Abstract
The impacts of climate change on cyanobacterial harmful algal blooms (cHABs) are paramount, promoting the widespread distribution, intensity, and toxicity of these phenomena in major freshwater bodies across the globe. Microcystins (MCs) and nodularins (NODs) are monocyclic peptides that produce hepatotoxic effects in living organisms. Despite efforts in understanding their molecular toxicological mechanisms, we do not fully have a grasp on the human health impacts associated with these toxins derived from freshwater cHABs. We seek to provide a current update on the toxicity and epidemiology of MCs and NODs, integrating key evidence from in vitro, in vivo, and epidemiological studies. The primary objective of this work is to understand the human health impacts of MC and NOD-producing cHABs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climatic patterns and anthropogenic activities promote eutrophication in freshwater bodies, leading to rapid multiplication of photosynthetic cyanobacteria termed cyanobacterial harmful algal blooms (cHABs). Current trends in atmospheric and water temperatures are expected to increase the incidence and expand the biogeography of toxic cyanobacteria worldwide [1, 2]. In addition, cHAB formation in freshwater environments is stimulated by various abiotic factors such as light intensity, nutrient levels (nitrogen and phosphorus), pH, temperature, pollutants, and short-wavelength radiations [3,4,5]. Globally, cHABs are increasing in frequency, duration, and severity, posing significant health hazards to wildlife, recreation, and public health [1, 6]. Several major freshwater lakes have been impacted by cHABs including Lake Erie, USA; Lake Winnipeg, Canada; Lake Victoria Kenya; and Lake Taihu, China [7]. The dominant and toxin-producing cyanobacteria in lakes are species belonging to the genus Microcystis [8].
Microcystins (MCs) are considered the most abundant and toxic cyanobacterial toxins (cyanotoxins). MC concentrations in surface waters are variable, though elevated levels can impair water quality used for recreation and consumption. MC-LR, the most potent form of MC, is regarded as one potential carcinogen to humans [9]. MC-LR toxicity depends upon active transport into hepatic tissue via organic anion transporting polypeptides (OATPs). The World Health Organization (WHO) has adopted provisional guidelines for MC-LR in drinking water (1.0 µg/L) and recreational water (12 µg/L) [10, 11]. Nodularins (NODs) constitute a similar group of cyanotoxins, where NOD-R is the most frequently detected variant [12, 13]. NODs predominately exist in brackish waters; however, they can appear in conjunction with MCs in freshwater. The presence of the extremely toxic ADDA (3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-4,6-decadienoic acid) moiety in their cyclic structures makes them an excellent target for quantitation [12]. Since toxicological data for NODs remain sparse, the level of exposure and toxicity are based on MCs (0.04 µg/kg body weight/d) [14].
Other notable cyanotoxins produced by cyanobacteria include hepatotoxic cylindrospermopsins, neurotoxic anatoxins and saxitoxins, and the non-proteinogenic amino acid, β-N-methylamino-L-alanine (BMAA) [15]. A comprehensive database called “CyanoMetDB” offers detailed information on their biosynthesis, identification, occurrence, and toxicological risks [16].
Direct or indirect exposure to cyanotoxins inflicts harm on aquatic organisms, wild and domestic animals, plants, and humans. With respect to animal and human intoxications, direct exposure typically occurs from consuming toxin-producing cyanobacterial cells or ingesting contaminated drinking water harboring cyanotoxins [17].
Epidemiological studies in China and Serbia established a potential association between MC-contaminated drinking water and primary liver cancer [18, 19]. Two other studies linked elevated levels of alanine and aspartate transaminase in sera of fishermen and children to liver damage, perhaps from chronic exposure to contaminated drinking water and aquatic foods [20, 21]. More recently, a case–control study in China discovered an elevated risk of chronic kidney disease among cases jointly exposed to high levels of MC and cadmium, suggesting the likelihood of synergistic effect of environmental pollutants in drinking water [22].
