Abstract
In this study, the influence of adding Yttrium iron garnet (Y3Fe5O12) nanoparticles (NPs) on the microstructure and fluctuation-induced conductivity (FIC) of Cu0.5Tl0.5Ba2Ca2Cu3O10-δ (CuTl-1223) superconductor was studied. Y3Fe5O12 NPs were produced by the co-precipitation technique. By solid state route, (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ composites, with x = 0.00, 0.02, 0.04, 0.06, 0.08, and 0.10 wt. % were prepared. The tetragonal unit cell parameters of (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ composites were found to be invariable with Y3Fe5O12 content. The volume fraction of the host phase was increased with Y3Fe5O12 addition till x = 0.04 wt. %. The different vibrational modes of the samples were identified through Fourier transform infrared spectroscopy (FTIR). The transition from normal to the superconducting state, for the prepared composites, was done through d.c resistivity measurements from room temperature down to zero critical temperature (T0). The Aslamazov–Larkin (AL) model was used to examine fluctuation regions in resistivity-temperature curves. At high temperatures, short wave fluctuation was observed. A cross-over between short wave fluctuation and the mean-field region was spotted at lower temperatures. The mean field region for the examined composites was composed of two-dimensional fluctuations along with one-dimensional fluctuation. The coherence length along the c-axis ζc(0), interlayer coupling (J), and anisotropy parameter (γ) were estimated from the Lawrence–Doniach (LD) model as a function of Y3Fe5O12 content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
From an application point of view, intergrain connections and vortex pinning play a major role in enhancing the superconducting critical parameters (i.e., superconducting transition temperature Tc, critical current density Jc, critical magnetic field, Hc) of high-temperature superconductors (HTSCs). As a consequence, it is vital to enhance intergrain connectivity and develop additional efficient pinning centers in the synthesized HTSCs.
Numerous approaches have been explored to solve this matter, but one of the most straightforward and effective is the insertion of appropriate nanosized structures in bulk HTSCs. This technique, known as chemical pinning, has certain advantages including a less complicated preparation procedure, high thermal stability, and cheap costs. The superconductor may include these nanoscale materials as required, and the pinning effect is much greater as a result. However, finding chemically inert materials that don't react with the superconducting phases is in fact a difficult task.
It was found that the addition of ZnFe2O4 nanoparticles increased the onset critical temperature Tc(0) and critical current density (Jc) of GdBa2Cu3O7 superconductor [1]. Sauzlina et al. [2] reported that the addition of Y2O3 nanoparticles up to 0.7 wt. % improved the superconducting properties of the Bi1.6Pb0.4Sr2CaCu2Oy phase. Improvements in microstructure and superconducting parameters were reported for the CuTl-1223 phase added with Fe2O3 nanoparticles [3]. The inclusion of BaSnO3 nanoparticles in the CuTl-1223 matrix was found to enhance its superconductor properties such as relative volume fraction, Tc(0), and Jc [4]. Tiny contents of Zn0.91Mn0.03Co0.06O (x = 0.02 wt. %) in the CuTl-1223 phase [5] have enhanced the intergrain connectivity by healing microcracks, improved the phase percentage, increased the critical temperature and ac conductivity (σac). Mumtaz et al. [6] found that the addition of Zn nanoparticles led to enhance the infield superconducting properties, improved carrier’s density to the optimal level, increased coherence length along the c-axis (ξc), and suppressed the anisotropy parameter (γ) of CuTl-1223 phase. Imran et al. [7] reported that the crystal structure of the bulk CuTl-1223 superconducting phase did not change with the inclusion of Co3O4 nanoparticles.
Following the review above, the most plausible candidates for use as artificial pinning centers in Cuprate CuTl-1223 superconductor are chemically inert nano-materials with low concentrations. The main aspect of choosing the CuTl-1223 superconductor is its low-cost ambient fabrication conditions, particularly pressure, as well as excellent critical parameter values. The Cu0.5Tl0.5Ba2Ca2Cu3O10-δ phase is among the most favourable phases in Cu0.5Tl0.5Ba2Can-1CunO2n+4-δ (n = 2, 3, etc.) superconducting group, with a comparatively high Jc (> 105 A/cm2), high superconducting transition temperature Tc (Tc ~ 106–132 K), long coherence length along the c-axis (ζc), and low superconducting anisotropy (γ = ζab/ζc) [8]. All the superior characteristics mentioned for the CuTl-1223 phase make it promising for many practical applications, especially for cables, coils, and tape usage.
