Abstract
Objective
Plastic pollution, particularly polystyrene, significantly threatens aquatic ecosystems worldwide. Furthermore, plastic leachates have been documented to be detrimental to some aquatic organisms; however, understanding the toxicity mechanism remains limited. This study aimed to investigate the ecotoxicological effects of polystyrene leachate on neonate and adult Daphnia magna, a keystone species in freshwater ecosystems.
Methods
The effects of the leachate were studied by employing the novel technique of separating daphnids from the polystyrene microplastic fragments via dialysis tubing, which was prepared 24 and 72 h before organism exposure. Acute toxicity was assessed as effects on organism mobility, oxidative stress (reactive oxygen species), antioxidative enzyme responses (superoxide dismutase and catalase), as well as the effects on the biotransformation enzyme glutathione S-transferase’s activity.
Results
Under the experimental conditions, the mobility and oxidative status of the daphnids were unaffected, irrespective of the organisms’ age or leaching time. In adults exposed for 24 h, the antioxidant defense enzyme activities were elevated, contributing to cellular homeostasis maintenance. However, the catalase activity was reduced for neonates and adults exposed to the prolonged pre-leached treatment, thus making them less capable of retaining homeostasis when exposed to toxicant mixtures.
Conclusion
This study highlights the vulnerability of D. magna to polystyrene leachate and underscores the need for continued research on the ecotoxicological effects of plastic pollution in aquatic ecosystems. Findings from this investigation contribute to understanding the ecological consequences of plastic pollution, which can inform mitigation strategies and policy decisions to preserve the health and integrity of freshwater ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of plastic has increased exponentially over time [1], with the majority ultimately being discarded instead of recycled. Therefore, plastic pollution in terrestrial and aquatic environments has become an escalating concern in recent decades, particularly as it poses threats to various organisms through multiple pathways, including entanglement, ingestion, and bioaccumulation [2, 3].
Among the myriad of plastic pollution types, microplastics (MPs) have garnered particular attention due to their ubiquitous global distribution [4,5,6] and potential environmental impacts [7, 8]. MPs cause devastating effects due to intestinal blockage following ingestion and its overall toxicity [8, 9]. Furthermore, as these minuscule particles continue to accumulate in various ecosystems, they have the potential to leach harmful chemicals [10] and synergistically interact with other pollutants [11], potentially leading to complex ecological and health ramifications; however, our current understanding is inadequate.
Leachates from MPs, containing inherent chemicals and absorbed environmental contaminants, have raised significant concerns due to their potential to impact aquatic life, soil health, and human well-being. Various additives are incorporated into plastics intended for diverse applications [10, 12]. The additives generally applied include plasticizers, flame retardants, and thermal stabilizers, which can represent up to 70, 20, and 8% by weight of the plastic, respectively [13]. Other known additives include photostabilizers, antioxidants, and pigments [14]. Additives enable and facilitate processing and impart desirable properties to the product or allow retaining the original plastic properties during molding [15, 16]. However, some of the used additives are known carcinogens, mutagens, and endocrine disruptors and, thus, are potentially hazardous to many organisms [17,18,19,20]. Moreover, residual additive monomers may migrate into the environment because they are not covalently bound to the plastic matrix [21,22,23,24]. Leaching has been shown to occur in various media, including sea- and freshwater, digestive fluid, and stomach oil [25,26,27], with the detection of plastic additives in aquatic systems reported by several studies (as reviewed by Gunaalan et al. [28]).
The processes involved in the liberation of additives from plastics were studied by Do et al. [10]. The leaching process has been shown to be influenced by factors such as the type of plastic, surface characteristics, the environment, and time. Thermal and photodegradation, as well as biodegradation, play significant roles. Thus, the specific types and amounts of chemicals leached from MPs likely depend on the plastic’s composition, size, and environmental conditions. Furthermore, the media in which plastics leach could contribute to toxicity as their properties could facilitate the liberation of more toxicants [29].
Knowledge of the potential toxic impacts of chemicals leaching from plastic into the environment is currently limited [30]. Nevertheless, due to the potential impact of plastic leachate on aquatic organisms, it is an area of active research, and our understanding of its effects is still evolving. Some studies have provided valuable insights into the potential impacts of plastic leachate on various aquatic organisms [28]. Despite several studies reporting on the toxicity of plastic leachates on daphnids, especially regarding reproduction [31], understanding of the physiological effects of these leachates on the primary consumer and keystone species Daphnia magna is lacking [8]. Daphnids filter feeds small, suspended particles, such as unicellular algae, and serves as prey for diverse invertebrates, playing a significant role in aquatic food chains transferring energy to higher trophic levels and thus deemed an invaluable study organism along with its recognized role as model bioindicator organism in ecotoxicology [32]. Given the prevalent detection of polystyrene (PS) MPs [4], this study focuses on the effects of PS leachates on D. magna.