Based on a literature review on cyanotoxin poisonings, recreational activities contribute to nearly 50% of all human intoxications worldwide [23]. Recreational exposures to cyanobacteria and their associated cyanotoxins (i.e., MCs) include contact with bloom-infested water, inhalation of aerosolized sprays, and accidental ingestion of contaminated water. Past surveys of participants engaging in water recreation reported a wide range of health effects including allergic reactions, headache, fever, gastroenteritis, hay fever-like symptoms, mouth sores, and pyritic skin rashes [24,25,26]. On the other hand, indirect exposure can occur when organisms of higher trophic levels consume contaminated animal or plant tissue. This is particularly concerning as concentrated toxin in tissue can bioaccumulate in the food chain and subsequently affect human health [23].
This mini-review seeks to enhance our current understanding of the toxicological and health risks associated MC and NOD toxins derived from freshwater cHABs.
Cyanotoxins
Cyanotoxins are potent secondary metabolites produced by cyanobacteria, with over 2000 identified to date [16]. Structurally, cyanotoxins are categorized as cyclic peptides (microcystins and nodularins), alkaloids (anatoxins, cylindrospermopsins, and lyngbyatoxins), and the non-proteinogenic amino acid, β-N-methylamino-L-alanine (BMAA) [27]. From a toxicological perspective, cyanotoxins are grouped by the organs they affect in living organisms, which include hepatotoxins, neurotoxins, dermatotoxins, irritant toxins, and cytotoxins [28]. These cyanotoxins vary in structure, mechanism, and toxicity (Table 1). For the purposes of this review, special attention is paid to the hepatotoxic microcystins and nodularins.
Microcystin toxicity
Microcystins (MCs) are monocyclic heptapeptides (cyclo-(D-Ala-L-X-D-MeAsp-L-Z-Adda-D-Glu-Mdha)) produced by cyanobacteria in freshwater, estuarine, and marine environments [43]. Various genera synthesize MCs in eutrophic waters including Aphanizomenon, Dolichospermum (formerly planktic Anabaena), Microcystis, Nostoc, Oscillatoria, and Planktothrix [44]. The Adda moiety (3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-4,6-decadienoic acid) is critical to hepatotoxicity induced by MCs. More than 300 variants have been identified, with many others yet to be explored. These variants mainly differ in two variable amino acid positions (2 and 4), although some exhibit modifications or substitutions at other positions [45]. Most of these occurrences involve replacement of a methoxy group with an acetyloxy or hydroxy group at C-9, resulting in 9-O-acetylDMAdda (ADMadda) and 9-O-desmethylAdda (DMAdda), respectively. Microcystin-LR (MC-LR), microcystin-RR (MC-RR), and microcystin-YR (MC-YR) are highly potent and well-studied toxins in freshwater bodies [39]. Of these three variants, MC-LR is arguably the most ubiquitous, analyzed, and toxic in surface waters. The WHO’s provisional guidance value of MC-LR in drinking water is 1 µg/mL, equivalent to a tolerable daily intake (TDI) of 0.04 µg/kg body mass [10]. MC-LR is displayed in Fig. 1, where leucine (L) and arginine (R) occupy C-2 and C-4 in the heterocyclic ring, respectively.
The primary route of MC exposure is oral consumption, and organic anion transporting peptides (OATPs) mediate its uptake into hepatocytes [46]. OATPs are differentially expressed in several essential organs including the brain, kidney, small and large intestines, and stomach [46, 47]. These membrane-bound transporters facilitate the uptake of endogenous compounds such as bile salts, hormones, toxins, drugs, and xenobiotics [48]. Differential expression of hepatic OATP 1B1 and 1B3 isoforms facilitates cellular uptake of MCs, which may explain varying degrees in uptake and toxicity of MC variants [49].
