Abstract
Objective
The present study aims to investigate early morphological and mineral density changes in bone tissues of Swiss albino mice, one month after the intravenous treatment of thorium [thorium nitrate (1 mg/100 µl); 40 mg/Kg)].
Methods
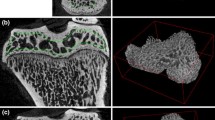
Synchrotron X-ray micro-tomographic imaging along with detailed quantitative analysis was applied to visualize and quantify morphological and mineral density changes in proximal and distal epiphysis regions of mice femur bone. Serum ALP analysis was done to assess the mechanisms of thorium-induced bone parameter changes.
Results
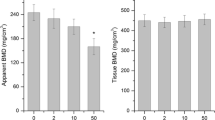
2D and 3D SR-µCT images are shown to depict morphological changes due to Th. Analysis of calibrated SR-µCT images shows the changes in trabecular bone thickness, volume fractions and connectivity and bone mineral density. The measured level of serum ALP showed a decrease in ALP level in thorium-treated mice.
Conclusions
Our study provides novel insights about the effect of thorium on mice bone tissues morphology and mineral density which may have significant implications in the study of mechanism of thorium interaction with bone tissues. The study also suggests the usefulness of SR-µCT technique for the characterization of heavy metal toxicity-induced structure and mineral density changes in bone samples.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [AKA] upon reasonable request.
References
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28:271–298. https://doi.org/10.1146/annurev.matsci.28.1.271
Koester KJ, Barth HD, Ritchie RO (2011) Effect of aging on the transverse toughness of human cortical bone: evaluation by R-curves. J Mech Behav Biomed Mater 4:1504–1513. https://doi.org/10.1016/j.jmbbm.2011.05.020
Patra JK, Das G, Fraceto LF et al (2018) Nano based drug delivery systems: Recent developments and future prospects 10 technology 1007 nanotechnology 03 chemical sciences 0306 physical chemistry (incl. structural) 03 chemical sciences 0303 macromolecular and materials chemistry 11 medical and he. J Nanobiotechnology 16:1–33. https://doi.org/10.1186/s12951-018-0392-8
Wu CT, Lu TY, Chan DC et al (2014) Effects of arsenic on osteoblast differentiation in vitro and on bone mineral density and microstructure in rats. Environ Health Perspect 122:559–565. https://doi.org/10.1289/ehp.1307832
Duranova H, Martiniakova M, Omelka R et al (2014) Changes in compact bone microstructure of rats subchronically exposed to cadmium. Acta Vet Scand 56:64. https://doi.org/10.1186/s13028-014-0064-0
Rodríguez J, Mandalunis PM (2018) A review of metal exposure and its effects on bone health. J Toxicol. https://doi.org/10.1155/2018/4854152
Lim H-S, Lee H-H, Kim T-H, Lee B-R (2016) Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J Bone Metab 23:223. https://doi.org/10.11005/jbm.2016.23.4.223
Anantharaman K, Shivakumar V, Saha D (2008) Utilisation of thorium in reactors. J Nucl Mater 383:119–121. https://doi.org/10.1016/j.jnucmat.2008.08.042
Schaffer MB (2013) Abundant thorium as an alternative nuclear fuel Important waste disposal and weapon proliferation advantages. Energy Policy 60:4–12. https://doi.org/10.1016/j.enpol.2013.04.062
Kumar A, Ali M, Mishra P et al (2009) Thorium-induced neurobehavioural and neurochemical alterations in Swiss mice. Int J Radiat Biol 85:338–347. https://doi.org/10.1080/09553000902781071
Kumar A, Mishra P, Ghosh S et al (2008) Thorium-induced oxidative stress mediated toxicity in mice and its abrogation by diethylenetriamine pentaacetate. Int J Radiat Biol 84:337–349. https://doi.org/10.1080/09553000801983133
Stehney AF, Lucas HF (2000) Thorium isotopes in autopsy samples from thorium workers. Health Phys 78:8–14. https://doi.org/10.1097/00004032-200001000-00003
Harrist TJ, Schiller AL, Trelstad RL et al (1979) Thorotrast-associated sarcoma of bone. A case report and review of the literature. Cancer 44:2049–2058. https://doi.org/10.1002/1097-0142(197912)44:6
Muramatsu Y, Ishikawa Y, Yoshida S, Mori T (1999) Determination of thorium in organs from Thorotrast patients by inductively coupled plasma mass spectroscopy and X-ray fluorescence. Radiat Res 152:S97. https://doi.org/10.2307/3580123