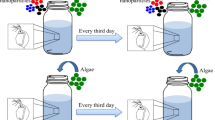
Differentiating adverse effects caused by MPs and those by leached substances is challenging, with factors such as photodegradation and biodegradation potentially influencing the mixture of leached chemicals. Thus, our study aims to control these variables by exposing D. magna to PS leachates and isolating them from the MPs using dialysis tubing. All leaching prior to exposure would be conducted in the dark, at four degrees Celsius, with pristine PS fragments to eliminate the effects of photodegradation, thermal degradation, and biodegradation. To evaluate the toxic effects of the leachate, immobilization/survival, oxidative stress status, and antioxidant defense responses, as well as the response of the biotransformation enzyme glutathione S-transferase (GST) were used as biomarkers in both neonates and adults, considering sensitivity at various life stages.
This investigation pioneers a novel approach to evaluating the impacts of plastic leachates on living organisms. A recent review by Yin et al. [33] highlighted the need for studies and methods distinguishing whether observed effects are due to MP particles or leachate. Furthermore, this study provides compelling evidence that chemicals released from polystyrene within 24–72 h can induce physiological effects, especially regarding oxidative stress and biotransformation enzymes. The insights garnered from this research could significantly advance our understanding of the environmental hazards associated with plastic pollution, particularly in the context of MP leachates’ impact on aquatic ecosystems.
Materials and methods
Daphnia magna cultivation
D. magna ephippia were purchased from Aboatox Oy (Masku, Finland) and hatched under a 16:8 h light–dark cycle (8500 lx) at 22.0 ± 1.0 °C in ISO medium [34]. The daphnids were fed daily with Chlorella vulgaris (~ 1.5 × 108 cells/mL) for seven days, followed by a combination of C. vulgaris and TetraMin® Baby afterward. Neonates (newly hatched daphnids) were removed daily, and culture media were renewed weekly. The pH and dissolved oxygen content were monitored and maintained according to the OECD guidelines [35]. For the present study, neonates used for experimentation were ≤ 24 h old, and adult daphnids were at least 10 days old and fully developed.
Consumables
Polystyrene beads (LS555533; batch C3586; Goodfellow, Huntingdon, UK) were ground into smaller fragments employing low-temperature ball milling on a Retsch Cryomill (Haan, Germany) maintained between −196 and −100 °C using liquid nitrogen. A 50-mL cell and a 2-cm-diameter steel ball were used for mechanical cryogenic grinding. A third of the cells were filled with the plastic material and ground for approximately 30 min, with 5 min of pre-cooling. The plastic fragments were separated using a Vibratory Sieve shaker (AS 300 Control; Retsch GmbH, Haan, Germany) via four sieves with mesh sizes of 100, 63, 45, and 25 µm according to ISO 3310–01 [36]. The size fraction containing fragments with diameters between 45 and 63 µm was used for exposure via dialysis tubing.
All other consumables were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) unless stated otherwise and were of analytical grade.
Exposure to PS leachates
Dialysis tubing was used to separate the daphnids from the PS MP fragments to exclude the effect of direct contact with the fragments and only assess toxic effects associated with leached chemicals during acute exposure. For the treatments, 5 mg of 45 to 63 µm PS fragments was placed in 15 kD Spectra/Por® 6 dialysis tubing (2 cm length), tied off with twine at both ends. The dialysis tubing containing the PS was placed in 100 mL ISO media [34] in glass beakers (n = 3). The control (n = 3) consisted of the same setup without PS fragments in the dialysis tubing. The control and exposure sets were prepared in duplicate sets, one for exposing adult daphnia and the other for neonates. Prior to adding the daphnids, these treatments were kept at 4 °C in the dark for 24 h to facilitate leaching but excluding photodegradation and thermal degradation of the plastics to aid leaching. A third exposure set (treatments and control, n = 3) was prepared and leached at 4 °C for 72 h before adding the daphnids (adult daphnids only). After allowing the treatments to return to room temperature (21 ± 1 °C), 20 adult daphnids and 45 neonates were added per replicate per respective exposure set. The daphnids were well fed before experimentation, with no additional feeding over the exposure period, and the media was not changed during this time. After 24 h, immobile daphnids were counted [35] to assess whether the daphnids were physically affected by exposure to PS leachates over 24 h. Mobile daphnids were collected, using one-third for reactive oxygen species (ROS) analysis (oxidative stress status marker) and the other two-thirds for assessing the activities of the antioxidative enzymes, superoxide dismutase (SOD), and catalase (CAT) (antioxidant defense response marker), as well as evaluate the enzymatic response of glutathione S-transferase (GST) as a measure of the activation of the biotransformation system.