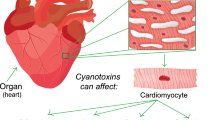
MCs inhibit protein phosphatases (PP) 1 and 2A in tissue via covalent binding to serine and threonine amino acids. Hepatotoxic MCs exert a stronger attraction towards PP1 and PP2A compared to PP2B [50]. A two-step mechanism has been proposed for interaction between PP1 and PP2B and MC-LR: (1) rapid binding and inactivation of PP-1c and PP-2Ac catalytic subunits and (2) formation of adducts through prolonged covalent interactions [51]. Since PP1 and PP2 regulate protein dephosphorylation, their inhibition can induce hyperphosphorylation of intracellular proteins, culminating in hepatocyte disintegration, internal hemorrhage, and hepatic necrosis or apoptosis [49]. PP1 and PP2 inactivation can also lead to cytoskeleton disruption, DNA damage, oxidative stress, and mitogen-activated protein kinase (MAPK) deregulation [52]. MC toxicity extends beyond the liver and has been implicated in organs such as the kidneys, lungs, and heart [40]. Experimental studies on MC toxicity in these organs are detailed in Table 2.
Microcystin epidemiology
Sporadic epidemiological investigations on MC exposure have been conducted across various continents including Asia, North America, and South America (Table 3).
Hemodialysis
Arguably the most renown outbreak of MC poisoning occurred in a hemodialysis center in Caruaru, Brazil, where 101 case patients received MC-contaminated dialysate, 50 whom had acute liver failure and succumbed after exposure. Affected patients who died were older than survived patients (median age, 47 vs. 35 years, p < 0.001). Toxicological analysis of MCs in liver tissue of 17 case patients revealed MC concentrations ranging from 0.03 to 0.60 g/kg [60].
Drinking water
Additionally, multiple studies in China previously associated MC exposure with primary liver cancer [18], colorectal cancer [61], and liver damage [20, 21]. A three-trial survey in Haimen city correlated MCs in drinking water sources to primary liver cancer incidence. A similar survey in Fusui, Guangxi Province, reported a high occurrence of MC contamination in water samples collected from ponds/ditches and rivers [18]. In the case of colorectal cancer, the relative risk (RR) was significantly higher among those who consumed pond (RR = 7.70) and river water (RR = 7.94). MC concentrations in these drinking water sources also positively correlated with colorectal cancer incidence (ρ = 0.881, p < 0.01). Overall, consistent findings were observed between studies, with higher exposures of MCs in drinking water sourced from ponds and rivers.
Another study examined chronic exposure to MCs in a population of fishermen who lived on fishing ships and consumed drinking water from Lake Chaohu. The presence of MCs in fishermen sera (average 0.39 ng/mL), accompanied by elevated serum enzymes (alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST), indicated the possibility of liver damage [20]. Similarly, a cross-sectional study in the Three Gorges Region investigated chronic exposure to MCs through drinking water among high and low exposed children in relation to liver damage. High exposed children had elevated levels of ALP and AST compared to low exposed children when analyses excluded subjects who used hepatotoxic medications or were positive for hepatitis B infection [21].
A more recent case–control study attempted to understand the combined effect of MC and cadmium (Cd) in drinking water with chronic kidney disease (CKD). For combined high exposures of MC and Cd, the odds of developing CKD 2.58 times greater than the reference group (low MC and low Cd) [22]. Findings of the study demonstrated that the combined effect between environmental pollutants in drinking water can significantly increase the risk of chronic disease.
Recreation
Recreational exposure to MCs during water activities has also received attention in epidemiology. In a small lake enduring an algal bloom, exposed participants reported more respiratory symptoms (i.e., cough and sore) 7 days before partaking in recreational activities than 7–10 days after partaking in recreational activities. Unexposed participants complained of dermatologic complaints immediately prior to engaging in recreational activities compared to after engaging in recreational activities. However, MC levels in aerosol (< 0.1 ng/m3), water (2–5 µg/L), and blood samples of participants (< 0.147 µg/L) were relatively low. The results indicated that low-level exposure to MC aerosols might occur during recreational activities [25]. In a different field study, exposed participants reported more upper respiratory symptoms 7 days before partaking in water recreational activities [26]. MC concentrations in two ‘Bloom Lakes’ varied from as low as < 10 µg/L to as high as > 500 µg/L, while nasal swabs (< 0.1–5 ng/m3) and blood samples (< 1.0 µg/L) contained low levels of toxin. These findings coincided with the earlier study, supporting inhalation as one potential exposure route for MCs during recreational activities.
