa. Child Adolesc Psychiatry Ment Health. 2008;2:33.
Nakai Y, Hosoda H, Nin K, Ooya C, Hayashi H, Akamizu T, et al. Short-term secretory regulation of the active form of ghrelin and total ghrelin during an oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol. 2004;150:913–4. Erratum in: Eur J Endocrinol. 2005;152:163.
Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88:109–16.
Harada T, Nakahara T, Yasuhara D, Kojima S, Sagiyama K, Amitani H, et al. Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biol Psychiatry. 2008;63:245–7.
Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–73.
Nakahara T, Harada T, Yasuhara D, Shimada N, Amitani H, Sakoguchi T, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64:252–5.
Germain N, Galusca B, Grouselle D, Frere D, Tolle V, Zizzari P, et al. Ghrelin/obestatin ratio in two populations with low bodyweight: constitutional thinness and anorexia nervosa. Psychoneuroendocrinology. 2009;34:413–9.
Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89:755–65.
Otto B, Tschöp M, Frühauf E, Heldwein W, Fichter M, Otto C, et al. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology. 2005;30:577–81.
Haas V, Onur S, Paul T, Nutzinger DO, Bosy-Westphal A, Hauer M, et al. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am J Clin Nutr. 2005;81:889–96.
Monteleone P, Serritella C, Martiadis V, Scognamiglio P, Maj M. Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa. J Clin Endocrinol Metab. 2008;93:4418–21.
Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, et al. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85:967–71.
Golden NH, Kreitzer P, Jacobson MS, Chasalow FI, Schebendach J, Freedman SM, et al. Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr. 1994;125:655–60.
Argente J, Caballo N, Barrios V, Muñoz MT, Pozo J, Chowen JA, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82:2084–92.
Støving RK, Flyvbjerg A, Frystyk J, Fisker S, Hangaard J, Hansen-Nord M, et al. Low serum levels of free and total insulin-like growth factor I (IGF-I) in patients with anorexia nervosa are not associated with increased IGF-binding protein-3 proteolysis. J Clin Endocrinol Metab. 1999;84:1346–50.
Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–23.
Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes Lond. 2005;29:1130–6.
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992.
Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007;12:594–600.
Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J. 2009;56:1119–28.
Miljic D, Pekic S, Djurovic M, Doknic M, Milic N, Casanueva FF, et al. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1491–5.
Miljic D, Djurovic M, Pekic S, Doknic M, Stojanovic M, Milic N, et al. Glucose metabolism during ghrelin infusion in patients with anorexia nervosa. J Endocrinol Invest. 2007;30:771–5.
Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, et al. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301:275–9.
Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, et al. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53:84–8.
Chance WT, Dayal R, Friend LA, Thomas I, Sheriff S. Continuous intravenous infusion of ghrelin does not stimulate feeding in tumor-bearing rats. Nutr Cancer. 2008;60:75–90.
DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–12.
Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–8.
Karapanagiotou EM, Polyzos A, Dilana KD, Gratsias I, Boura P, Gkiozos I, et al. Increased serum levels of ghrelin at diagnosis mediate body weight loss in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2009;66:393–8.
Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–6.
Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschöp M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–8.
Legakis I, Stathopoulos J, Matzouridis T, Stathopoulos GP. Decreased plasma ghrelin levels in patients with advanced cancer and weight loss in comparison to healthy individuals. Anticancer Res. 2009;29:3949–52.
Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447–55.
Papotti M, Cassoni P, Volante M, Deghenghi R, Muccioli G, Ghigo E. Ghrelin-producing endocrine tumors of the stomach and intestine. J Clin Endocrinol Metab. 2001;86:5052–9.
Rayhan N, Sano T, Qian ZR, Obari AK, Hirokawa M. Histological and immunohistochemical study of composite neuroendocrine-exocrine carcinomas of the stomach. J Med Invest. 2005;52:191–202.
Iwakura H, Hosoda K, Doi R, Komoto I, Nishimura H, Son C, et al. Ghrelin expression in islet cell tumors: augmented expression of ghrelin in a case of glucagonoma with multiple endocrine neoplasm type I. J Clin Endocrinol Metab. 2002;87:4885–8.
Volante M, Allìa E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab. 2002;87:1300–8.
Kim K, Arai K, Sanno N, Osamura RY, Teramoto A, Shibasaki T. Ghrelin and growth hormone (GH) secretagogue receptor (GHSR) mRNA expression in human pituitary adenomas. Clin Endocrinol Oxf. 2001;54:759–68.
Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:881–7.