Oxidative stress status and enzymatic activity assays
For the ROS determination, as a measure of the oxidative stress status, one-third of the mobile daphnids were snap-frozen and thoroughly homogenized in 200 µL of 20 mM sodium phosphate buffer, followed by centrifugation at 5,000 × g at 4 °C for 3 min. Then, 4 μL of supernatant was used to determine the protein content using Bradford solution [37]. The ROS levels were assessed by measuring the excitation and emission spectra at 540 and 570 nm, respectively, using the ROS Assay (Kit MAK 144-1KT; Sigma-Aldrich, St. Louis, Missouri, USA) as per the manufacturer’s instructions on a Tecan Infinite 200 Pro M Nano + reader (Spark, Tecan, Switzerland) using 50 μL of supernatant from each sample. The fluorescence intensities were normalized by the protein content (mg/mL) of each specimen.
For extracting the S9 enzyme fraction, the other two-thirds of the mobile daphnids were frozen and homogenized with a micro-pestle in 20 mM potassium phosphate buffer (pH 7) on ice. Cell debris was removed by centrifugation at 13,000 × g for 10 min, and the supernatant was used to assess the enzyme activities [38]. The SOD activity was evaluated using the SOD Assay Kit (19,160-1KT-F; Sigma-Aldrich, St. Louis, Missouri, USA). CAT (E.C. 1.11.1.6) activity was assayed using hydrogen peroxide as substrate as per Claiborne [39] on a Tecan Infinite 200 Pro Infinite M Nano + (Tecan GmbH, Grödig, Austria). GST (EC 2.5.1.18) activity was assessed by photometrically measuring the conjugation rate of 1-chloro-2,4-dinitrobenzene (CDNB) with GSH at 340 nm (extinction coefficient Ɛ = 9.6 L/mmol/cm) according to Habig et al. [40]. CAT and GST activities were normalized against the protein content, determined as per Bradford [37], i.e., enzyme activity was calculated as katal per milligram protein (kat/mg).
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics 28.0.0.0 (190) (2021). Descriptive analysis was performed on all data sets, followed by normality, sphericity, and homogeneity analysis. For data sets meeting the requirements of homogeneity and normality, a one-way analysis of variance (ANOVA) was performed. Tukey post hoc tests were performed. The nonparametric Kruskal–Wallis test with pairwise comparisons was used for data sets that did not meet the requirements. An alpha value of 0.05 was observed for all data sets, with p < 0.050 indicating significance [41]. Figures were prepared using Microsoft® Excel® for Microsoft 365 MSO (version 2308 Build 16.0.16731.20496).
Results
Daphnia magna immobilization
Using immobilization as an end-point marker for acute toxicity, it can be seen from Fig. 1 that with exposure to all treatments, compared to their respective controls, the leachate did not significantly affect the mobility of D. magna (p > 0.05). After the 24 h exposure, 98.5 ± 0.6% of both neonates (p = 0.3) and adults (p = 1) remained mobile regardless of the presence (treatment) or absence (control) of PS in the dialysis tubing. However, following 48 h of exposure to the 72 h pre-leached treatment and its corresponding control, significantly fewer adults exhibited activity (p < 0.05), with a 23.3% decrease in the control group and a 26.7% decrease in the treatment. Nonetheless, the difference in the number of mobile adult daphnids in the treatment compared to the control after 48 h was not statistically significant (p = 0.8).
Percentage of mobile neonate and adult Daphnia magna exposed for 24 h to PS MP leachate placed in dialysis tubing 24 h before the introduction of the daphnids. The percentage survival after 48 h of exposure of adult daphnids exposed to PS MP leachate via dialysis tubing prepared 72 h prior to the introduction of daphnids. Bars denote the average survival of mobile daphnids ± standard deviation (n = 3)
Oxidative stress status and enzymatic antioxidative response
Irrespective of the treatments, organism age, or exposure time (Fig. 2), none of the relative cellular ROS levels differed from their corresponding controls (p > 0.05). Notably, the neonates generally exhibited significantly higher ROS levels than adults (p > 0.05). The ROS levels for all adults in all treatments and controls were statistically the same (p > 0.05).
Relative cellular reactive oxygen species (ROS) in neonate and adult Daphnia magna exposed for 24 h to PS MP leachate placed in dialysis tubing 24 h before the introduction of the daphnids and after 48 h of exposing adult daphnids PS MP leachate via dialysis tubing prepared 72 h prior to the exposure. Bars denote the average relative cellular ROS ± standard deviation (n = 3)
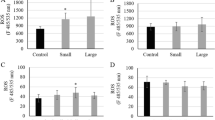
The SOD activities in neonates exposed to the PS leachate for 24 h (Fig. 3A) and the adults exposed for 48 h (Fig. 3C) were not statistically different from those in the control (p > 0.05). However, in adults exposed for 24 h to PS leachate (Fig. 3B), the SOD activity increased by 35.8% (p = 0.003). In general, the basal SOD activity in neonates was lower than that in adults.
