Abstract
Purpose
Keratinocytes are epithelial cells in epidermis tightly connected to each other by cell-cell junction. During the process of wound re-epithelization, these epithelial cells undergo significant phenotypic changes that are reminiscent of the EMT process occurring during development and tumor progression. As the wound healing progresses, the reepithelization is initiated by activation and migration of keratinocytes from the edges of the wound. As they migrate toward the wound center, keratinocytes set off from their laminin-rich niche, and are forced to experience the abrupt changes in ECM microenvironment consisting of fibronectin and collagen matrices with locally elevated stiffness along the interface of the blood clot and the granulation tissue layer. In this study, thus, we tried to elucidate the effect of alteration of ECM proteins and substrate stiffness in keratinocytes during wound re-epithelization.
Methods
We expose primary normal human epidermal keratinocytes (NHEK) to the culture microenvironment, rich in fibronectin or collagen with varying stiffness, to investigate the role of the physiochemical stimuli on induction of EMTlike changes in NHEKs.
Results
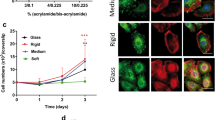
Our results show that a stiffer substrate coated with either fibronectin or collagen induces faster migration with elevated expressions of mesenchymal genes (vimentin, MMP1), and proteins (vimentin, FAK) while suppressing those of the epithelial origin (CK10, CK14, Fillagrin, and Ecadherin) when compared to the laminin coated surfaces.
Conclusions
Various physicochemical stimuli inherently present at the wounds must orchestrate to induce the EMT process in NHEKs, promoting the re-epithelization.
Similar content being viewed by others
References
Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010; 176(5):2247–58.
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008; 68(4):989–97.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009; 139(5):871–90.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009; 9(4):239–52.
Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFbetainduced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008; 314(1):143–52.
Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001; 159(3):1009–20.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007; 127(3):526–37.
O’Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001; 26(6):525–30.
Woodley DT, Okeefe EJ, Prunieras M. Cutaneous woundhealing - a model for cell-matrix interactions. J Am Acad Dermatol. 1985; 12(2 Pt2):420–33.
Woodley DT, Bachmann PM, Okeefe EJ. Laminin inhibits human keratinocyte migration. J Cell Physiol. 1988; 136(1): 140–6.
O’Toole EA, Marinkovich MP, Hoeffler WK, Furthmayr H, Woodley DT. Laminin-5 inhibits human keratinocyte migration. Exp Cell Res. 1997; 233(2):330–9.
Clark RA, Lanigan JM, Dellapelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal-cell migration during wound reepithelialization. J Invest Dermatol. 1982; 79(5):264–269.
Markowski MC, Brown AC, Barker TH. Directing epithelial to mesenchymal transition through engineered microenvironments displaying orthogonal adhesive and mechanical cues. J Biomed Mater Res A. 2012; 100(8):2119–27.
Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008; 30(5):599–606.
Hendriks FM, Brokken D, van Eemeren JT, Oomens CW, Baaijens FP, Horsten JB. A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Res Technol. 2003; 9(3):274–83.
Diridollou S, Vabre V, Berson M, Vaillant L, Black D, Lagarde JM, Gregoire JM, Gall Y, Patat F. Skin ageing: changes of physical properties of human skin in vivo. Int J Cosmet Sci. 2001; 23(6):353–62.
Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005; 13(5):468–79.
Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990; 97(Pt 1):39–50.
Ivaska J. Vimentin: central hub in EMT induction? Small GTPases. 2011; 2(1):51–3.
Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell. 2012; 23(6):1068–79.
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005; 6(1):56–68.
Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010; 123(Pt 7):1007–13.
Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000; 2(12):E231–6.
Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001; 20(44):6459–72.
Kim JH, Serra-Picamal X, Tambe DT, Zhou EH, Park CY, Sadati M, Park JA, Krishnan R, Gweon B, Millet E, Butler JP, Trepat X, Fredberg JJ. Propulsion and navigation within the advancing monolayer sheet. Nat Mater. 2013; 12(9):856–63.
Boyer B. The Ras and Src signaling cascades involved in epithelial cell scattering. In: Savagner P, editor. Rise and fall of epithelial phenotype: concepts of epithelial-mesenchymal transition (molecular biology intelligence unit). New York: Springer; 2005. pp. 245–54.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006; 126(4):677–89.
Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005; 310(5751): 1139–43.
Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motility Cytoskeleton. 2005; 60(1):24–34.
Elosegui-Artola A, Bazellieres E, Allen MD, Andreu I, Oria R, Sunyer R, Gomm JJ, Marshall JF, Jones JL, Trepat X, Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat Mater. 2014; 13(6):631–7.
Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through alpha(v)beta(3) integrins and RPTP alpha. Biophys J. 2006; 90(5):1804–9.
Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010; 19(2):194–206.
Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011; 1:30–47.
Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006; 119(Pt 19):3901–3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Two authors contributed equally.
Rights and permissions
About this article
Cite this article
Kim, M., Gweon, B., Koh, U. et al. Matrix stiffness induces epithelial mesenchymal transition phenotypes of human epidermal keratinocytes on collagen coated two dimensional cell culture. Biomed. Eng. Lett. 5, 194–202 (2015). https://doi.org/10.1007/s13534-015-0202-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-015-0202-2