High-temperature superconductors are used in a variety of highly precise and dependable high-technology applications, including in vivo live body measurements in medicine, terahertz equipment for security systems, quantum bit applications in quantum computers, and bolometers for some types of space research. In addition, high-temperature superconductors have been consistently used in magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), magneto encephalography (MEG), and magnetocardiography (MCG) for analyzing the magnetic activity of various human body regions, such as the brain and heart wave activities, as well as for the early diagnosis of a number of diseases [6, 8].
Y3Fe5O12 is a versatile garnet ferrite with unique electromagnetic properties [9]. Y3Fe5O12 is a ferrimagnetic material with a cubic structure. Each unit cell consists of eight chemical formula units. Chemically Yttrium Iron garnet can be represented as Y3Fe2Fe3O12 where Y3+ ions occupy 24 sites, Fe3+ ions occupy 40 sites, and O2− ions occupy 96 sites [10]. There are three crystallographic lattice sites labelled a, c, and d that are surrounded by O octahedron, dodecahedron, and tetrahedron, respectively. The c sites are occupied by Y3+ ions, and the magnetic Fe3+ ions occupy both the a and d sites, while oxygen ions are distributed at the interstitial sites [11]. The ferrimagnetic behaviour of Y3Fe5O12 is primarily due to the opposite spin orientation of Fe3+ ions at the a and d sites [12].
In this study, further investigations on the Cu0.5Tl0.5Ba2Ca2Cu3O10-δ superconducting phase were done by adding proper contents of Y3Fe5O12 NPs to it. Moreover, the main drawback of the ambient pressure synthesis of the CuTl-1223 phase is the existence of voids and holes in increased density, which affects total superconducting critical parameters. Furthermore, a significant improvement in CuTl-1223 superconducting characteristics can be attained when crucial factors including the size, shape, and orientation of the added Y3Fe5O12 NPs are carefully adjusted. As a consequence, this study is targeting the ability of Y3Fe5O12 NPs to act as intergrain connectivity/pinning center enhancers in the CuTl-1223 matrix. The effect of adding Y3Fe5O12 NPs on the microstructure and fluctuation-induced conductivity (FIC) of Cu0.5Tl0.5Ba2Ca2Cu3O10-δ phase was studied in detail. Numerous important superconducting parameters, such as fluctuation dimensionality, coherence length along c-axis ζc(0), interlayer coupling (J), and anisotropy parameter (γ) were deduced from the excess conductivity data as a function of Y3Fe5O12 NP addition.
2 Materials and Method
2.1 Y3Fe5O12 Preparation
The co-precipitation method was used to synthesize Y3Fe5O12 from YCl3.6H2O and FeCl3.6H2O as starting materials. The powder was of high purity and purchased from Sigma-Aldrich. In 100 ml of pure water, the powders in the stoichiometric ratio of Y: Fe → 3:5 were dissolved. NaOH of molarity 5 M was added to the solution dropwise with magnetic stirring until the pH reached 12. The solution is then heated at 80 °C for 4 h while being continuously stirred. The samples were then washed until the pH reached 7, as measured by a digital pH meter. The samples were then dried for 18 h at 100 °C. Finally, these samples were calcined at 1050 °C for 2 h.
2.2 Y3Fe5O12/CuTl-1223 Preparation
Cu0.5Tl0.5Ba2Ca2Cu3O10-δ superconducting samples were prepared at ambient pressure using a solid-state reaction method. In their nominal compositions, high-grade starting materials of Tl2O3, BaO2, CaO, and CuO were crushed and sifted through a 65 μm sieve. To obtain (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ composites, Y3Fe5O12 nanoparticles (particle size 50–80 nm) with weight percent 0.00, 0.02, 0.04, 0.06, 0.08 and 0.10 wt. % were added to the sifted powder then mixed and crushed to ensure homogeneity. To reduce Tl loss during the heating procedure, the powder was compressed into a disc and then wrapped in a silver sheet. The samples were sintered in a sealed quartz tube at a rate of 2 °C/min to 760 °C, then at a rate of 1 °C/min to 850 °C, and finally held at this temperature for 6 h. At the end of this step, the samples were slowly cooled to room temperature.