Nodularin toxicity and epidemiology
Nodularins (NODs) are cyclic pentapeptides produced by planktonic, filamentous Nodularia spumigena and benthic Nodularia sphaerocarpa in brackish waters, and less commonly, in freshwater [42]. NOD-producing blooms have occurred in many parts of the world including the Baltic Sea, Northern Europe, Australia, and the USA [40]. Like those in MCs, NODs contain the amino acid residues D-erythro-β-methylaspartic acid, L-arginine, Adda, D-glutamic acid, and N-methyldehydrobutyrine [42]. Because of their shared chemical properties, NODs and MCs are usually co-studied by the same analytical method. Currently, ten variants of NODs are recognized, with NOD-R (arginine) being the most common (Fig. 2) [63]. The presence of arginine (R) as opposed to valine (V) at C-2 differentiates NOD-R from mutoporin, a hepatotoxin derived from the marine sponge Theonella swinhoei [64].
Few studies on NOD toxicokinetics are currently available. However, cellular uptake and biological activity of NOD are similar to MC. Uptake transporters (OATPs), Oatp1d1 and Oatp2b1, are mainly expressed in the liver of zebrafish. A study demonstrated that Oatp1d1 (drOatp1d1) mediates uptake of NOD in the permanent zebrafish cell line ZFL. As for PP inhibition, NOD non-covalently binds to PP2A, which can ultimately enhance the production of tumor necrosis factor α (TNF-α), as evidenced by a study in primary rat hepatocytes [65, 66]. Additionally, exposure to 2.5 nM NOD for 24 h was shown to induce TNF-α in human primary liver. Consequently, molecular induction of TNF-α stimulates the expression of interleukin-8 (IL-8) and activation of MAPK, thereby contributing to the toxicity and tumor-promoting activities of NOD in hepatocytes [67]. Because of the lack of exposure data, the International Agency for Research on Cancer (IARC) classifies NOD as non-carcinogenic to humans [13].
NODs are regarded as important hepatotoxins to human health. However, no epidemiological studies have explicitly investigated the relationship between NOD exposure and health outcomes at the population level. Health effects of NODs are generally inferred from limited epidemiological studies on MCs (Table 3). NOD exposure may therefore cause a variety of signs and symptoms including allergic reactions, skin rashes, gastrointestinal illness, nausea, liver damage, and bleeding [68]. Future epidemiological studies could start by simultaneously assessing the co-exposure of MC and NOD in freshwater environments and examining their relationship with liver disease.
Conclusion
The findings of this review emphasize the health impacts of microcystin (MC) and nodularin (NOD) toxins derived from freshwater cyanobacterial harmful algal blooms (cHABs). It is imperative to gain a deeper understanding of the pathways through which these cyanotoxins are encountered in the aquatic environment, as this knowledge is essential for mitigating toxic exposures. Moreover, it can pave the way for the implementation of regulatory guidelines to ensure appropriate levels of exposures and toxicities to humans. Given recent epidemiological findings, the synergistic effect of MC and NOD with other environmental pollutants on chronic health conditions merits further exploration. In short, there is a pressing need to conduct toxicological experiments, exposure assessments, and epidemiological investigations to appreciate the human health impacts of chronic MC and NOD exposures.