Superoxide dismutase (SOD) activity in A neonate and B adult Daphnia magna exposed for 24 h to PS MP leachate placed in dialysis tubing 24 h before the introduction of the daphnids and C after 48 h of exposing adult daphnids PS MP leachate via dialysis tubing prepared 72 h prior to the exposure. Bars denote the average SOD activity ± standard deviation (n = 3). Statistical significance compared to the corresponding control is indicated as an asterisk (*) when p < 0.050
The CAT activity in neonates exposed for 24 h was inhibited by 39.5% (p = 0.007; Fig. 4A) and 23.3% in adults exposed for 48 h (p = 0.04; Fig. 4C). However, in adults exposed to PS leachate for 24 h, the CAT activity increased 37.6% (p = 0.05).
Catalase (CAT) activity in A neonate and B adult Daphnia magna exposed for 24 h to PS MP leachate placed in dialysis tubing 24 h before the introduction of the daphnids and C after 48 h of exposing adult daphnids PS MP leachate via dialysis tubing prepared 72 h prior to the exposure. Bars denote the average CAT activity ± standard deviation (n = 3). Statistical significance compared to the corresponding control is indicated as an asterisk (*) when p < 0.050
For the neonates, exposure did not affect GST activity (p = 0.6; Fig. 5A). However, the adults had diverging responses. In those exposed for 24 h (Fig. 5B), the GST activities increased by 42.2% (p = 0.004), whereas in adults exposed for 48 h (Fig. 5C), GST activities were inhibited by 27.6% (p = 0.02).
Glutathione S-transferase (GST) activity in A neonate and B adult Daphnia magna exposed for 24 h to PS MP leachate placed in dialysis tubing 24 h before the introduction of the daphnids and C after 48 h of exposing adult daphnids PS MP leachate via dialysis tubing prepared 72 h prior to the exposure. Bars denote the average GST activity ± standard deviation (n = 3). Statistical significance compared to the corresponding control is indicated as an asterisk (*) when p < 0.05
Discussion
Daphnia magna immobility
In the present study, exposure to PS leachate did not affect either the neonate or the adult D. magna, irrespective of the time the plastics leached prior to exposure or contact time. Lithner et al. [42] leached plastics from old electronic products (100.0 g/L) in water for three days and exposed D. magna for 24 and 48 h. They found that it was non-toxic to the test organisms; however, the specific types of plastics were not reported. A previous study by Kim et al. [43] investigated the effect of plastic (synthetic rubber) shoe sole leachate on the immobility of neonate D. magna. Using dilutions of the leachate prepared by an accelerated artificial leaching protocol using 2.0 g/L material, immobility significantly increased from 24 to 48 h. Compared to the current study, this higher immobilization rate might be attributed to the release of more toxicants through thermal and photodegradation processes facilitating artificial leaching. In addition, the concentration of 0.05 g/L PS used in the present study, given the 50.0% dilution of the leachate used by Kim et al. [43], is 20-fold lower.
For the leaching of waste materials with particles smaller than 4 mm, a liquid-to-solid ratio of 10:1 has been suggested [44] and used to test plastic leachate toxicity [45, 46]. However, in freshwater aquatics in Europe and Asia, up to 2561 MP particles/m3 have been detected [47]. Using the conversion presented by Besseling et al. [48], i.e., supposing a weight of 5.0 µg per particle, this converts to 12.8 µg/L. Specifically for PS, concentrations up to 13.0 ng/L have been reported in the environment [49]. Thus, the concentrations used thus far to evaluate the toxicity of MP leachates greatly exceed the environmental concentrations.
Nevertheless, Li et al. [30] reported low toxicities for leachate from PS compared to other plastic types concerning Amphibalanus amphitrite as a bioindicator organism. Similarly, Lithner et al. [50] reported no immobility of daphnids with exposure to leachate from PS plastic products (100.0 g/L).
Oxidative status and antioxidative response
Physiological parameters often serve as more sensitive indicators of toxicological effects. The antioxidant response system is essential in maintaining homeostasis, i.e., the balance of reactive oxygen species (ROS), a natural by-product of cellular processes, to minimize biomolecule damage. SOD initiates the detoxification process by transforming superoxide radicals to hydrogen peroxide, which, in turn, is converted by enzymes, such as CAT, among others, to water and oxygen. Elevated ROS levels are a well-known consequence of toxicant exposure and indicative of oxidative stress [51]. However, considering the ROS levels in Fig. 2, oxidative stress was not promoted in any organisms in the present study. This might be attributed to the possibility that ROS levels, which could have initially increased at the onset of exposure, were either neutralized by the enzymatic or non-enzymatic antioxidative system or that exposure to the leachate did not lead to an elevation in ROS. Nevertheless, the activities of antioxidative enzymes such as SOD (Fig. 3B), CAT (Fig. 4B), and the biotransformation enzyme GST (Fig. 5B) were only elevated in adults exposed for 24 h. Furthermore, the enzyme activities of these enzymes likely returned to their baseline levels after 48 h of exposure. It is well documented that CAT expression can be upregulated by ROS [52]. However, CAT activities in neonates (Fig. 4A) and adults exposed for 48 h (Fig. 4C) were significantly reduced. This observation aligns with the theory proposed by Liu et al. [53], which stated that continued exposure to MPs could potentially result in amplified ROS, leading to the indiscriminate oxidation of proteins and causing downregulation of CAT [54]. Similarly, the neonates could have been overwhelmed by the ROS level at the start of the exposure, suggesting that the non-enzymatic antioxidant system aided in neutralizing the ROS (Fig. 2). Schiavo et al. [55] reported elevated ROS in Dunaliella tertiolecta exposed to PS leachate for 72 h (100.0 g/L); however, the PS concentration the leachate was produced from was 2000-fold higher than in the present study.
