Abstract
Sepsis presents a major health care problem and remains one of the leading causes of death within the intensive care unit (ICU). Therapeutic approaches against severe sepsis and septic shock focus on early identification. Adequate source control, administration of antibiotics, preload optimization by fluid resuscitation and further hemodynamic stabilisation using vasopressors whenever appropriate are considered pivotal within the early—golden—hours of sepsis. However, organ dysfunction develops frequently in and represents a significant comorbidity of sepsis. A considerable amount of patients with sepsis will show signs of severe muscle wasting and/or ICU-acquired weakness (ICUAW), which describes a frequently observed complication in critically ill patients and refers to clinically weak ICU patients in whom there is no plausible aetiology other than critical illness. Some authors consider ICUAW as neuromuscular organ failure, caused by dysfunction of the motor unit, which consists of peripheral nerve, neuromuscular junction and skeletal muscle fibre. Electrophysiologic and/or biopsy studies facilitate further subclassification of ICUAW as critical illness myopathy, critical illness polyneuropathy or critical illness myoneuropathy, their combination. ICUAW may protract weaning from mechanical ventilation and impede rehabilitation measures, resulting in increased morbidity and mortality. This review provides an insight on the available literature on sepsis-mediated muscle wasting, ICUAW and their potential pathomechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Sepsis
1.1 Epidemiology and definition of sepsis
Sepsis is a leading cause of death in intensive care units (ICU) [1–3]. In order to establish consensus definitions, the terms systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock have been proposed [4–7]. These terms define gradual stages of disease severity that correlate with mortality. Whereas SIRS defines a rather unspecific inflammatory host response due to both infectious and non-infectious origin, severe sepsis refers to a proven systemic infection associated with acute organ dysfunction. Criteria of septic shock are met once additional volume-refractory hemodynamic failure occurs [4, 5]. Recently, there has been great effort in studying the epidemiology of sepsis [2]. Data from the United States report 750,000 cases/year of severe sepsis [8] and an overall mortality of 29% for 2001. A 9% annual increase of severe sepsis incidence between 1977 and 2000 was reported [9]. Epidemiological trials from Germany indicate that 79,000 inhabitants (116/100,000) will suffer from sepsis each year and an additional 75,000 inhabitants (110/100,000) will be diagnosed with severe sepsis. Ninety-day mortality of severe sepsis amounts to 54%. With 60,000 sepsis-related deaths per year in Germany, sepsis remains a leading cause of death and a considerable burden for health care systems [10–12].
1.2 Fundamental clinical and pathophysiological considerations in sepsis
Treatment of patients with sepsis should be implemented according to international guidelines and recommendations, e.g., as proposed by the Surviving Sepsis Campaign [4]. Early identification and therapy initiation seem of pivotal importance and were shown to significantly improve patients’ prognoses. In general, guidelines for the treatment of severe sepsis or septic shock aim to initiate early symptomatic organ support therapies; e.g. early optimization of cardiac preload, afterload and contractility in order to balance oxygen delivery with oxygen demand influences survival rates in sepsis [13]. Over the past years, several treatment approaches have been associated with beneficial outcomes in large-scale, mostly single-centre trials, including low-dose hydrocortisone [14], glycemic control [15, 16] and recombinant activated protein C [17]. Yet, these benefits could not be replicated by confirmative studies [18–20].
Pathophysiologically, the first response to a severe infection consists in the activation of antigen-presenting immune cells. This occurs via pattern recognition receptors [1, 21–23] and is accompanied by other immune mechanisms such as cytokine liberation, endothelium and complement activation, and release of oxygen radicals. Cytokines (tumour-necrosis factor alpha (TNF-α), interleukin (IL)-6 and IL-1) and complement factors (C3a and C5a) may then act as key mediators during this stage of inflammation [21]. Clinical signs of infection such as leukocytosis, increased respiratory rate or acute organ failure may then develop [1] and, as a protective measure, the immune system coordinates an immunological and endocrine counter-regulation which is mediated by up-regulation of key anti-inflammatory cytokines (e.g. IL-10, tumour-growth factor-ß) [7, 24–27]. This may lead to prolonged hypo-inflammatory states (“immunoparalysis”) [27], which have been linked to secondary/nosocomial infection, prolonged ICU stay, chronic multiple organ failure and an increased mortality [21–31]. Ongoing clinical trials investigate measures of immunostimulation [28–30] or removal of inhibitory factors [31] to reverse this immunological condition.