References
Wells ML, Karlson B, Wulff A, Kudela R, Trick C, Asnaghi V et al (2020) Future HAB science: directions and challenges in a changing climate. Harmful Algae 91:101632. https://doi.org/10.1016/j.hal.2019.101632
Wells ML, Trainer VL, Smayda TJ, Karlson BS, Trick CG, Kudela RM et al (2015) Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae 49:68–93. https://doi.org/10.1016/j.hal.2015.07.009
Neilan BA, Pearson LA, Muenchhoff J, Moffitt MC, Dittmann E (2013) Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ Microbiol 15(5):1239–1253. https://doi.org/10.1111/j.1462-2920.2012.02729.x
Hader DP, Villafane VE, Helbling EW (2014) Productivity of aquatic primary producers under global climate change. Photochem Photobiol Sci 13(10):1370–1392. https://doi.org/10.1039/c3pp50418b
Rastogi RP, Sinha RP, Moh SH, Lee TK, Kottuparambil S, Kim YJ et al (2014) Ultraviolet radiation and cyanobacteria. J Photochem Photobiol B 141:154–169. https://doi.org/10.1016/j.jphotobiol.2014.09.020
Glibert PM (2020) Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 91:101583. https://doi.org/10.1016/j.hal.2019.03.001
Davis TW, Gobler CJ (2016) Preface for special issue on “Global expansion of harmful cyanobacterial blooms: diversity, ecology, causes, and controls.” Harmful Algae 54:1–3
Lad A, Breidenbach JD, Su RC, Murray J, Kuang R, Mascarenhas A et al (2022) As we drink and breathe: adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life (Basel) 12(3):418. https://doi.org/10.3390/life12030418
Lone Y, Koiri RK, Bhide M (2015) An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol Rep 2:289–296. https://doi.org/10.1016/j.toxrep.2015.01.008
Chorus IBJ (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. World Health Organization, London
Farrer D, Counter M, Hillwig R, Cude C (2015) Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins (Basel) 7(2):457–477. https://doi.org/10.3390/toxins7020457
Chen Y, Shen D, Fang D (2013) Nodularins in poisoning. Clin Chim Acta 425:18–29. https://doi.org/10.1016/j.cca.2013.07.005
Frontiers | Detection and Occurrence of Microcystins and Nodularins in Lake Manatee and Lake Washington-Two Floridian Drinking Water Systems (frontiersin.org)
Chen GWL, Wang M, Hu T (2021) Comprehensive insights into the occurrence and toxicological issues of nodularins. Mar Pollut Bull 162:111884
Metcalf JS, Banack SA, Wessel RA, Lester M, Pim JG, Cassani JR et al (2021) Toxin analysis of freshwater cyanobacterial and marine harmful algal blooms on the West Coast of Florida and implications for estuarine environments. Neurotox Res 39(1):27–35. https://doi.org/10.1007/s12640-020-00248-3
Jones MR, Pinto E, Torres MA, Dorr F, Mazur-Marzec H, Szubert K et al (2021) CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res 196:117017. https://doi.org/10.1016/j.watres.2021.117017
Rastogi RP, Madamwar D, Incharoensakdi A (2015) Bloom dynamics of cyanobacteria and their toxins: environmental health impacts and mitigation strategies. Front Microbiol 6:1254. https://doi.org/10.3389/fmicb.2015.01254
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD et al (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17(6):1317–1321. https://doi.org/10.1093/carcin/17.6.1317
Svircev Z, Krstic S, Miladinov-Mikov M, Baltic V, Vidovic M (2009) Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27(1):36–55. https://doi.org/10.1080/10590500802668016
Chen J, Xie P, Li L, Xu J (2009) First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol Sci 108(1):81–89. https://doi.org/10.1093/toxsci/kfp009
Li Y, Chen JA, Zhao Q, Pu C, Qiu Z, Zhang R et al (2011) A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region. China Environ Health Perspect 119(10):1483–1488. https://doi.org/10.1289/ehp.1002412