Remarkably, SOD activity was affected only in adult daphnids exposed for 24 h in the present study (Fig. 3B). Sunil et al. [56] reported increased SOD activity in Donax faba with exposure to a 25.0% dilution of leachate derived from PET bottles (100.0 g/L) after 72 h but inhibition with higher concentrations of the leachate. However, the inhibition coincided with substantially elevated ROS levels. Another possibility is that the concentration of toxicants in the leachate used in the current study was not sufficiently high to induce an increase in ROS levels and, consequently, did not continuously stimulate SOD activity. Nevertheless, CAT was inhibited in neonates and adults exposed for 48 h despite no corresponding elevated ROS concentration. CAT activity was unchanged in Mytilus galloprovincialis, exposed to PET, PS, PP, PVC, and car tire leachate (80.0 g/L) [57]. Sunil et al. [56] reported considerable inhibition of CAT activity in D. faba exposed to PET leachate. Some plastic additives, such as hydroxylamine, used as a UV stabilizer, and resorcinol, as a flame retardant, are potent catalase inhibitors [14, 58, 59]. Potentially, in the more sensitive neonates and with more prolonged exposure, these compounds were responsible for the observed reduction in CAT activity and should be tested for in future studies.
In the present study, GST in adults was elevated after 24 h of exposure to 24 h leachate but inhibited after 48 h of exposure to 72 h leachate. In M. galloprovincialis, leachates from polypropylene and polyethylene terephthalate increased GST activity in digestive glands, while polypropylene leachate was the only treatment to enhance GST in the gills. PS, however, did not affect GST activity in either tissue [57], demonstrating the variable sensitives of bioindicator organisms to different toxicants. Glutathione protects against trace metal toxicity and is involved in enzymatic antioxidant defense reactions as well as the biotransformation of toxic compounds [60]. However, toxic metals can directly bind to glutathione, resulting in decreased availability and thus affecting GST activities [61]. Therefore, leached heavy metals [62, 63] may be responsible for the decreased GST activity observed after 48 h of exposure to the PS pre-leachate for 72 h.
One of the limitations of this study is that the chemical compounds in the leachate were neither identified nor quantified; thus, speculations must be made based on studies that previously characterized leachates. Furthermore, the concentration of PS used is higher than environmentally relevant but significantly lower than in previous literature. This study did not account for plastic surface alterations due to weathering or biofilms, factors that can substantially impact equilibrium, kinetics, and the transformation of additives [10]. Hence, the results may underestimate the environmental effect of leachate despite the higher PS concentration used in this study.
Conclusion
The study pioneers a novel approach to evaluate the impacts of plastic leachates on living organisms via dialysis membranes. This method could greatly aid in elucidating the toxicity mechanism of leachates from plastics. Furthermore, this study found that acute exposure to PS leachate did not result in reduced mobility in D. magna, suggesting that, under short-term exposure, the leachate did not have a significant immediate toxic impact on the organisms. Nonetheless, even without elevated ROS levels, certain antioxidative response enzymes were found to be inhibited with prolonged exposure. This inhibition could increase the vulnerability of D. magna to additional risks when concurrently exposed to other toxicants due to the synergistic effects of mixture toxicity. The resulting compromised antioxidant defense system might be unable to effectively regulate ROS levels under stress from other environmental pollutants, rendering the organisms vulnerable. Our findings extend beyond laboratory conditions and have significant ecological ramifications. Thus, D. magna, a key organism in freshwater food webs, has life stages with differential sensitivities to oxidative stress, which may be exacerbated by continuous exposure to pollutants like PS leachate. This research data enriches the scientific community’s understanding of the consequences of plastic pollution, serving as a crucial reference for environmental risk assessment. Moreover, the data extend the reach of this research by underpinning the need for policies aimed at reducing plastic waste and by informing conservation strategies that consider the cumulative impacts of multiple stressors on aquatic ecosystems, potentially affecting biodiversity and ecological function on a broader scale.
Data availability
The corresponding author can be contacted to request access to the data, which will be made available.