Inflammation-mediated organ dysfunction represents a detrimental comorbidity of sepsis. As sepsis is frequently accompanied by short- and long-term affection of neuromuscular function, it has been postulated that the “motor unit” presents another system affected by inflammation-mediated multiple organ dysfunction: Sepsis is not only associated with involuntary loss of muscle mass, which is frequently referred to as muscle wasting, but also severe neuromuscular dysfunction resulting in “ICU-acquired weakness” (ICUAW). Patients with acute respiratory distress syndrome show a particularly high incidence for ICU-acquired weakness [32].
2 Sepsis-induced muscle wasting and ICUAW: an imbalance in protein metabolism
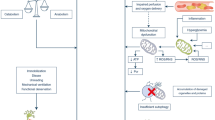
William Osler first commented on the “rapid loss of flesh” that occurs with severe sepsis in 1892 [33]. Today, this phenomenon remains a frequent complication of critical illness—particularly sepsis—and is often referred to as muscle wasting. However, because sepsis led to a fairly rapid death in Osler’s days, the primary focus was rather survival than sepsis-associated long-term complications. Yet, once ICU and artificial organ support therapies are available, more patients survive the initial stages of systemic inflammation/sepsis and critical care specialists are increasingly confronted with profound muscle weakness, which is often accompanied by difficulties in weaning from respiratory support. Sepsis-induced multiple organ failure has been identified as one of the primary risk factors for this major and frequent complication of critical illness [34]. As a confusing number of various terminologies referred to similar or identical clinical presentations of this neuromuscular disorder, a recent round table conference held in 2009 proposed the mutual term “ICUAW” [35]. Whereas ICUAW is usually accompanied by muscle wasting, muscle wasting does not necessarily lead to neuromuscular dysfunction, since overall muscle strength depends both on total muscle mass and force generating capacity (force per cross-sectional area), which is affected in ICUAW but not necessarily in muscle wasting syndromes [36] (Fig. 1).
2.1 Sepsis-associated muscle wasting
As mentioned above, muscle force capacity may remain stable in muscle wasting syndromes [36]. Muscle wasting can be triggered by other conditions than sepsis, including disuse, denervation, fasting, cancer, cardiac failure and renal dysfunction. As these conditions frequently coincide with sepsis, systematic research of sepsis-specific muscle wasting and ICUAW may be challenging in humans. This seems especially the case as sepsis is often accompanied by prolonged bedrest/immobilisation, application of sedatives, acute or chronic organ dysfunction, malignancy as an underlying disease, medication using glucocorticoids and others.
An imbalance between muscle protein synthesis and muscle protein degradation causing net loss of muscle mass is considered to present the main mechanism of muscle atrophy in muscle wasting and may result from decreased protein synthesis and/or increased protein degradation [36] (Fig. 1).
2.2 Decreased muscle protein synthesis during sepsis
Even though studies on sepsis-induced muscle protein degradation received more attention, animal models of sepsis clearly indicate that sepsis also decreases protein synthesis in skeletal muscles [37] and preferentially inhibits myofibrillar and sarcoplasmatic protein synthesis within fast twitch muscles [38].
One central mechanism is decreased protein translation by lower amounts of active translation initiation factor complex eIF4E/eIF4G, possibly resulting from decreased phosphorylation of eIF4G and mammalian target of rapamycin (mTOR) [37]. Interestingly, mTOR activation presents an important downstream target of anabolic insulin/insulin like growth factor 1 (IGF-1) signalling, which may be impaired in sepsis since decreased insulin sensitivity presents a frequently observed complication of sepsis [39–44]. Further indirect evidence for contribution of impaired insulin signalling to decreased protein synthesis comes from the observation that the proinflammatory cytokines IL-6 and TNF-α have been linked to both insulin resistance [45, 46] and muscle atrophy [47–50] and that local IGF-1 application prevents sepsis-induced muscle atrophy [37], possibly by inhibition of sepsis-induced increases of muscle atrogin-1 and the proinflammatory cytokine IL-6 [51]. IL-1 may lead to decreased protein synthesis as well, as an animal model of abdominal sepsis showed cytokine-dependent decreases of phosphorylated eIF4G that was primarily attributed to this cytokine [52]. There is evidence that systemic cytokine response in sepsis results in local amplification of proinflammatory cytokines within muscle [36], possibly aggravating decreased protein synthesis in skeletal muscle during sepsis.
Nutritional aspects may contribute to decreased muscle protein synthesis as well. Administration of the essential branched chain amino acid leucine has been shown to increase protein synthesis in rat skeletal muscles during ageing, exercise or food-deprivation [53–56]. However, under certain conditions including sepsis, the muscle may be resistant to leucine-stimulated protein synthesis [37]. Although the exact mechanisms of sepsis-induced leucine resistance remain to be elucidated, this phenomenon was accompanied by an 80% reduction in the amount of active eIF4E/eIF4G complex (besides other factors) and could be completely reversed by the combination of TNF binding protein and glucocorticoid receptor antagonist RU486 [57]. Exploring the underlying mechanism of sepsis-induced resistance to leucine-stimulated protein synthesis may contribute to a better understanding of the mechanisms involved in sepsis-mediated reductions of active translation initiation factor complexes, particularly the eIF4E/eIF4G complex.