Arnaldi G, Mancini T, Kola B, Appolloni G, Freddi S, Concettoni C, et al. Cyclical Cushing’s syndrome in a patient with a bronchial neuroendocrine tumor (typical carcinoid) expressing ghrelin and growth hormone secretagogue receptors. J Clin Endocrinol Metab. 2003;88:5834–40.
Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C, et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab. 2004;89:400–9.
Lainscak M, Andreas S, Scanlon PD, Somers VK, Anker SD. Ghrelin and neurohumoral antagonists in the treatment of cachexia associated with cardiopulmonary disease. Intern Med. 2006;45:837.
Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med. 2006;45:127–34.
Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61.
Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:961–6.
Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:879–82.
Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156:1800–6.
Nagaya N, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of chronic heart failure. Regul Pept. 2003;114:71–7.
Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. Erratum in: Lancet. 1997;349:1258.
Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–75.
Saccà L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev. 1994;15:555–73.
Saccà L, Fazio S. Cardiac performance: growth hormone enters the race. Nat Med. 1996;2:29–31.
Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34.
Niebauer J, Pflaum CD, Clark AL, Strasburger CJ, Hooper J, Poole-Wilson PA, et al. Deficient insulin-like growth factor I in chronic heart failure predicts altered body composition, anabolic deficiency, cytokine and neurohormonal activation. J Am Coll Cardiol. 1998;32:393–7.
Yang R, Bunting S, Gillett N, Clark R, Jin H. Growth hormone improves cardiac performance in experimental heart failure. Circulation. 1995;92:262–7.
Yang R, Bunting S, Gillett N, Clark RG, Jin H. Effects of growth hormone in rats with postinfarction left ventricular dysfunction. Cardiovasc Drugs Ther. 1995;9:125–31.
Cittadini A, Stromer H, Katz SE, et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat: a combined in vivo and in vitro evaluation. Circulation. 1996;93:800–9.
Fazio S, Sabatini D, Capaldo B, et al. A preliminary study of growth hormone in the treatment of dilated cardiomyopathy. N Engl J Med. 1996;334:809–14.
Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–5.
Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–37.
Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1483–7.
Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol. 2010;25:711–24.
Mak RH, Cheung W. Adipokines and gut hormones in end-stage renal disease. Perit Dial Int. 2007;27 Suppl 2:S298–302.
Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, et al. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol. 2002;13:2748–52.
Jarkovská Z, Rosická M, Krsek M, Sulková S, Haluzík M, Justová V, et al. Plasma ghrelin levels in patients with end-stage renal disease. Physiol Res. 2005;54:403–8.
Jarkovská Z, Hodková M, Sazamová M, Rosická M, Dusilová-Sulková S, Marek J, et al. Plasma levels of active and total ghrelin in renal failure: a relationship with GH/IGF-I axis. Growth Horm IGF Res. 2005;15:369–76.
Rodriguez Ayala E, Pecoits-Filho R, Heimbürger O, Lindholm B, Nordfors L, Stenvinkel P. Associations between plasma ghrelin levels and body composition in end-stage renal disease: a longitudinal study. Nephrol Dial Transplant. 2004;19:421–6.
Wu R, Zhou M, Cui X, Simms HH, Wang P. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. Int J Mol Med. 2003;12:777–81.
Deboer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–35.
Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–8.
Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199–206.
Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–8.
Xin X, Ren AJ, Zheng X, Qin YW, Zhao XX, Yuan WJ, et al. Disturbance of circulating ghrelin and obestatin in chronic heart failure patients especially in those with cachexia. Peptides. 2009;30:2281–5.
Aygen B, Dogukan A, Dursun FE, Aydin S, Kilic N, Sahpaz F, et al. Ghrelin and obestatin levels in end-stage renal disease. J Int Med Res. 2009;37:757–65.
Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–6.
Wolf I, Sadetzki S, Kanety H, Kundel Y, Pariente C, Epstein N, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. 2006;106:966–73.
Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol. 2003;3:146–51.
Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, et al. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H1643–51.
von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [122].
Sources of funding
This work was supported by grants from the Hilda and Preston Davis Foundation—Davis Foundation Postdoctoral Fellowship Program in Eating Disorders.
Disclosures
The authors confirm that there is no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Müller, T.D., Perez-Tilve, D., Tong, J. et al. Ghrelin and its potential in the treatment of eating/wasting disorders and cachexia. J Cachexia Sarcopenia Muscle 1, 159–167 (2010). https://doi.org/10.1007/s13539-010-0012-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-010-0012-4