Warnakulasuriya T, Williams S, Dabarera M et al (2017) Frequency of micronuclei among persons resident in the vicinity of a mineral sand processing factory in Pulmoddai, Sri Lanka. Mutagenesis 32:511–516. https://doi.org/10.1093/MUTAGE/GEX019
Conibear SA (1983) Long term health effects of thorium compounds on exposed workers: the complete blood count. Health Phys 44(Suppl 1):231–237. https://doi.org/10.1097/00004032-198306001-00020
Chen XA, Cheng YE, Rong Z (2005) Recent results from a study of thorium lung burdens and health effects among miners in China. J Radiol Prot 25:451–460. https://doi.org/10.1088/0952-4746/25/4/007
Farid I, Conibear SA (1983) Hepatic function in previously exposed thorium refinery workers as compared to normal controls from the health and nutrition survey. Health Phys 44(Suppl 1):221–230. https://doi.org/10.1097/00004032-198306001-00019
Creff G, Safi S, Roques J et al (2016) Actinide(IV) deposits on bone: potential role of the osteopontin-thorium complex. Inorg Chem 55:29–36. https://doi.org/10.1021/acs.inorgchem.5b02349
Frantellizzi V, Cosma L, Brunotti G et al (2020) Targeted alpha therapy with thorium-227. Cancer Biother Radiopharm 35:437–445. https://doi.org/10.1089/CBR.2019.3105
Ogawa K, Washiyama K (2012) Bone target radiotracers for palliative therapy of bone metastases. Curr Med Chem 19:3290–3300. https://doi.org/10.2174/092986712801215865
Taylor GN, Jee WSS, Christensen WR et al (1966) Thorium-228 induced fractures in beagles. Health Phys 12:889–893. https://doi.org/10.1097/00004032-196607000-00002
Babosova R, Duranova H, Omelka R et al (2016) Structural changes in femoral bone microstructure of female rabbits after intramuscular administration of quercetin. Acta Vet Scand 58:1–6. https://doi.org/10.1186/s13028-016-0225-4
Freese M, Rizzo LY, Pohlmann JD et al (2019) Bone resorption and body reorganization during maturation induce maternal transfer of toxic metals in anguillid eels. Proc Natl Acad Sci USA 166:11339–11344. https://doi.org/10.1073/pnas.1817738116
Larrue A, Rattner A, Peter ZA et al (2011) Synchrotron radiation micro-CT at the micrometer scale for the analysis of the three-dimensional morphology of microcracks in human trabecular bone. PLoS ONE 6:e21297. https://doi.org/10.1371/journal.pone.0021297
Ma S, Boughton O, Karunaratne A et al (2016) Synchrotron imaging assessment of bone quality. Clin Rev Bone Miner Metab 14:150–160. https://doi.org/10.1007/s12018-016-9223-3
Bayat S, Apostol L, Boller E et al (2005) In vivo imaging of bone micro-architecture in mice with 3D synchrotron radiation micro-tomography. Nucl Instrum Methods Phys Res Sect A Accel Spectrom, Detect Assoc Equip 548:247–252. https://doi.org/10.1016/j.nima.2005.03.097
Tang L, Chen X, Bao Y et al (2016) CT imaging biomarkers of bone damage induced by environmental level of cadmium exposure in male rats. Biol Trace Elem Res 170:146–151. https://doi.org/10.1007/s12011-015-0447-8
Sankaramanivel S, Jeyapriya R, Hemalatha D et al (2006) Effect of chromium on vertebrae, femur and calvaria of adult male rats. Hum Exp Toxicol 25:311–318. https://doi.org/10.1191/0960327105ht627oa
Ha C, M Y, G H, et al (2013) Differential development of the distal and proximal femoral epiphysis and physis in mice. Bone 52:337–346. https://doi.org/10.1016/J.BONE.2012.10.011
Lavado-García JM, Puerto-Parejo LM, Roncero-Martín R, et al (2017) Dietary intake of cadmium, lead and mercury and its association with bone health in healthy premenopausal women. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph14121437
Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L (2008) Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol 231:34–42. https://doi.org/10.1016/j.taap.2008.03.017
Lavery TJ, Kemper CM, Sanderson K et al (2009) Heavy metal toxicity of kidney and bone tissues in South Australian adult bottlenose dolphins (Tursiops aduncus). Mar Environ Res 67:1–7. https://doi.org/10.1016/j.marenvres.2008.09.005
Rodríguez J, Mandalunis PM (2016) Effect of cadmium on bone tissue in growing animals. Exp Toxicol Pathol Off J Ges Toxikol Pathol 68:391–397. https://doi.org/10.1016/j.etp.2016.06.001