2.3 Increased muscle protein degradation during sepsis
Data from studies in cultured cells, animals and humans indicate an increase of sepsis-associated muscle protein degradation by several mechanisms, including the ubiquitin proteasome system (UPS) [58–61] and lysosomal systems [50, 62–64]. Calcium-dependent non-lysosomal calpains and pro-apoptotic pathways (caspases) have also been associated with sepsis-induced muscle atrophy [66–68].It has been postulated that caspases and calpains are responsible for the cleavage of myofibrillar proteins preceding their proteasomal degradation [65–67] and that cleavage is necessary as the proteasome cannot degrade intact myofibrillar proteins. Protein degradation by proteasomal and/or lysosomal systems may not be sepsis-specific as various other conditions associated with muscle wasting share similar or identical biochemical and transcriptional pathways.
During muscle wasting, defective insulin signalling may not only be involved in decreased muscle protein synthesis, but also in increased muscle proteolysis, since mTOR inhibits autophagy-induced lysosomal proteolysis [68]. mTOR activation is regulated by phosphorylated Akt, another key downstream effector of insulin signalling. Phosphorylated Akt leads to phosphorylation of FOXO3 transcription factors, which results in FOXO3 translocation from nucleus to cytoplasm and a thereby decreased transcription of atrophy gene atrogin-1 [69].
E3 ubiqitin ligases, such as atrogin-1 and muscle ring finger protein 1 (MuRF-1), represent substrate specific enzymes involved in the UPS that prevent unselective degradation by the proteasome and have been shown to be markedly induced during experimental atrophic conditions [70, 71]. Whereas myosin heavy chain and other myofibrillar proteins including myosin binding proteins were identified as MuRF-1 substrates [72, 73], the only atrogin-1 substrates identified so far include MyoD and translation initiation factor eIF3-f, which are both known to regulate protein synthesis [74, 75]. It is therefore possible, that atrogin-1 leads to muscle atrophy by selective breakdown of key regulators of protein synthesis [76]. We emphasise once more, that sepsis-mediated muscle wasting and ICU-acquired weakness should not be used synonymously, as ICUAW weakness represents its own entity, characterised by additional factors.
3 ICUAW: clinical presentation, relevance, risk factors, treatment options and subclassifications
3.1 General considerations on ICUAW
ICUAW presents a severe and frequent complication of critical illness, confronting intensivists around the globe with various difficulties. It is believed that ICUAW can affect more than half of all ICU patients [77]. This substantial neuromuscular complication of critical illness is associated with increased rates of morbidity and mortality, substantially affecting both short- and long-term clinical outcomes in septic patients [78–85]. By causing prolongation of ICU stay and rehabilitation, ICUAW leads to an increased risk of secondary complications and a higher demand on already limited resources of health care systems. ICUAW is further associated with decreases in health-related quality of life (HRQOL) which may be impaired for years after ICU discharge [85, 86]. Modern critical care must therefore no longer solely focus on survival, but particularly consider HRQOL after ICU discharge.
3.2 Clinical presentation and diagnostic limitations of ICUAW
ICUAW refers to the bedside diagnosis of pronounced weakness in ICU patients without plausible aetiology other than critical illness [35]. Whereas the patients’ history usually reveal exposure to ICUAW-associated risk factors (see below), physical examination of awake and cooperative patients with typical ICUAW shows symmetric weakness and decreased tone. This primarily affects the lower limbs but may extend to tetraplegia in more severe cases, explaining its prior terminology of acute quadriplegic myopathy. Muscles innervated by cranial nerves are normally—yet not necessarily—spared, while respiratory muscle function is frequently abnormal. Deep tendon reflexes may be normal, decreased or absent [35]. Whenever suspecting ICUAW, it is fundamental to rule out prolonged neuromuscular blockade (involvement of cranial nerve-innervated muscles), pre-existing neuromuscular dysfunction and other conditions as alternative causes. Currently, ICUAW is frequently evidenced by difficulties in weaning from mechanical ventilation or unexpected problems with mobilisation. However, as weaning from respiratory support may not be initiated for several days or even weeks, it seems important to consider possible ICUAW before development of its full extent in order to prevent or at least attenuate underlying pathology by minimising risk factor exposure [34].