2.3 Characterization and Measurements
For Y3Fe5O12 nanoparticles; structural characterization was achieved using x-ray powder diffraction (XRD) technique with XRD-D2 phaser Bruker CuKα radiation (λ = 1.5406 Å) in the range of 10° ≤ 2θ ≤ 80°. Transmission electron microscope (TEM) model JEOL JEM 1400 Plus was used to observe particle size and sample morphology.
(Y3Fe5O12)x/CuTl-1223 (x = 0.02, 0.04, 0.06, 0.08 and 0.10 wt.%) composites were identified using Shimadzu-7000 XRD with CuKα radiation (λ = 1.5418 Å) in 2θ range of 10°–70°. JEOL scanning electron microscope model JSM-IT200 was utilized to study the sample morphology. The device operated at a high voltage of 20 kV, at which low pressure was attained using a rotary pump. The electron source was at a distance of 15 mm from the surface of the sample. The value of magnification at which the pictures were taken is 5000. The different modes of vibration were investigated using Fourier transform infrared spectrometer model SHIMADZU-8400S. The electrical resistivity of the prepared composites was measured using the traditional four-point probe procedure from room temperature down to T0 using a closed cryogenic cooling system (Displex).
3 Results and Discussion
3.1 XRD and TEM of Y3Fe5O12
Figure 1a and b display the XRD pattern and TEM micrograph for Y3Fe5O12 nanoparticles, respectively. XRD pattern has affirmed that all peaks are properly indexed with the cubic structure of Y3Fe5O12 with space group la-3d (standard sheet number ICDD: 43–0507) [13]. The relevant peaks for garnet structure (400), (420), and (422) are sharply reflected. Spherical grains were observed from the TEM micrograph with an average particle size of ~ 50–80 nm, as shown in Fig. 1b.
3.2 XRD and SEM of (Y3Fe5O12)x/CuTl-1223
Figure 2a displays XRD patterns for (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ with x = 0.00, 0.02, 0.04, 0.06, 0.08, and 0.10 wt.%. The (hkl) indices of most diffraction peaks clarify that the prepared samples possess a tetragonal structure with P4/mmm symmetry [14]. The small diffraction peaks marked by asterisk and number sign assign the presence of CuTl-1212 and BaCuO2 phases. No peaks were reflected for Yttrium or iron-based compounds. Thus, the tetragonal structure of the CuTl-1223 phase is not influenced by Y3Fe5O12 addition as these NPs will prefer to enter interstitial places and will not enter the structure of the host phase. The relative volume fraction percentages for CuTl-1223, CuTl-1212, and BaCuO2 phases are determined as follows:
where ƩI is the summation of the diffraction peaks intensities [15]. Table 1 shows the relative volume fraction percentages for (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ with x = 0.00, 0.02, 0.04, 0.06, 0.08 and 0.10 wt.%. The volume fraction percentage of the CuTl-1223 phase increases from 87.9 to 91.4% when the Y3Fe5O12 NPs is adjusted from 0.00 to 0.04 wt. %. Higher Y3Fe5O12 addition contents, x > 0.04 wt. %, cause a delay in the main CuTl-1223 phase percentage and enhance CuTl-1212 and BaCuO2 phases percentages. Similar results were reported for the addition of Al2O3 and MgO NPs to the Bi-2223 phase [16, 17]. The decrease in volume fraction for higher Y3Fe5O12 NP additions can be attributed to many reasons such as the ability of these NPs to delay the phase growth to a certain limit and to the ferrimagnetic nature of Y3Fe5O12 NPs, which will cause Cooper pairs damage. The tetragonal unit cell parameters (a) and (c) were estimated, and their values are shown in Table 1. Nearly invariable tetragonal unit cell parameters were seen after the addition of Y3Fe5O12 NPs to the host CuTl-1223 phase. This suggests that the low concentration of the added NPs is insufficient to cause any change in the unit cell of the host phase. Furthermore, this demonstrates that Y3Fe5O12 NPs will prefer to occupy interstitial spaces between the grains. Similar results were observed for the Bi-2223 phase [18, 19] added with NiFe2O4 and NiO NPs, respectively.
