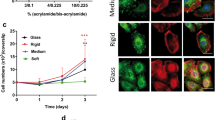
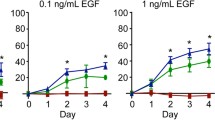
Abstract
Restoration of epidermal organization and function in response to a variety of pathophysiological insults is critically dependent on coordinated keratinocyte migration, proliferation, and stratification during the process of wound healing. These processes are mediated by the reconfiguration of both cell–cell (desmosomes, adherens junctions) and cell–matrix (focal adhesions, hemidesmosomes) junctions and the cytoskeletal filament networks that they serve to interconnect. In this study, we investigated the role of substrate elasticity (stiffness) on keratinocyte colony formation in vitro during the process of nascent epithelial sheet formation as triggered by the calcium switch model of keratinocyte culture. Keratinocytes cultured on pepsin digested type I collagen coated soft (nominal E = 1.2 kPa) polyacrylamide gels embedded with fluorescent microspheres exhibited (i) smaller spread contact areas, (ii) increased migration velocities, and (iii) increased rates of colony formation with more cells per colony than did keratinocytes cultured on stiff (nominal E = 24 kPa) polyacrylamide gels. As assessed by tracking of embedded microsphere displacements, keratinocytes cultured on soft substrates generated large local substrate deformations that appeared to recruit adjacent keratinocytes into joining an evolving colony. Together with the observed differences in keratinocyte kinematics and substrate deformations, we developed two ad hoc analyses, termed distance rank and radius of cooperativity, that help to objectively ascribe what we perceive as increasingly cooperative behavior of keratinocytes cultured on soft vs. stiff gels during the process of colony formation. We hypothesize that the differences in keratinocyte colony formation observed in our experiments could be due to cell–cell mechanical signaling generated via local substrate deformations that appear to be correlated with the increased expression of β4 integrin within keratinocytes positioned along the periphery of an evolving cell colony.










Similar content being viewed by others
References
Achterberg, V. F., L. Buscemi, H. Diekmann, J. Smith-Clerc, H. Schwengler, J. J. Meister, et al. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134(7):1862–1872, 2014.
Anon, E., X. Serra-Picamal, P. Hersen, N. C. Gauthier, M. P. Sheetz, X. Trepat, et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl. Acad. Sci. USA 109(27):10891–10896, 2012.
Aratyn-Schaus, Y., P. W. Oakes, J. Stricker, S. P. Winter, and M. L. Gardel. Preparation of complaint matrices for quantifying cellular contraction. J. Visualized Exp. 46:2170, 2010
Boudou, T., J. Ohayon, C. Picart, R. I. Pettigrew, and P. Tracqui. Nonlinear elastic properties of polyacrylamide gels: implications for quantification of cellular forces. Biorheology 46(3):191–205, 2009.
Butler, J. P., I. M. Tolic-Norrelykke, B. Fabry, and J. J. Fredberg. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282(3):C595–C605, 2002.
Chan, S. H., D. T. Võ, and T. Q. Nguyen, (ed.). Subpixel Motion Estimation Without Interpolation. IEEE International Conference on Acoustics Speech and Signal Processing (ICASSP), IEEE, 2010.
Cras, J., C. Rowe-Taitt, D. Nivens, and F. Ligler. Comparison of chemical cleaning methods of glass in preparation for silanization. Biosensors Bioelectron. 14(8):683–688, 1999.
Doyle, A. D., F. W. Wang, K. Matsumoto, and K. M. Yamada. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184(4):481–490, 2009.
Eming, S. A. Biology of Wound Healing. In: Dermatology, edited by J. L. Bolognia, J. L. Jorizzo, and J. V. Schaffer. Philadephia: Elsevier Saunders, 2012.
Evans, N. D., R. O. Oreffo, E. Healy, P. J. Thurner, and Y. H. Man. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 28:397–409, 2013.