After all, earlier detection of ICUAW is possible and can be obtained by assessment of voluntary maximum strength in ICU patients, either by hand dynamometry or according to the Medical Research Council (MRC)-score [35]. The MRC-score grades manually tested strength from 0 (no movement observed) to 5 (muscle contracts normally against full resistance) in three functional muscle groups of each extremity, with mean MRC-scores of <4 (antigravity strength) indicating ICUAW. It has shown good interobserver reliability in patients with Guillan–Barré syndrome [87]. Values of <11-kg force for men and <7-kg force for women at dominant-hand dynamometry have also been described to identify ICU-acquired weakness in previously healthy individuals [88]. Nevertheless—and in spite of daily wake up calls, both approaches share one major limitation—the requirement for patient cooperation and consciousness, which may be inadequate due to delirium resulting from sedation and/or septic encephalopathy. Electrophysiologic testing offers an additional approach to estimate neuromuscular dysfunction in unconscious patients incapable of voluntary contraction. Moreover, electrophysiologic testing after direct muscle stimulation can be conducted in unconscious/sedated patients and has been shown to predict ICUAW with high sensitivity and specificity in mechanically ventilated, sedated patients and differentiates between primary nerve or muscle dysfunction [89]. However, as it requires some expertise and certain devices to perform these measurements, use of this technique is currently restricted to larger facilities or experts.
3.3 Risk factors involved in ICUAW
While the exact molecular mechanisms contributing to ICUAW remain to be elucidated, five central risk factors of ICUAW have been repeatedly reported [34]. As there are currently no specific therapies, minimising exposure to these risk factors is crucial in order to prevent this devastating neuromuscular complication. Possibly the most important risk factor complex comprises conditions leading to multiple organ failure, particularly severe sepsis and septic shock. Some authors actually consider ICUAW an additional organ failure following severe sepsis and septic shock. The other four risk factors of ICUAW involve muscle inactivity, disturbances of glucose metabolism resulting in hyperglycaemia, administration of corticosteroids, and use of neuromuscular blocking agents (Fig. 1).
3.4 Implications for minimising risk factor exposure and treatment options
3.4.1 Sepsis
In addition to the optimum realisation of early goal-directed sepsis therapy [13], there are a number of hypothetical approaches targeting inflammation-mediated multiple organ failure, such as reducing levels of distinct cytokines by extracorporeal measures [31, 90–94] or restoring the immunological equilibrium by pharmaceutical immunomodulation [28–30]. However, the current literature on ICUAW has not considered sepsis-induced affections in cellular immunity to participate directly in the induction of neuromuscular dysfunction.
3.4.2 Immobilisation
Schweickert and colleagues [95] recently reported that patients undergoing an ambitious protocol of early and determined mobilisation were more frequently able to get out of bed, stand and occasionally walk with assistance during mechanical ventilation whereas standard regimens of physical therapy led to longer impairment of functional status and recovery time. Besides, early mobilisation was associated with a shorter duration of delirium. It is possible, that additional electrical muscle stimulation (EMS) assists in preventing ICUAW, since studies indicate that EMS partially prevents muscle atrophy in critically ill patients [96] and mitigates increased proteasome activation besides stimulating insulin like growth factor in patients after major abdominal surgery [97]. Preliminary data of our ongoing trial on ICUAW prevention by daily EMS supports this, as it indicates improvement of muscle membrane excitability in critically ill patients at high risk for ICUAW (unpublished data). Importantly, EMS can be initiated immediately after ICU admission and could facilitate faster mobilisation progress. Implementation of protocol-based sedation and weaning measures may further reduce the incidence of ICUAW and weanig failure (Fig. 2).
Suggested beneficial effects of electrical muscle stimulation (EMS) with regard to muscle hypertrophy, atrophy, aerobic capacity, membrane excitability and membrane translocation of GLUT4. EMS may preserve membrane excitability. Membrane translocation of GLUT4 is regulated by IGF-1, AMPK, PGC-1α and its downstream targets, which may all be affected by EMS. Atrophy gene expression (MuRF-1, atrogin-1) increases upon desphosphorylation of FoxO3 trancription factors, which is inhibited by downstream insulin signalling. EMS electrical muscle stimulation, IGF-1 insulin growth factor-1, GLUT4 glucose transporter 4, IRS-1 insulin receptor substrate 1, AMPK AMP-activated protein kinase, PI3K phopshoinositide 3-kinase, PPAR peroxisome proliferator-activated receptor, PGC-1α PPAR-γ coactivator 1α, pAKT phosphorylated Akt protein kinase B, mTOR mammalian target of rapamycin, FoxO3 forkhead box O3, MuRF muscle-specific ring finger protein
3.4.3 Intensive insulin therapy
Increased serum glucose levels are typical findings in patients with severe sepsis and septic shock. Studies from van den Berghe and colleages reported that intensive insulin therapy (IIT) was associated with a lower incidence of ICUAW [98, 99]. However, recent data from the NICE-SUGAR trial demonstrates that 90-day mortality rate is significantly lower in critically ill patients with liberal glycemic control (serum glucose ≤180 mg/dl) vs. those patients receiving IIT, the risk of severe hypoglycemia being significantly higher in the IIT group [19, 20]. Although tight glycemic control as a general approach in ICU patients is currently controversial, it seems reasonable that protocol-based glycemic control targeting prevention of excessive blood glucose levels and variability help reduce the incidence of ICUAW.