References
Plastics Europe (2019) Plastics—the Facts 2019. An analysis of European plastics production, demand and waste data. Published on the occasion of the special show of K 2019, Messe Düsseldorf
JGB Derraik 2002 The pollution of the marine environment by plastic debris: a review Mar Pollut Bull https://doi.org/10.1016/S0025-326X(02)00220-5
JM Silva Da LM Alves MI Laranjeiro F Bessa AV Silva AC Norte MF Lemos JA Ramos SC Novais FR Ceia 2022 Accumulation of chemical elements and occurrence of microplastics in small pelagic fish from a neritic environment Environ Pollut https://doi.org/10.1016/j.envpol.2021.118451
C Scopetani D Chelazzi A Cincinelli M Esterhuizen-Londt 2019 Assessment of microplastic pollution: occurrence and characterisation in Vesijärvi lake and Pikku Vesijärvi pond Finland Environ Monit Assess https://doi.org/10.1007/s10661-019-7843-z
N Ajith S Arumugam S Parthasarathy S Manupoori S Janakiraman 2020 Global distribution of microplastics and its impact on marine environment—a review Environ Sci Pollut Res https://doi.org/10.1007/s11356-020-09015-5
W Li X Li J Tong W Xiong Z Zhu X Gao S Li M Jia Z Yang J Liang 2023 Effects of environmental and anthropogenic factors on the distribution and abundance of microplastics in freshwater ecosystems Sci Total Environ https://doi.org/10.1016/j.scitotenv.2022.159030
H Du J Wang 2021 Characterization and environmental impacts of microplastics Gondwana Res https://doi.org/10.1016/j.gr.2021.05.023
A Samadi Y Kim SA Lee YJ Kim M Esterhuizen 2022 Review on the ecotoxicological impacts of plastic pollution on the freshwater invertebrate Daphnia Environ Toxicol https://doi.org/10.1002/tox.23623
Y Deng J Wu J Chen K Kang 2023 Overview of microplastic pollution and its influence on the health of organisms J Environ Sci Health, Part A https://doi.org/10.1080/10934529.2023.2190715
ATN Do Y Ha JH Kwon 2022 Leaching of microplastic-associated additives in aquatic environments: a critical review Environ Pollut https://doi.org/10.1016/j.envpol.2022.119258
SH Joo Y Liang M Kim J Byun H Choi 2021 Microplastics with adsorbed contaminants: mechanisms and treatment Environ Chall https://doi.org/10.1016/j.envc.2021.100042
H Wiesinger Z Wang S Hellweg 2021 Deep dive into plastic monomers, additives, and processing aids Environ Sci Technol https://doi.org/10.1021/acs.est.1c00976
AL Andrady N Rajapakse 2016 Additives and chemicals in plastics H Takada H Karapanagioti Eds Hazardous chemicals associated with plastics in the marine environment, the handbook of environmental chemistry 78 Springer Cham 1 17
MP Stevens 1990 Polymer chemistry 2 Oxford University Press New York
OECD (Organisation for Economic Co-operation and Development) (2009) Emission scenario document on plastic additives: series on emission scenario documents. OECD environmental health and safety publications No. 3, OECD Environment Directorate, Paris
JN Hahladakis CA Velis R Weber E Iacovidou P Purnell 2018 An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling J Hazard Mater https://doi.org/10.1016/j.jhazmat.2017.10.014
European Chemicals Bureau (ECB) (2008) ESIS – European chemical substances information system. http://ecb.jrc.it/esis/. Accessed 10 Sep 2023
KJ Groh T Backhaus B Carney-Almroth B Geueke PA Inostroza A Lennquist HA Leslie M Maffini D Slunge L Trasande AM Warhurst 2019 Overview of known plastic packaging-associated chemicals and their hazards Sci Total Environ https://doi.org/10.1016/j.scitotenv.2018.10.015
I Blinova A Lukjanova H Vija M Mortimer M Heinlaan 2023 Toxicity of plastic additive 1-hydroxycyclohexyl phenyl ketone (1-HCHPK) to freshwater microcrustaceans in natural water Water https://doi.org/10.3390/w15183213
A Li C Yan J Qiu Y Ji Y Fu W Yan 2023 Adverse effects of plastic leachate and its component 2, 4-DTBP on the early development of zebrafish embryos Sci Total Environ https://doi.org/10.1016/j.scitotenv.2023.167246
TR Crompton 1979 Additive migration from plastics into food 1 Pergamon Press New York
PHH Araújo C Sayer R Giudici JGR Poço 2002 Techniques for reducing residual monomer content in polymers: a review Polym Eng Sci https://doi.org/10.1002/pen.11043
D Lithner Å Larsson G Dave 2011 Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition Sci Total Environ https://doi.org/10.1016/j.scitotenv.2011.04.038
CS Kwan H Takada 2016 Release of additives and monomers from plastic wastes H Takada H Karapanagioti Eds Hazardous chemicals associated with plastics in the marine environment. The handbook of environmental chemistry 78 Springer Cham