SEM micrographs of (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ composites with x = 0.00 and 0.04 wt.% were used to examine the morphology, as shown in Fig. 2b. Solid-state diffusional phase transformation, which includes nucleation, growth, and impingement, is directly responsible for the arrangements of constituent composites, which include a specific volume fraction of spatially distributed phases with various compositions, grain size, and morphology with random orientation, and their spatial distribution. The CuTl-1223 phase formation was confirmed from the observed plate-like grains. Some irregular-shaped grains were assigned for impurity phases such as CuTl-1212 and BaCuO2. Furthermore, even after high-temperature sintering, there are fine spherical grains that may indicate the presence of Y3Fe5O12 nanoparticles in the CuTl-1223 matrix. Also, the micrograph for x = 0.04 wt.% showed apparent suppression in the intergrain voids and improvement in the intergrain weak links that can be visualized in comparison with the pure sample.
3.3 FTIR of (Y3Fe5O12)x/CuTl-1223
FTIR is used to indicate the molecular bonding process in the sample. Moreover, the various oxygen vibrational phonon modes, which play an important role in superconductivity, may be seen via FTIR spectroscopy. Figure 3 depicts room temperature FTIR spectra for the Pure sample and the inset shows the grouped spectra for (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ with x = 0.00, 0.04, 0.06, 0.08, and 0.10 wt. % in the 400 to 4000 cm−1 range. Apical oxygen atoms and CuO2 planar oxygen atoms of the prepared samples appeared in wavenumber ranges from 400 to 540 cm−1 and from 541 to 600 cm−1, respectively. Peaks in the 670 to 700 cm−1 range are caused by Oδ atoms in the charge reservoir layer. It is clear that the inclusion of Y3Fe5O12 NPs causes a minor shift in the apical modes of CuTl-1223 superconductors, which may be related to the impact of stresses and strains induced in the superconductor matrix on the bond lengths [20]. The existence of a peak at 3778.94 cm−1 and a particular peak at 3485.85 cm−1 corresponds to the stretching vibration of the intermolecular hydrogen bond (O–H), showing that the OH groups are basically stretched [21, 22]. The emergence of a peak at 1617.76 cm−1 confirms the complicated creation of the CuTl-1223 phase, as previously reported [23]. The existence of two consecutive peaks at 1055.26 cm−1 and 1421.38 cm−1 verifies the bond stretching of other metal oxides and carbonates such as CaCo3, and CuO [23]. The findings of FTIR and XRD confirm that no changes in the structure of the host CuTl-1223 superconductor occur following the addition of Y3Fe5O12 NPs. These investigations demonstrate that Y3Fe5O12 nanoparticles do not break down or substitute any lattice site in the unit cell of CuTl-1223, but instead reside at grain boundaries.