Goffin, J. M., P. Pittet, G. Csucs, J. W. Lussi, J. J. Meister, and B. Hinz. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell. Biol. 172(2):259–268, 2006.
Grzesiak, J. J., and M. D. Pierschbacher. Changes in the concentrations of extracellular Mg++ and Ca++ down-regulate E-cadherin and up-regulate alpha 2 beta 1 integrin function, activating keratinocyte migration on type I collagen. J. Invest. Dermatol. 104(5):768–774, 1995.
Guo, W. H., M. T. Frey, N. A. Burnham, and Y. L. Wang. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 90(6):2213–2220, 2006.
Hamill, K. J., S. B. Hopkinson, P. DeBiase, and J. C. Jones. BPAG1e maintains keratinocyte polarity through beta4 integrin-mediated modulation of Rac1 and cofilin activities. Mol. Biol Cell 20(12):2954–2962, 2009.
Hartwig, B., B. Borm, H. Schneider, M. J. Arin, G. Kirfel, and V. Herzog. Laminin-5-deficient human keratinocytes: defective adhesion results in a saltatory and inefficient mode of migration. Exp. Cell Res. 313(8):1575–1587, 2007.
Hunyadi, J., B. Farkas, C. Bertenyi, J. Olah, and A. Dobozy. Keratinocyte grafting: a new means of transplantation for full-thickness wounds. J. Dermatol. Surg. Oncol. 14(1):75–78, 1988.
Kim, J. H., X. Serra-Picamal, D. T. Tambe, E. H. Zhou, C. Y. Park, M. Sadati, et al. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 12(9):856–863, 2013.
Kirsner, R. S., W. A. Marston, R. J. Snyder, T. D. Lee, D. I. Cargill, and H. B. Slade. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 380(9846):977–985, 2012.
Lange, T. S., A. K. Bielinsky, K. Kirchberg, I. Bank, K. Herrmann, T. Krieg, et al. Mg2+ and Ca2+ differentially regulate beta 1 integrin-mediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins. Exp. Cell Res. 214(1):381–388, 1994.
Lange, T. S., J. Kirchberg, A. K. Bielinsky, A. Leuker, I. Bank, T. Ruzicka, et al. Divalent cations (Mg2+, Ca2+) differentially influence the beta 1 integrin-mediated migration of human fibroblasts and keratinocytes to different extracellular matrix proteins. Exp. Dermatol. 4(3):130–137, 1995.
Leigh, I. M., and F. M. Watt. The Culture of Human Epidermal Keratinocytes. The Keratinocyte Handbook. Cambridge: Cambridge University Press, pp. 43–51, 1994.
Martin, P. Wound healing-aiming for perfect skin regeneration. Science 276(5309):75–81, 1997.
Mertz, A. F., Y. Che, S. Banerjee, J. M. Goldstein, K. A. Rosowski, S. F. Revilla, et al. Cadherin-based intercellular adhesions organize epithelial cell–matrix traction forces. Proc. Natl. Acad. Sci. USA 110(3):842–847, 2013.
NiAnnaidh, A., K. Bruyere, M. Destrade, M. D. Gilchrist, and M. Ottenio. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 5(1):139–148, 2012.
Pelham, Jr, R. J., and Y. Wang. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94(25):13661–13665, 1997.
Peyton, S. R., and A. J. Putnam. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 204(1):198–209, 2005.
Raghupathy, R., C. Witzenburg, S. P. Lake, E. A. Sander, and V. H. Barocas. Identification of regional mechanical anisotropy in soft tissue analogs. J. Biomech. Eng. 133(9):091011, 2011.
Reinhart-King, C. A., M. Dembo, and D. A. Hammer. Cell–cell mechanical communication through compliant substrates. Biophys. J. 95(12):6044–6051, 2008.
Rudnicki, M. S., H. A. Cirka, M. Aghvami, E. A. Sander, Q. Wen, and K. L. Billiar. Nonlinear strain stiffening is not sufficient to explain how far cells can feel on fibrous protein gels. Biophys. J. 105(1):11–20, 2013.