Feng S, Deng S, Tang Y, Liu Y, Yang Y, Xu S et al (2022) Microcystin-LR combined with cadmium exposures and the risk of chronic kidney disease: a case-control study in central China. Environ Sci Technol 56(22):15818–15827. https://doi.org/10.1021/acs.est.2c02287
Wood R (2016) Acute animal and human poisonings from cyanotoxin exposure—a review of the literature. Environ Int 91:276–282. https://doi.org/10.1016/j.envint.2016.02.026
Stewart I, Webb PM, Schluter PJ, Shaw GR (2006) Recreational and occupational field exposure to freshwater cyanobacteria—a review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ Health 5:6. https://doi.org/10.1186/1476-069X-5-6
Backer LC, Carmichael W, Kirkpatrick B, Williams C, Irvin M, Zhou Y et al (2008) Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar Drugs 6(2):389–406. https://doi.org/10.3390/md20080018
Backer LC, McNeel SV, Barber T, Kirkpatrick B, Williams C, Irvin M et al (2010) Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 55(5):909–921. https://doi.org/10.1016/j.toxicon.2009.07.006
Du X, Liu H, Yuan L, Wang Y, Ma Y, Wang R et al (2019) The diversity of cyanobacterial toxins on structural characterization. Distrib Identif: Syst Rev Toxins (Basel) 11(9):530. https://doi.org/10.3390/toxins11090530
Blaha L, Babica P, Marsalek B (2009) Toxins produced in cyanobacterial water blooms—toxicity and risks. Interdiscip Toxicol 2(2):36–41. https://doi.org/10.2478/v10102-009-0006-2
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11(1):268–287. https://doi.org/10.3390/ijms11010268
Schreidah CM, Ratnayake K, Senarath K, Karunarathne A (2020) Microcystins: biogenesis, toxicity, analysis, and control. Chem Res Toxicol 33(9):2225–2246. https://doi.org/10.1021/acs.chemrestox.0c00164
McLellan NL, Manderville RA (2017) Toxic mechanisms of microcystins in mammals. Toxicol Res (Camb) 6(4):391–405. https://doi.org/10.1039/c7tx00043j
Plata-Calzado C, Diez-Quijada L, Medrano-Padial C, Prieto AI, Camean AM, Jos A (2023) In vitro mutagenic and genotoxic assessment of anatoxin-a alone and in combination with cylindrospermopsin. Toxins (Basel) 15(7):458. https://doi.org/10.3390/toxins15070458
Ziesemer S, Meyer S, Edelmann J, Vennmann J, Gudra C, Arndt D et al (2022) Target mechanisms of the cyanotoxin cylindrospermopsin in immortalized human airway epithelial cells. Toxins (Basel) 14(11):785. https://doi.org/10.3390/toxins14110785
Cevallos-Cedeno RE, Quinones-Reyes G, Agullo C, Abad-Somovilla A, Abad-Fuentes A, Mercader JV (2022) Rapid immunochemical methods for anatoxin-a monitoring in environmental water samples. Anal Chem 94(30):10857–10864. https://doi.org/10.1021/acs.analchem.2c01939
Christensen VG, Khan E (2020) Freshwater neurotoxins and concerns for human, animal, and ecosystem health: a review of anatoxin-a and saxitoxin. Sci Total Environ 736:139515. https://doi.org/10.1016/j.scitotenv.2020.139515
Lopicic S, Svircev Z, Palanacki Malesevic T, Kopitovic A, Ivanovska A, Meriluoto J (2022) Environmental neurotoxin beta-N-methylamino-L-alanine (BMAA) as a widely occurring putative pathogenic factor in neurodegenerative diseases. Microorganisms 10(12):2418. https://doi.org/10.3390/microorganisms10122418
Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S et al (2003) Activation of cutaneous protein kinase C alpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol 171(5):2703–2713. https://doi.org/10.4049/jimmunol.171.5.2703
Stewart I, Schluter PJ, Shaw GR (2006) Cyanobacterial lipopolysaccharides and human health—a review. Environ Health 5:7. https://doi.org/10.1186/1476-069X-5-7