K Tanaka H Takada R Yamashita K Mizukawa M Fukuwaka Y Watanuki 2015 Facilitated leaching of additive-derived PBDEs from plastic by seabirds’ stomach oil and accumulation in tissues Environ Sci Technol https://doi.org/10.1021/acs.est.5b01376
S Coffin GY Huang I Lee D Schlenk 2019 Fish and seabird gut conditions enhance desorption of estrogenic chemicals from commonly ingested plastic items Environ Sci Technol https://doi.org/10.1021/acs.est.8b07140
JH Bridson EC Gaugler DA Smith GL Northcott S Gaw 2021 Leaching and extraction of additives from plastic pollution to inform environmental risk: a multidisciplinary review of analytical approaches J Hazard Mater https://doi.org/10.1016/j.jhazmat.2021.125571
K Gunaalan E Fabbri M Capolupo 2020 The hidden threat of plastic leachates: a critical review on their impacts on aquatic organisms Water Res https://doi.org/10.1016/j.watres.2020.116170
M Esterhuizen SA Lee Y Kim R Järvinen Y Kim 2024 Ecotoxicological consequences of polystyrene naturally leached in pure, fresh, and saltwater: lethal and nonlethal toxicological responses in Daphnia magna and Artemia salina Front Mar Sci https://doi.org/10.3389/fmars.2024.1338872
H-X Li GJ Getzinger PL Ferguson B Orihuela M Zhu D Rittschof 2016 Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle Amphibalanus amphitrite Environ Sci Technol https://doi.org/10.1021/acs.est.5b02781
O Pikuda ER Dumont Q Chen JR Macairan SA Robinson D Berk N Tufenkji 2023 Toxicity of microplastics and nanoplastics to Daphnia magna: current status, knowledge gaps and future directions Trends Anal Chem https://doi.org/10.1016/j.trac.2023.117208
K Reilly LA Ellis HH Davoudi S Supian MT Maia GH Silva Z Guo DST Martinez I Lynch 2023 Daphnia as a model organism to probe biological responses to nanomaterials-from individual to population effects via adverse outcome pathways Front Toxicol https://doi.org/10.3389/ftox.2023.1178482
J Yin Y Long W Xiao D Liu Q Tian Y Li 2023 Ecotoxicology of microplastics in Daphnia: a review focusing on microplastic properties and multiscale attributes of Daphnia Ecotoxicol Environ Saf https://doi.org/10.1016/j.ecoenv.2022.114433
International Organization of Standardization (ISO) (2012) ISO 6341:2012 - Water quality - determination of the inhibition of the mobility of Daphnia magna straus (Cladocera, Crustacea) - acute toxicity test. International Standard ISO 6341 (3rd ed.). ISO, Geneva, Switzerland. https://www.iso.org/standard/54614.html. Accessed 12 Mar 2022.
OECD (Organisation for Economic Co-operation and Development) (2004) Test No. 202: Daphnia sp. Acute immobilisation test, OECD guidelines for the testing of chemicals. OECD Publishing: Paris
International Organization of Standardization (ISO) (2016) ISO 3310–1:2016 - test sieves — technical requirements and testing — part 1: test sieves of metal wirecloth. ISO, Geneva, Switzerland. https://www.iso.org/standard/14030.html. Accessed 16 Mar 2022.
MM Bradford 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem https://doi.org/10.1006/abio.1976.9999
M Esterhuizen MA Wolff von YJ Kim S Pflugmacher 2022 Ecotoxicological implications of leachates from concrete demolition debris on oligochaetes: survival and oxidative stress status Heliyon https://doi.org/10.1016/j.heliyon.2022.e11237
A Claiborne 1985 Catalase activity RA Greenwald Eds CRC handbook of methods for oxygen radical research CRC Press Boca Raton 283 284
W Habig MJ Pabst WB Jacoby 1974 Glutathione S-transferase: the first step in mercapturic acid formation J Biol Chem 249 1730 1739
RR Sokal FJ Rohlf 1987 Introduction to biostatistics Francise & Co New York
D Lithner M Halling G Dave 2012 Toxicity of electronic waste leachates to Daphnia magna: screening and toxicity identification evaluation of different products, components, and materials Arch Environ Contam Toxicol https://doi.org/10.1007/s00244-011-9729-0
L Kim D Kim SA Kim H Kim TY Lee YJ An 2022 Are your shoes safe for the environment?–Toxicity screening of leachates from microplastic fragments of shoe soles using freshwater organisms J Hazard Mater https://doi.org/10.1016/j.jhazmat.2021.126779
European Committee for Standardization (2002) Characterization of waste–leaching–compliance test for leaching of granular waste materials and sludges. Part 2: one stage batch test at a liquid to solid ratio of 10 L/kg for materials with particle size below 4 mm (without or with size reduction). EN 12457–2:2002. Brussels, Belgium
D Lithner I Nordensvan G Dave 2012 Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile–butadiene–styrene, and epoxy to Daphnia magna Environ Sci Pollut Res https://doi.org/10.1007/s11356-011-0663-5
S Bejgarn M MacLeod C Bogdal M Breitholtz 2015 Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes Chemosphere https://doi.org/10.1016/j.chemosphere.2015.03.010