3.4 Electrical Resistivity of (Y3Fe5O12)x/CuTl-1223
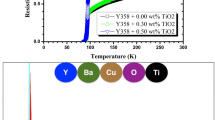
Figure 4 shows the variation of d.c resistivity (ρ) with temperature (T) for (Y3Fe5O12)x/ Cu0.5Tl0.5Ba2Ca2Cu3O10-δ with x = 0.00,0.02, 0.04, 0.06, 0.08 and 0.10 wt.%. All the samples show a linear ρ-T relationship in the normal state region that starts from room temperature down to the onset critical temperature Tc(0), where Tc(0) was determined as the peak point in dρ/dT versus T curves. At Tc(0) there is a transition from normal to superconducting state and the samples completely lose their resistivity (ρ = 0) at a temperature equal to the zero critical temperature (T0). The change in Tc(0) with Y3Fe5O12 NPs for (Y3Fe5O12)x/ Cu0.5Tl0.5Ba2Ca2Cu3O10-δ samples is shown in the inset of Fig. 4. Tc(0) rises initially up to x = 0.04 wt. % then falls for additions greater than 0.04 wt. %. The rise in Tc(0) might be attributed to the higher volume fraction of the host CuTl-1223 phase at low Y3Fe5O12 contents ( x ≤ 0.04 wt. %). Typically, Tc(0) of cuprates is controlled by the density of the carriers in their CuO2 planes [24]. The carriers supplied to CuO2 planes by the charge reservoir layer are determined by the oxygen content of the charge reservoir layer [25]. With the inclusion of these nanoparticles, Tc(0) rises, perhaps owing to optimizing carrier concentration in the CuO2 planes provided by the charge reservoir layer. The decreasing trend in Tc(0) after a certain optimum limit of these NPs addition (x > 0.04 wt. %) may be due to their aggregation and segregation at the grain boundaries in the CuTl-1223 matrix, which can cause the samples’ quality to degrade and the superconducting volume fraction to decrease [26, 27]. Table 2 lists the values of room temperature resistivity ρroom with x. The increase of ρroom with Y3Fe5O12 addition may strongly depend on impurities and crystal defects scattering by the charge carriers in the CuO2 plane.
3.5 Excess Conductivity of (Y3Fe5O12)x/CuTl-1223
The fluctuation-induced conductivity (FIC) behaviour can have a significant impact on the superconducting characteristics [28]. At high temperatures (above Tc(0)), the fluctuation comprises the divergence of the electrical resistivity from the linear metallic normal state behaviour. Superconducting fluctuation, which is formed as a result of Cooper pair production, is thought to initiate the excess conductivity area [29]. FIC may be used to calculate the dimensionality of the superconducting fluctuation and the coherence length [30]. Because of the short ζc(0), a strong fluctuation may be seen in the cuprates. Because of the rapid rise in conductivity caused by superconducting oscillations, the resistivity curve might deviate slightly above the critical temperature [31]. The superconducting fluctuations may be analyzed using Aslamazov–Larkin (AL) [32, 33] model, Lawrence–Doniach (LD) model, and Maki–Thomson [34,35,36] model. Excess conductivity (\(\Delta\upsigma )\) is determined as follows;
where σm (T) and \({\upsigma }_{\mathrm{n}}\left(\mathrm{T}\right)\) are the measured and normal state conductivities, respectively.
The normal state resistivity ρn (T) is calculated in a temperature range of 170 K ≤ T ≤ 300 K using the following relation [37]
where ρ0 and β are the residual resistivity due to impurity scattering and the temperature resistivity coefficient, respectively. The values of ρ0 and β are listed in Table 2 with x. The residual resistivity ρ0 was found to increase with x which is attributed to the increase in impurity scattering in the CuO2 plane and localization of carriers across these magnetic nanoparticles.
Excess conductivity may be represented using the AL model [32] as follows:
where σroom is the conductivity at 300 K (1/ρroom) and t is the reduced temperature \(\mathbf{t}=\frac{\mathbf{T}-{\mathbf{T}}_{\mathbf{c}}}{{\mathbf{T}}_{\mathbf{c}}}\).
The conductivity exponent α in Eq. (4) is related to the conduction dimensionality D by the following relations:
where cr, 3D, 2D, 1D, and SW denoted the dynamic critical, three-dimensional, two-dimensional, one-dimensional, and short wave fluctuations, respectively. The temperature independent amplitude A in Eq. (4) is given by [38]:
where ξc(0) is the coherence length along the c-axis at T = 0 K, d is the effective layer thickness of 2D systems usually equal to the c-axis length of the unit cell and s is the cross-sectional area of 1D system. The LD model introduces the idea of interlayer coupling, J, which is based on Josephson coupling as a result of \({\xi }_{\mathrm{c}}\left(0\right)\) contact with the superconducting layers. The relation between \({\xi }_{\mathrm{c}}\left(0\right)\) and interlayer coupling, J is given by [39]:
The anisotropy, γ of layered superconducting systems is estimated from [40]
where \({\xi }_{\mathrm{ab}}\left(0\right)\) is the ab coherence length which is generally between 10 Å and 20 Å for HTSC [41].