Toporowska M (2022) Degradation of three microcystin variants in the presence of the macrophyte Spirodela polyrhiza and the associated microbial communities. Int J Environ Res Public Health 19(10):6086. https://doi.org/10.3390/ijerph19106086
Abdallah MF, Van Hassel WHR, Andjelkovic M, Wilmotte A, Rajkovic A (2021) Cyanotoxins and food contamination in developing countries: review of their types, toxicity, analysis, occurrence and mitigation strategies. Toxins (Basel) 13(11):786. https://doi.org/10.3390/toxins13110786
Arman T, Clarke JD (2021) Microcystin toxicokinetics, molecular toxicology, and pathophysiology in preclinical rodent models and humans. Toxins (Basel) 13(8):537. https://doi.org/10.3390/toxins13080537
Pearson L, Mihali T, Moffitt M, Kellmann R, Neilan B (2010) On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar Drugs 8(5):1650–1680. https://doi.org/10.3390/md8051650
Preece EP, Hardy FJ, Moore BC, Bryan M (2017) A review of microcystin detections in estuarine and marine waters: environmental implications and human health. Harmful Algae 61:276–282
Svircev Z, Lalic D, Bojadzija Savic G, Tokodi N, Drobac Backovic D, Chen L et al (2019) Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch Toxicol 93(9):2429–2481. https://doi.org/10.1007/s00204-019-02524-4
Baliu-Rodriguez D, Peraino NJ, Premathilaka SH, Birbeck JA, Baliu-Rodriguez T, Westrick JA et al (2022) Identification of novel microcystins using high-resolution MS and MS(n) with python code. Environ Sci Technol 56(3):1652–1663. https://doi.org/10.1021/acs.est.1c04296
Greer B, Meneely JP, Elliott CT (2018) Uptake and accumulation of Microcystin-LR based on exposure through drinking water: an animal model assessing the human health risk. Sci Rep 8(1):4913. https://doi.org/10.1038/s41598-018-23312-7
Zegura B, Gajski G, Straser A, Garaj-Vrhovac V, Filipic M (2011) Microcystin-LR induced DNA damage in human peripheral blood lymphocytes. Mutat Res 726(2):116–122. https://doi.org/10.1016/j.mrgentox.2011.10.002
Zhi H, Yuan Y, Zhang C, Jiang Y, Zhang H, Wang C et al (2020) Importance of OATP1B1 and 1B3 in the liver uptake of luteolin and its consequent glucuronidation metabolites. J Agric Food Chem 68(7):2063–2070. https://doi.org/10.1021/acs.jafc.9b06954
Chowdhury RR, Rose S, Ezan F, Sovadinova I, Babica P, Langouet S (2024) Hepatotoxicity of cyanotoxin microcystin-LR in human: insights into mechanisms of action in the 3D culture model Hepoid-HepaRG. Environ Pollut 342:123047. https://doi.org/10.1016/j.envpol.2023.123047
Fontanillo M, Kohn M (2018) Microcystins: synthesis and structure-activity relationship studies toward PP1 and PP2A. Bioorg Med Chem 26(6):1118–1126. https://doi.org/10.1016/j.bmc.2017.08.040
Craig M, Luu HA, McCready TL, Williams D, Andersen RJ, Holmes CF (1996) Molecular mechanisms underlying he interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem Cell Biol 74(4):569–578. https://doi.org/10.1139/o96-061
Rai AK, Chaturvedi R, Kumar A (2018) Proteomic evidences for microcystin-RR-induced toxicological alterations in mice liver. Sci Rep 8(1):1310. https://doi.org/10.1038/s41598-018-19299-w
Piyathilaka MA, Pathmalal MM, Tennekoon KH, De Silva BG, Samarakoon SR, Chanthirika S (2015) Microcystin-LR-induced cytotoxicity and apoptosis in human embryonic kidney and human kidney adenocarcinoma cell lines. Microbiology (Reading) 161(Pt 4):819–828. https://doi.org/10.1099/mic.0.000046
Wang Z, Li G, Wu Q, Liu C, Shen J, Yan W (2019) Microcystin-LR exposure induced nephrotoxicity by triggering apoptosis in female zebrafish. Chemosphere 214:598–605. https://doi.org/10.1016/j.chemosphere.2018.09.103