3.4.4 Glucocorticoid exposure
Although de Jonghe et al. identified corticosteroid administration as the strongest predictor for ICU-acquired weakness [100], data from a recent meta-analysis does not indicate a clear relationship between systemic levels of corticosteroids and myotoxic effects in patients with sepsis [81]. Development of steroid induced myopathy [101, 102] may be dependent on steroid doses applied, which is in line with findings from our group, that do not indicate an association between low-dose hydrocortisone application and impaired muscle membrane excitability—one of the key features in ICUAW patients with predominant myopathy [103]. Yet, as this observation has to be confirmed by other groups, strict indication is still warranted considering low-dose hydrocortisone administration.
3.4.5 Neuromuscular blockers
Treatment with neuromuscular blocking agents has been reported to contribute to ICUAW development. Initial reports stated that vecuronium and pancuronium may be particularly harmful in regard to critical illness neuromuscular abnormalities [104]. It remains unclear, however, whether this is simply attributable to the rather widespread use of these substances at that time. Importantly, renal function must be taken into account, as some neuromuscular blocking agents undergo excretion via the kidneys. As firm conclusions regarding specific deleterious effects of neuromuscular blockers are precluded, cautious use is still warranted [34].
3.5 Subclassification of ICUAW
Depending on electrophysiologic or histological documentation of neuropathy and/or myopathy, ICUAW can be further subclassified as critical illness myopathy (CIM), critical illness polyneuropathy (CIP), or critical illness myoneuropathy (CIMN), which is more frequent than previously thought and applies to ICUAW patients with coinciding neuropathy and myopathy [35]. Myopathy is likely to present the predominant feature of ICUAW and has been shown to precede neuropathy in patients with CIMN [89]. Yet, as both histology and electrophysiology are currently not obtained during clinical routine, subclassification of ICUAW may seem of less importance to clinicians dealing with this complication. We just recently demonstrated that myopathy occurs earlier and more frequent, whereas additional polyneuropathy develops later and less frequent. By showing that additional polyneuropathy was associated with longer ICU length of stay, we suggest that differential diagnosis of ICUAW becomes more important to clinicians [105].
3.6 Sepsis-induced myopathy: CIM and CIMN
Three characteristics are commonly described in patients with CIM or CIMN [106–108]:
-
Selective thick filament loss
-
Predominant type II muscle fibre atrophy (Fig. 3)
-
Muscle membrane inexcitability
Unspecific but predominant type II (fast twitch) muscle fibre atrophy has been repeatedly described within muscle tissue from CIM/CIMN biopsies [35]. Selective but patchy loss of myosin filaments can be visualised by electron microscopy and is considered a hallmark of CIM, explaining its additional terminology of thick filament myopathy [35]. In contrast to these morphologic alterations, non-excitable muscle membrane is detected by electrophysiologic testing [43, 108, 109] and has been shown to predict ICUAW in mechanically ventilated, sedated patients with high sensitivity and specificity [89]. All three characteristics may result in different mechanisms leading to ICUAW, as loss of muscle mass (atrophy) correlates with decreased maximum force, thick filament loss represents an additional reduction of force generating capacity by additional myofilament dysfunction, and non-excitable muscle membrane may be considered as an incapability of the muscle to generate contraction-preceding action potentials. Less frequently described histological signs of CIM/CIMN include acute necrosis [110, 111], regeneration [112] as well as loss of myofibrillar ATPase staining [113], the latter affecting both type I and II muscle fibres.