Plots of ln Δσ/σroom against ln t for (Y3Fe5O12)x/ Cu0.5Tl0.5Ba2Ca2Cu3O10-δ with x = 0.00,0.02, 0.04, and 0.10 wt.% are shown in Fig. 5a–d. The plots show different fluctuation regions. The conductivity exponent values were estimated by fitting the data according to Eq. (4) at each region. Three primary fluctuating regions are observed for 2D, 1D, and SW fluctuations. Table 3 lists the conductivity exponent values (α2D, α1D, and αSW) and crossover temperatures (T2D-1D and T1D-SW) for the observed regions. The region that occurs at high temperatures denotes the termination of the Ginzburg–Landau (GL) approach and the dominance of SW fluctuations [42]. In this temperature range, ln Δσ/σroom decreases rapidly, with an exponent αSW ranging between 2.63 and 3.74, which corresponded extremely well with theoretical expectations. Because there are changes in ρn, Tc and therefore in the oxygen content of the current samples, structural band fluctuations in the Fermi surface may be accountable for SW behaviour [43]. When the temperature is reduced, a shift from SW fluctuations to the mean-field regime (MFR) occurs at T1D- SW. The MFR for various produced composites consists of two separate linear components. The conductivity exponent α1D values range from 1.99 to 1.67 in the first portion at temperatures ranging between T1D-SW and T2D- 1D, indicating the presence of 1D fluctuations and hence the existence of 1D conduction channels. The existence of 1D fluctuation region arises in CuTl-1223 due to the presence of conducting charge stripes [44].
Just below T2D -1D, the values of the conductivity exponent are varying from 0.92 to 0.99, implying the presence of 2D fluctuations. The mobility of the carriers in the conducting CuO2 planes indicates the conductivity in the 2D fluctuating area, due to the layered structure of HTSCs materials [45].
Coherence length along the c-axis at T = 0 K \(\left({\xi }_{\mathrm{c}}\left(0\right)\right)\), interlayer coupling (J) and anisotropy parameter (γ) values are estimated according to Eqs. (6)–(7) and are listed in Table 3 with x. It can be seen that \({\xi }_{\mathrm{c}}\left(0\right)\) decreases as x increases from 0.00 to 0.10 wt. % for (Y3Fe5O12)x/ Cu0.5Tl0.5Ba2Ca2Cu3O10-δ samples. Khurram et al. [46] reported a lower value of \({\xi }_{\mathrm{c}}\left(0\right)\) for pure sample \(({\xi }_{\mathrm{c}}\left(0\right)\)= 10 Å) than our reported value \({\xi }_{c}\left(0\right)=6\)Å. All the values of interlayer coupling J are less than one, indicating a weak coupling between the CuO2-planes. Also, J decreases as the Y3Fe5O12 content increases. At x = 0.10 wt. %, the shortest \({\xi }_{\mathrm{c}}\left(0\right)\) was estimated as 2.45 Å. This sample also has the lowest J value (0.082) as well as the greatest anisotropy (4.07). As a result, Y3Fe5O12 inclusions reduced \({\xi }_{\mathrm{c}}\left(0\right)\), increased the anisotropy of the samples and decreased the interlayer coupling strength.