Yi X, Xu S, Huang F, Wen C, Zheng S, Feng H et al (2019) Effects of chronic exposure to microcystin-LR on kidney in mice. Int J Environ Res Public Health 16(24):5030. https://doi.org/10.3390/ijerph16245030
Wang C, Gu S, Yin X, Yuan M, Xiang Z, Li Z et al (2016) The toxic effects of microcystin-LR on mouse lungs and alveolar type II epithelial cells. Toxicon 115:81–88. https://doi.org/10.1016/j.toxicon.2016.03.007
Liu H, Zeng X, Wang Y, Losiewicz MD, Chen X, Du X et al (2022) Chronic exposure to environmentally relevant concentrations of microcystin-leucine arginine causes lung barrier damage through PP2A activity inhibition and claudin1 ubiquitination. J Agric Food Chem 70(35):10907–10918. https://doi.org/10.1021/acs.jafc.2c05207
Martins ND, Yunes JS, McKenzie DJ, Rantin FT, Kalinin AL, Monteiro DA (2019) Microcystin–LR exposure causes cardiorespiratory impairments and tissue oxidative damage in trahira. Hoplias malabaricus Ecotoxicol Environ Saf 173:436–443. https://doi.org/10.1016/j.ecoenv.2019.02.053
Yan C, Liu Y, Yang Y, Massey IY, Cao L, Osman MA et al (2023) Cardiac toxicity induced by long-term environmental levels of MC-LR exposure in Mice. Toxins (Basel) 15(7):427. https://doi.org/10.3390/toxins15070427
Jochimsen EM, Carmichael WW, An J, Cardo DM, Cookson ST, Holmes CEM, Antunes MB, de Melo Filho DA, Lyra TM, Barreto VS, Azevedo SM, Jarvis WR (1998) Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med 13:873
Zhou LYH, Chen K (2002) Relationship between microcystin in drinking water and colorectal cancer. BES 15:166–171
Giannuzzi L, Sedan D, Echenique R, Andrinolo D (2011) An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam. Argentina Mar Drugs 9(11):2164–2175. https://doi.org/10.3390/md9112164
Fujiki H, Suganuma M (2011) Tumor promoters–microcystin-LR, nodularin and TNF-alpha and human cancer development. Anticancer Agents Med Chem 11(1):4–18. https://doi.org/10.2174/187152011794941163
Beattie KA, Kaya K, Codd GA (2000) The cyanobacterium nodularia PCC 7804, of freshwater origin, produces [L-Har2]nodularin. Phytochemistry 54(1):57–61. https://doi.org/10.1016/s0031-9422(00)00045-5
Cornell TT, Hinkovska-Galcheva V, Sun L, Cai Q, Hershenson MB, Vanway S et al (2009) Ceramide-dependent PP2A regulation of TNFalpha-induced IL-8 production in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol 296(5):L849–L856. https://doi.org/10.1152/ajplung.90516.2008
Sueoka E, Sueoka N, Okabe S, Kozu T, Komori A, Ohta T et al (1997) Expression of the tumor necrosis factor alpha gene and early response genes by nodularin, a liver tumor promoter, in primary cultured rat hepatocytes. J Cancer Res Clin Oncol 123(8):413–419. https://doi.org/10.1007/BF01372544
Meili N, Christen V, Fent K (2016) Nodularin induces tumor necrosis factor-alpha and mitogen-activated protein kinases (MAPK) and leads to induction of endoplasmic reticulum stress. Toxicol Appl Pharmacol 300:25–33. https://doi.org/10.1016/j.taap.2016.03.014
Wharton RE, Cunningham BR, Schaefer AM, Guldberg SM, Hamelin EI, Johnson RC (2019) Measurement of microcystin and nodularin activity in human urine by immunocapture-protein phosphatase 2A assay. Toxins (Basel) 11(12):729. https://doi.org/10.3390/toxins11120729
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rajesh Melaram, Amanda Rose Newton, Anna Lee, Scott Herber, Anthony El-Khouri, and Jennifer Chafin declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Melaram, R., Newton, A.R., Lee, A. et al. A review of microcystin and nodularin toxins derived from freshwater cyanobacterial harmful algal blooms and their impact on human health. Toxicol. Environ. Health Sci. (2024). https://doi.org/10.1007/s13530-024-00220-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s13530-024-00220-0