3.7 Suggested subcellular abnormalities in sepsis-induced myopathy
A number of subcellular sites involved in excitation contraction coupling may be affected in sepsis-induced myopathy [36]. These include the sarcolemma, the sarcoplasmatic reticulum, the contractile apparatus and the mitochondria. As described above, one of the key features of CIM/CIMN is that skeletal muscle becomes electrically inexcitable, which has led to the concept that CIM/CIMN could represent an acquired channelopathy involving dysregulation of sodium channels located at the sarcolemma. Experimental studies describe sarcolemmal injuries in diaphragm and limb muscle that were in part associated with excessive nitric oxide generation [114–116]. Altered calcium homeostasis has been observed in a number of studies on skeletal muscle during sepsis with calcium level increases in some subcellular compartments and decreases in others [36]. Contractile protein dysfunction resulting in reduced force pCa relationship has been reported in diaphragm and limb muscle in a number of sepsis models [117–120]. It is likely that free radical generation is involved in this mechanism, as either a superoxide scavenger or NO synthesis inhibitor significantly attenuated reduced endotoxin-induced force pCa relationships. TNF-α has been linked to decreases in titanic force generation due to changes in myofilament function, as well [121, 122]. A considerable amount of data indicates that sepsis also causes profound alterations in respiratory muscle mitochondrial function [36]. Limb muscle biopsies from septic patients show decreased mitochondrial content and lower concentrations of energy rich phosphates [123].
4 Conclusions and outlook
In conclusion, sepsis and systemic inflammation may lead to multiple organ failure, which is associated with loss of muscle mass and/or onset of ICUAW. ICUAW presents a significant clinical problem and is thought to affect up to 50% of all ICU patients. As an increasing amount of patients survives sepsis, long-term complications of critical care are likely to gain in significance. Short term complications of ICUAW include secondary complications due to prolonged ICU stay, protraction of rehabilitation measures, failure in weaning from mechanical ventilation and others. Currently, there is no causal therapy of ICUAW and measures are restricted to minimising risk factor exposure. Risk factors besides multiple organ failure—which is most likely due to sepsis and systemic inflammation—include excessive blood glucose levels, immobilisation, exposure to neuromuscular blockers and corticosteroid application. Therefore, measures such as identification of risk patients, avoidance of unnecessarily deep sedation, promotion of early mobilisation and EMS, prevention of excessive blood glucose levels, rational administration of glucocorticoids and/or neuromuscular blockers as well as early goal-directed therapy of sepsis seem to reduce the severity and incidence of ICUAW. Nutritional approaches, immunomodulatory interventions, antioxidant treatment or others might furthermore lower the incidence of muscle wasting and ICUAW in critically ill patients.
References
Annane D, Bellissant E, Cavaillon J. Septic shock. Lancet. 2005;365:63–78.
Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165–72.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74.
Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991;114:332–3.
Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–8.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10.
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54.
Engel C, Brunkhorst FM, Bone H, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–18.
Moerer O, Schmid A, Hofmann M, Herklotz A, Reinhart K, Werdan K, et al. Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use. Intensive Care Med. 2002;28:1440–6.
Schmid A, Burchardi H, Clouth J, Schneider H. Burden of illness imposed by severe sepsis in Germany. Eur J Health Econ. 2002;3:77–82.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Annane D, Sébille V, Charpentier C, Bollaert P, François B, Korach J, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71.
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24.
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97.
Finfer S, Heritier S. The NICE-SUGAR (normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation) study: statistical analysis plan. Crit Care Resusc. 2009;11:46–57.
Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91.
Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41:S504–12.
Schefold JC, Hasper D, Volk HD, Reinke P. Sepsis: time has come to focus on the later stages. Med Hypotheses. 2008;71:203–8.
Höflich C, Volk HD. Immunomodulation in sepsis. Chirurg. 2002;73:1100–4.
Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000;26:S124–8.
Schefold JC, Hasper D, Reinke P, Monneret G, Volk H. Consider delayed immunosuppression into the concept of sepsis. Crit Care Med. 2008;36:3118.
Volk HD, Reinke P, Döcke WD. Clinical aspects: from systemic inflammation to 'immunoparalysis’. Chem Immunol. 2000;74:162–77.
Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29:646–51.
Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81.
Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–8.
Schefold JC, von Haehling S, Corsepius M, Pohle C, Kruschke P, Zuckermann H, et al. A novel selective extracorporeal intervention in sepsis: immunoadsorption of endotoxin, interleukin 6, and complement-activating product 5a. Shock. 2007;28:418–25.
Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, et al. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:711–5.
Osler SW. The principles and practice of medicine: designed for the use of practitioners and students of medicine. New York: D. Appleton and Company; 1910.
de Jonghe B, Lacherade J, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37:S309–15.
Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37:S299–308.
Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37:S354–67.
Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–9.
Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol. 1992;262:C1513–9.
Thorell A, Rooyackers O, Myrenfors P, Soop M, Nygren J, Ljungqvist OH. Intensive insulin treatment in critically ill trauma patients normalizes glucose by reducing endogenous glucose production. J Clin Endocrinol Metab. 2004;89:5382–6.
Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–8.
Carlson GL. Insulin resistance in sepsis. Br J Surg. 2003;90:259–60.
Carlson GL. Hunterian Lecture: insulin resistance in human sepsis: implications for the nutritional and metabolic care of the critically ill surgical patient. Ann R Coll Surg Engl. 2004;86:75–81.