4 Conclusions
In this study, the advantages and disadvantages of adding Y3Fe5O12 NPs to the microstructure and superconducting properties of the CuTl-1223 superconductor were presented and interpreted. Selected amounts of Y3Fe5O12 NPs were implemented into the CuTl-1223 matrix through solid-state reaction method. From XRD and FTIR results, it was concluded that Y3Fe5O12 was a chemically inert material that did not react with the CuTl-1223 superconducting phase. The optimum content of Y3Fe5O12 (x = 0.04 wt. %) has a significant effect on improving the microstructure and the superconductivity of the CuTl-1223 phase. The microstructure was enhanced by filling gaps created by the inclusion of Y3Fe5O12 nanoparticles at the grain boundaries of the CuTl-1223 matrix. The onset critical temperature Tc(0) increased from 106 K to 119.5 K by raising the concentration of Y3Fe5O12 NPs from 0.00 to 0.04 wt. %. This increase in Tc(0) was attributed to the ability of Y3Fe5O12 NPs to act as an effective chemical pinning center. On the other hand, the retardation in superconductivity and microscopic parameters were drawbacks of adding large concentrations of Y3Fe5O12 NPs (x > 0.04 wt. %) to the CuTl-1223 matrix. The superconducting microscopic parameters determined by FIC analysis showed a reduced \({\xi }_{\mathrm{c}}\left(0\right)\), increased anisotropy and decreased interlayer coupling strength. These disadvantages happened due to the agglomeration and segregation of Y3Fe5O12 NPs at the grain boundaries of the CuTl-1223 matrix.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
R. Awad, A.I. Abou Aly, N.H. Mohammed, S. Isber, H.A. Motaweh, D.E. Bakeer, Journal of alloys and compounds. 610, 614 (2014)
M.A. Suazlina, S.Y. Yusainee, H. Azhan, R. Abd-Shukor, R.M. Mustaqim, Jurnal Teknologi. 69(2), 49 (2014)
N.H. Mohammed, A.I. Abou-Aly, R. Awad, I.H. Ibrahim, M. Roumie, M. Rekaby, J. Low Temp. Phys. 172(3), 234 (2013)
A. Srour, W. Malaeb, M. Rekaby, R. Awad, Phys. Scr. 92(10), 104002 (2017)
M. Rekaby, Appl. Phys. A 126(8), 1 (2020)
M. Mumtaz, L. Ali, S. Azeem, S. Ullah, G. Hussain, M.W. Rabbani, A. Jabbar, K. Nadeem, Journal of Advanced Ceramics. 5(2), 159 (2016)
M. Imran, M.Z. Khan, M. Waqee-Ur-Rehman, A. Ullah, S. Ahmed, K. Nadeem, M. Mumtaz, J. Low Temp. Phys. 200, 152 (2020)
H. Ihara, Y. Sekita, N. Terada, H. Obara, H. Tateai, IEEE Trans. Appl. Supercond. 9(2), 1551 (1999)
W. Zhang, C. Guo, R. Ji, C. Fang, Y. Zeng, Mater. Chem. Phys. 125(3), 646 (2011)
M.A. Gilleo, S. Geller, Physical Review. 110(1), 73 (1958)
M.N. Akhtar, A.B. Sulong, M.A. Khan, M. Ahmad, G. Murtaza, M.R. Raza, R. Raza, M. Saleem, M. Kashif, J. Magn. Magn. Mater. 401, 425 (2016)
R. Kumar, Z. Hossain, R.C. Budhani, J. Appl. Phys. 121(11), 113901 (2017)
S. Ravi, J. Am. Ceram. Soc. 101(11), 5046 (2018)
T. Hopfinger, O.O. Shcherban, P. Galez, R.E. Gladyshevkii, M. Lomello-Tafin, J.L. Jorda, M. Couach, J. Alloy. Compd. 333(1–2), 237–248 (2002)
I. Karaca, S. Celebi, A. Varilci, A.I. Malik, Supercond. Sci. Technol. 16(1), 100 (2002)
A. Ghattas, M. Annabi, M. Zouaoui, F.B. Azzouz, M.B. Salem, Physica C: Superconductivity and its applications. 468(1), 31 (2008)