Sehgal, B. U., P. J. DeBiase, S. Matzno, T. L. Chew, J. N. Claiborne, S. B. Hopkinson, et al. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 281(46):35487–35498, 2006.
Selby, J. C. Mechanobiology of Epidermal Keratinocytes: Desmosomes, Hemidesmosomes, Keratin Intermediate Filaments, and Blistering Skin Diseases. Mechanobiology of Cell–Cell and Cell–Matrix Interactions. Berlin: Springer, pp. 169–210, 2011.
Selby, J. C., and M. A. Shannon. Mechanical response of a living human epidermal keratinocyte sheet as measured in a composite diaphragm inflation experiment. Biorheology 44(5–6):319–348, 2007.
Tang, X., A. Tofangchi, S. V. Anand, and T. A. Saif. A novel cell traction force microscopy to study multi-cellular system. PLoS Comput. Biol. 10(6):e1003631, 2014.
Toyjanova, J., E. Bar-Kochba, C. Lopez-Fagundo, J. Reichner, D. Hoffman-Kim, and C. Franck. High resolution, large deformation 3D traction force microscopy. PloS ONE 9(4):e90976, 2014.
Trappmann, B., J. E. Gautrot, J. T. Connelly, D. G. Strange, Y. Li, M. L. Oyen, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11(7):642–649, 2012.
Trepat, X., M. R. Wasserman, T. E. Angelini, E. Millet, D. A. Weitz, J. P. Butler, et al. Physical forces during collective cell migration. Nat. Phys. 5(6):426–430, 2009.
Tsuruta, D., T. Hashimoto, K. J. Hamill, and J. C. Jones. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J. Dermatol. Sci. 62(1):1–7, 2011.
Wang, Y., G. Wang, X. Luo, J. Qiu, and C. Tang. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns 38(3):414–420, 2012.
Watt, F. M. Influence of cell shape and adhesiveness on stratification and terminal differentiation of human keratinocytes in culture. J. Sci. Suppl. 8:313–326, 1987.
Watt, F. M., P. W. Jordan, and C. H. O’Neill. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA 85(15):5576–5580, 1988.
Wen, J. H., L. G. Vincent, A. Fuhrmann, Y. S. Choi, K. C. Hribar, H. Taylor-Weiner, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13(10):979–987, 2014.
Winer, J. P., S. Oake, and P. A. Janmey. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PloS ONE 4(7):e6382, 2009.
Zamansky, G. B., U. Nguyen, and I. N. Chou. An immunofluorescence study of the calcium-induced coordinated reorganization of microfilaments, keratin intermediate filaments, and microtubules in cultured human epidermal keratinocytes. J. Invest. Dermatol. 97(6):985–994, 1991.
Acknowledgements
Support for this project was provided by the National Institutes of Health (R03-AR063967) and the Roy J. Carver Charitable Trust (#14-4384). We thank Kelly Messingham for assistance with immunolabeling, and Janet Fairley, George Giudice, and Thomas Magin for insightful discussions on this work.
Conflict of interest
Hoda Zarkoob, Sandeep Bodduluri, Sailahari V. Ponnaluri, John C. Selby, and Edward A. Sander declare that they have no conflict of interest.
Ethical Standards
No human or animal studies or were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Roger D. Kamm oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 35373 kb)
Supplementary material 2 (MP4 34992 kb)
Supplementary material 3 (MP4 36924 kb)
Supplementary material 4 (MP4 37305 kb)
Supplementary material 5 (MP4 29938 kb)
Supplementary material 6 (MP4 29142 kb)
Supplementary material 7 (MP4 78733 kb)
Rights and permissions
About this article
Cite this article
Zarkoob, H., Bodduluri, S., Ponnaluri, S.V. et al. Substrate Stiffness Affects Human Keratinocyte Colony Formation. Cel. Mol. Bioeng. 8, 32–50 (2015). https://doi.org/10.1007/s12195-015-0377-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0377-8