A Cera G Cesarini M Scalici 2020 Microplastics in freshwater: what is the news from the world? Diversity https://doi.org/10.3390/d12070276
E Besseling P Redondo-Hasselerharm EM Foekema AA Koelmans 2019 Quantifying ecological risks of aquatic micro-and nanoplastic Crit Rev Environ Sci Technol https://doi.org/10.1080/10643389.2018.1531688
GF Schirinzi M Llorca R Seró E Moyano D Barceló E Abad M Farré 2019 Trace analysis of polystyrene microplastics in natural waters Chemosphere https://doi.org/10.1016/j.chemosphere.2019.07.052
D Lithner J Damberg G Dave Å Larsson 2009 Leachates from plastic consumer products-screening for toxicity with Daphnia magna Chemosphere https://doi.org/10.1016/j.chemosphere.2008.11.022
F Regoli ME Giuliani 2014 Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms Mar Environ Res https://doi.org/10.1016/j.marenvres.2013.07.006
AA Franco RS Odom TA Rando 1999 Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle Free Radical Biol Med https://doi.org/10.1016/S0891-5849(99)00166-5
Y Liu J Zhang H Zhao J Cai Y Sultan H Fang B Zhang J Ma 2022 Effects of polyvinyl chloride microplastics on reproduction, oxidative stress and reproduction and detoxification-related genes in Daphnia magna Comp Biochem Physiol Part C Toxicol Pharmacol https://doi.org/10.1016/j.cbpc.2022.109269
B Venkatesan L Mahimainathan F Das N Ghosh-Choudhury G Ghosh Choudhury 2007 Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells J Cell Physiol https://doi.org/10.1002/jcp.20953
S Schiavo M Oliviero S Chiavarini S Dumontet S Manzo 2021 Polyethylene, polystyrene, and polypropylene leachate impact upon marine microalgae Dunaliella tertiolecta J Toxicol Environ Health, Part A https://doi.org/10.1080/15287394.2020.1860173
Z Sunil J Thomas A Mukherjee N Chandrasekaran 2023 Microplastics and leachate materials from pharmaceutical bottle: an in vivo study in Donax faba (Marine Clam) Environ Toxicol Pharmacol https://doi.org/10.1016/j.etap.2023.104205
M Capolupo K Gunaalan AM Booth L Sørensen P Valbonesi E Fabbri 2021 The sub-lethal impact of plastic and tire rubber leachates on the Mediterranean mussel Mytilus galloprovincialis Environ Pollut https://doi.org/10.1016/j.envpol.2021.117081
D Keilin E Hartree 1934 Inhibitors of catalase reaction Nature https://doi.org/10.1038/134933b0
H Blaschko 1935 The mechanism of catalase inhibitions Biochem J https://doi.org/10.1042/bj0292303
MMHCM Valko H Morris MTD Cronin 2005 Metals, toxicity and oxidative stress Curr Med Chem https://doi.org/10.2174/0929867053764635
H Sies 1999 Glutathione and its role in cellular functions Free Radic Biol Med https://doi.org/10.1016/S0891-5849(99)00177-X
N Ahmad M Nasibullah F Hassan AK Singh DK Patel AR Khan M Rahman 2012 Heavy metal assessment of leachates of some plastic toys purchased from different districts of UP, India Internal Res J Environ Sci 1 32 36
A Turner M Filella 2021 Hazardous metal additives in plastics and their environmental impacts Environ Int https://doi.org/10.1016/j.envint.2021.106622
Acknowledgements
This work was partly supported by the Nanomaterial Technology Development Program (NRF-2017M3A7B6052455), funded by the South Korean Ministry of Science and ICT and the National Research Council of Science & Technology (Global-23-004) grant by the Strategies for Establishing Global Research Networks. The University of Helsinki Library provided open-access funding. We also wish to thank Julia Honkanen (UH) for assistance in the laboratory.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). Ministry of Science and ICT, South Korea, NRF-2017M3A7B6052455 and the South Korean Ministry of Science and ICT and the National Research Council of Science & Technology (Global-23-004).
Author information
Authors and Affiliations
Contributions
ME, SAL, YK, and YJK contributed to the study’s conception and design. ME and MM performed material preparation, experimentation, data collection, and analysis. ME wrote the first draft of the manuscript, and all authors commented and contributed to completing the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to publish
There is no need for consent to publish this paper.
Ethical approval
This article does not contain any studies with human subjects or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esterhuizen, M., Monticelli, M., Lee, SA. et al. Oxidative stress status and antioxidative responses in neonate versus adult Daphnia magna exposed to polystyrene leachate. Toxicol. Environ. Health Sci. 16, 171–179 (2024). https://doi.org/10.1007/s13530-024-00211-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-024-00211-1