B. Zhao, X. Wan, W. Song, Y. Sun, J. Du, Physica C 337(1–4), 138 (2000)
W. Kong, R. Abd-Shukor, J. Supercond. Novel Magn. 23(2), 257 (2010)
B.A. Albiss, I.M. Obaidat, M. Gharaibeh, H. Ghamlouche, S.M. Obeidat, Solid State Commun. 150(33–34), 1542 (2010)
A. Jabbar, I. Qasim, K.M. Khan, Z. Ali, K. Nadeem, M. Mumtaz, J. Alloy. Compd. 618, 110 (2015)
M. Arshad, A. Qureshi, K. Masud, N. Qazi, J. Therm. Anal. Calorim. 89(2), 595 (2007)
R. Yanru, L. Hanpeng, L. Mingzhu, T. Qingyun, S. Lihua, L. Zhenjin, M. Xianren, L. Zhenxing, Physica C 156(5), 799 (1988)
R. Kumar, H.S. Singh, Y. Singh, In AIP Conference Proceedings. 1953(1), 030001 (2018)
N.A. Khan, M. Mumtaz, Phys. Rev. B 77(5), 054507 (2008)
M. Mumtaz, N.A. Khan, S. Khan, J. Appl. Phys. 107(10), 103905 (2010)
J. Zhang, F. Liu, G. Cheng, J. Shang, J. Liu, S. Cao, Z. Liu, Phys. Lett. A 201(1), 70 (1995)
P.F. Miceli, J.M. Tarascon, L.H. Greene, P. Barboux, F.J. Rotella, J.D. Jorgensen, Phys. Rev. B 37(10), 5932 (1988)
M.R. Cimberle, C. Ferdeghini, E. Giannini, D. Marre, M. Putti, A. Siri, F. Federici, A. Varlamov, Phys. Rev. B. 55(22), R14745 (1997)
M. Cardona, Physica C 317, 30 (1999)
M.R. Islam, M.H. Pramanik, Phys. Rev. B 55(10), 6621 (1997)
L.G. Aslamazov, A.I. Larkin, In30 Years Of The Landau Institute—Selected Papers vol 23 (1996)
L.G. Asmalazov, A.I. Larkin, Sov. Phys. Solid State. 10, 1104 (1968)
W.E. Lawrence, S. Doniach, Proceedings of the Twelfth International Conference on Low Temperature Physics./Kanda, E.(ed.). Tokyo Keigaku Publishing Co., Ltd. Stanford Univ. Calif. (1971)
R.S. Thompson, Phys. Rev. B 1(1), 327 (1970)
S. Hikami, A.I. Larkin, Mod. Phys. Lett. B 2(05), 693 (1988)
S. Ismail, A.K. Yahya, N.A. Khan, Ceram. Int. 39, S257 (2013)
S.V. Sharma, G. Sinha, T.K. Nath, S. Chakroborty, A.K. Majumdar, Physica C 242(3–4), 351 (1995)
T. Sato, H. Nakane, N. Mori, S. Yoshizawa, Physica C 357, 244 (2001)
A. Ali Yusuf, A.K. Yahya, N.A. Khan, F.M. Salleh, E. Marsom, N. Huda, Physica C Superconductivity. 471(11), 363 (2011)
N.A. Khan, A. Mumtaz, Physica B 405(13), 2772 (2010)
N.A. Khan, N. Hassan, M. Irfan, T. Firdous, Physica B 405(6), 1541 (2010)
A.R. Jurelo, R.M. Costa, P.R. Júnior, F.C. Serbena, J. Supercond. Novel Magn. 23(2), 247 (2010)
M.A. Rafique, S. Aziz, N. Hassan, N.A. Khan, J. Supercond. Novel Magn. 27(11), 2427 (2014)
R. Algarni, E. Hannachi, Y. Slimani, M.A. Almessiere, F.B. Azzouz, Ceram. Int. 47(5), 6675 (2021)
G. Hussain, A. Jabbar, I. Qasim, M. Mumtaz, K. Nadeem, M. Zubair, S.Q. Abbas, A.A. Khurram, J. Appl. Phys. 116(10), 103911 (2014)
A. Khurram, N.A. Khan, J. Electromagn. Anal. Appl. 2, 63 (2010)
Acknowledgements
This work was performed in the Superconductivity and metallic glasses laboratory, Faculty of Science, Alexandria University, Alexandria, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rekaby, M., Awad, R., Mohammed, N.H. et al. Infrared Spectroscopy and Excess Conductivity Analysis for (Y3Fe5O12)x/Cu0.5Tl0.5Ba2Ca2Cu3O10-δ Composites. Braz J Phys 52, 182 (2022). https://doi.org/10.1007/s13538-022-01183-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13538-022-01183-8