Mesotten D, Delhanty PJD, Vanderhoydonc F, Hardman KV, Weekers F, Baxter RC, et al. Regulation of insulin-like growth factor binding protein-1 during protracted critical illness. J Clin Endocrinol Metab. 2002;87:5516–23.
Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, et al. Severity of insulin resistance in critically ill medical patients. Metab Clin Exp. 2007;56:1–5.
Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7.
Xu J, Kim HT, Ma Y, Zhao L, Zhai L, Kokorina N, et al. Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor-alpha. Endocrinology. 2008;149:2369–82.
Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metab Clin Exp. 2007;56:49–57.
Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–70.
Steffen BT, Lees SJ, Booth FW. Anti-TNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J Appl Physiol. 2008;105:1950–8.
Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, et al. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci. 1995;89:431–9.
Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E501–12.
Vary TC, Deiter G, Lang CH. Cytokine-triggered decreases in levels of phosphorylated eukaryotic initiation factor 4G in skeletal muscle during sepsis. Shock. 2006;26:631–6.
Kobayashi H, Kato H, Hirabayashi Y, Murakami H, Suzuki H. Modulations of muscle protein metabolism by branched-chain amino acids in normal and muscle-atrophying rats. J Nutr. 2006;136:234S–6.
Freyssenet D, Berthon P, Denis C, Barthelemy JC, Guezennec CY, Chatard JC. Effect of a 6-week endurance training programme and branched-chain amino acid supplementation on histomorphometric characteristics of aged human muscle. Arch Physiol Biochem. 1996;104:157–62.
Ventrucci G, Ramos Silva LG, Roston Mello MA, Gomes Marcondes MCC. Effects of a leucine-rich diet on body composition during nutritional recovery in rats. Nutrition. 2004;20:213–7.
Sugawara T, Ito Y, Nishizawa N, Nagasawa T. Supplementation with dietary leucine to a protein-deficient diet suppresses myofibrillar protein degradation in rats. J Nutr Sci Vitaminol. 2007;53:552–5.
Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–9.
Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–905.
Hasselgren P, Menconi MJ, Fareed MU, Yang H, Wei W, Evenson A. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–68.
Rabuel C, Renaud E, Brealey D, Ratajczak P, Damy T, Alves A, et al. Human septic myopathy: induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology. 2004;101:583–90.
Klaude M, Fredriksson K, Tjäder I, Hammarqvist F, Ahlman B, Rooyackers O, et al. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci. 2007;112:499–506.
Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983;308:553–8.
Hummel RP, James JH, Warner BW, Hasselgren PO, Fischer JE. Evidence that cathepsin B contributes to skeletal muscle protein breakdown during sepsis. Arch Surg. 1988;123:221–4.
Voisin L, Breuillé D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, et al. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+ -activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest. 1996;97:1610–7.
Huang J, Forsberg NE. Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA. 1998;95:12100–5.
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23.
Tan FC, Goll DE, Otsuka Y. Some properties of the millimolar Ca2 + -dependent proteinase from bovine cardiac muscle. J Mol Cell Cardiol. 1988;20:983–97.
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412.
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8.
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–5.
Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–85.
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–95.
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–56.
Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, et al. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–76.
Attaix D, Baracos VE. MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab. 2010;13:223–4.
Vincent J, Norrenberg M. Intensive care unit-acquired weakness: framing the topic. Crit Care Med. 2009;37:S296–8.
Spitzer AR, Giancarlo T, Maher L, Awerbuch G, Bowles A. Neuromuscular causes of prolonged ventilator dependency. Muscle Nerve. 1992;15:682–6.
Latronico N, Fenzi F, Recupero D, Guarneri B, Tomelleri G, Tonin P, et al. Critical illness myopathy and neuropathy. Lancet. 1996;347:1579–82.
Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–96.
Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–91.
De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–21.
De Jonghe B, Lacherade J, Durand M, Sharshar T. Critical illness neuromuscular syndromes. Crit Care Clin. 2007;23:55–69.
De Jonghe B, Bastuji-Garin S, Durand M, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–15.
Herridge MS. Long-term outcomes after critical illness. Curr Opin Crit Care. 2002;8:331–6.
Herridge MS. Legacy of intensive care unit-acquired weakness. Crit Care Med. 2009;37:S457–61.
Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–9.
Latronico N, Rasulo FA. Presentation and management of ICU myopathy and neuropathy. Curr Opin Crit Care [Internet]. 2010 Jan 13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20075723. Accessed 4 Feb 2010
Weber-Carstens S, Koch S, Spuler S, Spies CD, Bubser F, Wernecke KD, et al. Nonexcitable muscle membrane predicts intensive care unit-acquired paresis in mechanically ventilated, sedated patients. Crit Care Med. 2009;37:2632–7.
Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–52.
Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, Corradi V, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 2007;11:R47.
Schefold JC, Hasper D, Jörres A. Organ crosstalk in critically ill patients: hemofiltration and immunomodulation in sepsis. Blood Purif. 2009;28:116–23.
Stegmayr B. Apheresis in patients with severe sepsis and multi organ dysfunction syndrome. Transfus Apher Sci. 2008;38:203–8.
Haase M, Bellomo R, Morgera S, Morger S, Baldwin I, Boyce N. High cut-off point membranes in septic acute renal failure: a systematic review. Int J Artif Organs. 2007;30:1031–41.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82.
Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13:R161.
Strasser EM, Stättner S, Karner J, Klimpfinger M, Freynhofer M, Zaller V, et al. Neuromuscular electrical stimulation reduces skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner: a randomized, controlled clinical trial in major abdominal surgical patients. Ann Surg. 2009;249:738–43.
Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–53.
Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175:480–9.
De Jonghe B, Sharshar T, Lefaucheur J, Authier F, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–67.
Sander HW, Golden M, Danon MJ. Quadriplegic areflexic ICU illness: selective thick filament loss and normal nerve histology. Muscle Nerve. 2002;26:499–505.
Amaya-Villar R, Garnacho-Montero J, García-Garmendía JL, Madrazo-Osuna J, Garnacho-Montero MC, Luque R, et al. Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2005;31:157–61.
Weber-Carstens S, Deja M, Koch S, Spranger J, Bubser F, Wernecke K, et al. Risk factors in Critical illness myopathy (CIM) during the early course of critical illness: a prospective observational study. Crit Care. 2010;14:R119.
Segredo V, Caldwell JE, Matthay MA, Sharma ML, Gruenke LD, Miller RD. Persistent paralysis in critically ill patients after long-term administration of vecuronium. N Engl J Med. 1992;327:524–8.
Koch S, Spuler S, Deja M, Bierbrauer J, Dimroth A, Behse F, et al. Critical illness myopathy is frequent—accompanying neuropathy protracts ICU discharge. JNNP. 2010 (in press).
Larsson L, Li X, Edström L, Eriksson LI, Zackrisson H, Argentini C, et al. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45.
Lacomis D, Giuliani MJ, Van Cott A, Kramer DJ. Acute myopathy of intensive care: clinical, electromyographic, and pathological aspects. Ann Neurol. 1996;40:645–54.
Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46:731–6.
Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve. 1997;20:665–73.
Hirano M, Ott BR, Raps EC, Minetti C, Lennihan L, Libbey NP, et al. Acute quadriplegic myopathy: a complication of treatment with steroids, nondepolarizing blocking agents, or both. Neurology. 1992;42:2082–7.
Ramsay DA, Zochodne DW, Robertson DM, Nag S, Ludwin SK. A syndrome of acute severe muscle necrosis in intensive care unit patients. J Neuropathol Exp Neurol. 1993;52:387–98.
Showalter CJ, Engel AG. Acute quadriplegic myopathy: analysis of myosin isoforms and evidence for calpain-mediated proteolysis. Muscle Nerve. 1997;20:316–22.
Sher JH, Shafiq SA, Schutta HS. Acute myopathy with selective lysis of myosin filaments. Neurology. 1979;29:100–6.
Lin MC, Ebihara S, El Dwairi Q, Hussain SN, Yang L, Gottfried SB, et al. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med. 1998;158:1656–63.
Comtois AS, Barreiro E, Huang PL, Marette A, Perrault M, Hussain SN. Lipopolysaccharide-induced diaphragmatic contractile dysfunction and sarcolemmal injury in mice lacking the neuronal nitric oxide synthase. Am J Respir Crit Care Med. 2001;163:977–82.
Ebihara S, Hussain SNA, Danialou G, Cho W, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165:221–8.
Supinski G, Stofan D, Callahan LA, Nethery D, Nosek TM, DiMarco A. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol. 1999;87:783–91.
Supinski G, Nethery D, Nosek TM, Callahan LA, Stofan D, DiMarco A. Endotoxin administration alters the force vs. pCa relationship of skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2000;278:R891–6.
Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54.
Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24:210–7.
Reid MB, Lännergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–84.
Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, et al. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694–9.
Fredriksson K, Hammarqvist F, Strigård K, Hultenby K, Ljungqvist O, Wernerman J, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–50.
von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2010;1:7–8.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [124].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schefold, J.C., Bierbrauer, J. & Weber-Carstens, S. Intensive care unit—acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 1, 147–157 (2010). https://doi.org/10.1007/s13539-010-0010-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-010-0010-6