Monir AU, Gundberg CM, Yagerman SE et al (2010) The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone 47:888–894. https://doi.org/10.1016/j.bone.2010.07.013
Ishikawa Y, Humphreys JAH, Collier CG et al (1999) Revised organ partition of thorium-232 in thorotrast patients. Radiat Res 152:S102. https://doi.org/10.2307/3580124
Lucas LK, de Oliveira MS, de Alencar MAV (2019) Evaluation of the effect of three constituent metals of monazita on the radiosensibility of human osteoblasts. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2019.106011
Agrawal AK, Singh B, Kashyap YS et al (2015) Design, development and first experiments on the X-ray imaging beamline at Indus-2 synchrotron source RRCAT, India. J Synchrotron Radiat 22:1531–1539. https://doi.org/10.1107/S1600577515016276
Agrawal A, Singh B, Kashyap Y et al (2017) Synchrotron-based X-ray microimaging facility for biomedical research. J Radiat Cancer Res 8:153. https://doi.org/10.4103/jrcr.jrcr_29_17
Herman GT (2009) Filtered backprojection for parallel beams. In: Fundamentals of computerized tomography. Advances in pattern recognition. Springer, London. https://doi.org/10.1007/978-1-84628-723-7_8
Limaye A (2012) Drishti: a volume exploration and presentation tool. In: Stock SR (ed) Developments in X-ray tomography, vol VIII. SPIE, p 85060X
Ketcham RA, Ryan TM (2004) Quantification and visualization of anisotropy in trabecular bone. J Microsc 213:158–171. https://doi.org/10.1111/j.1365-2818.2004.01277.x
Chiba K, Nango N, Kubota S et al (2012) Relationship between microstructure and degree of mineralization in subchondral bone of osteoarthritis: a synchrotron radiation μCT study. J Bone Miner Res 27:1511–1517. https://doi.org/10.1002/jbmr.1609
Yang X, Shen X, Long J, Chen H (2012) An improved median-based Otsu image thresholding algorithm. AASRI Procedia 3:468–473. https://doi.org/10.1016/j.aasri.2012.11.074
Doube M, Klosowski MM, Arganda-Carreras I et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47:1076–1079. https://doi.org/10.1016/j.bone.2010.08.023
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
da Silva AMH, Alves JM, da Silva OL, da Silva Junior NF (2014) Two and three-dimensional morphometric analysis of trabecular bone using X-ray microtomography (μCT). Rev Bras Eng Biomed 30:93–101. https://doi.org/10.1590/rbeb.2014.011
Odgaard A (1997) Three-dimensional methods for quantification of cancellous bone architecture. Bone 20:315–328. https://doi.org/10.1016/S8756-3282(97)00007-0
Doube M (2015) The ellipsoid factor for quantification of rods, plates, and intermediate forms in 3D geometries. Front Endocrinol (Lausanne) 6:15. https://doi.org/10.3389/fendo.2015.00015
Costantini M, Colosi C, Mozetic P et al (2016) Correlation between porous texture and cell seeding efficiency of gas foaming and microfluidic foaming scaffolds. Mater Sci Eng C 62:668–677. https://doi.org/10.1016/j.msec.2016.02.010
Acknowledgements
The authors acknowledge Dr. S.M. Yusuf, Director Physics Group, & Dr. L. M. Pant Head, Technical Physics Division, for his support and encouragement in beamline development and utilization activities of imaging beamline. AKA acknowledges the support of Dr. Mayank Shukla for development activities of imaging beamline. Authors also acknowledge the RRCAT, Indore, particularly Indus synchrotron operation division for providing the beam time for the experiments. This project did not receive any specific grant from funding agencies
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Ashish K. Agrawal, Rakhee Yadav, Balwant Singh, Manjoor Ali, Amit Kumar, Yogesh Kashyap, and Badri N. Pandey declare that we have no conflict of interest.
Ethical approval
All the experiments were adhered to the guidelines of the Institutional Animal Ethics Committee of BARC regarding the experimental use of animals.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agrawal, A.K., Yadav, R., Singh, B. et al. Study of thorium-induced micro-structural changes in mice femoral bone using SR-µCT. Toxicol. Environ. Health Sci. 15, 399–410 (2023). https://doi.org/10.1007/s13530-023-00191-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00191-8