Abstract
Objective
Infertility is one of the common problems in today’s modern world, it is estimated that 50% of infertility problems are related to men. Infertility in men has different causes. Oxidative stress and testis inflammation play an important role in sperm damage and infertility in men. The aim of the present study was to investigate the effect of Lawsonia inermis (L. inermis) on the changes made on the testicular tissue and sperm following the administration of lipopolysaccharide (LPS).
Methods
In this project, 40 mice were divided into four equal groups: (1) control: Tween 1% gavage, (2) Tween 1% + LPS (1 mg/kg; IP) and 3 and 4) L. inermis extract (300 and 500 mg/kg) along with LPS. After 7 days of the experimental period, the animals were anesthetized, and immediately, general sperm analysis was performed. Serum testosterone level, total antioxidant capacity and testicular histological changes were also investigated.
Results
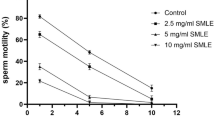
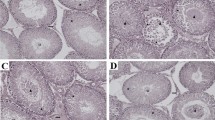
In examining the quality of sperms, it was observed that the number, motility and viability of sperms in the LPS group are significantly reduced compared to the sham group. Relative improvement in sperm parameters was observed in the LPS group treated with a dose of 500 mg/kg L. inermis extract. The percentage of inhibition of oxidants (DPPH) was significantly decreased in the LPS group compared to the sham group. L. inermis extract increased the level of DPPH in the LPS group. Serum testosterone level was significantly decreased in all LPS and treatment groups. Histological examination also showed abnormality in the germinal epithelium and a decrease in the number of sperm cells in the seminiferous tubules in the LPS group. In the group treated with 500 mg/kg L. inermis extract, the number of sperms increased, and the layers of germ cells became better organized. In other cell lines in the germinal epithelium and Leydig cells, no difference was observed between the groups.
Conclusion
In general, the results of this study showed that LPS decreases sperm quality and increases testicular tissue damage, and L. inermis extract probably by reducing oxidants improves the number, motility and viability of sperms and testicular tissue damage.



Similar content being viewed by others
References
Cox C, Thoma M, Tchangalova N, Mburu G, Bornstein M, Johnson C, Kiarie J (2022) Infertility prevalence and the methods of estimation from 1990 to 2021: a systematic review and meta-analysis. Hum Reprod Open 2022(4):hoac051
Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R (2018) Role of oxidative stress, infection and inflammation in male infertility. Andrologia 50(11):e13126. https://doi.org/10.1111/and.13126
Lykhmus O, Mishra N, Koval L, Kalashnyk O, Gergalova G, Uspenska K, Komisarenko S, Soreq H, Skok M (2016) Molecular mechanisms regulating LPS-induced inflammation in the brain. Front Mol Neurosci 9:19. https://doi.org/10.3389/fnmol.2016.00019
Liu XR, Wang YY, Dan XG, Kumar A, Ye TZ, Yu YY, Yang LG (2016) Anti-inflammatory potential of β-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod Toxicol 60:148–155. https://doi.org/10.1016/j.reprotox.2015.11.003
Palladino MA, Fasano GA, Patel D, Dugan C, London M (2018) Effects of lipopolysaccharide-induced inflammation on hypoxia and inflammatory gene expression pathways of the rat testis. Basic Clin Androl 28(1):12–21. https://doi.org/10.1016/j.reprotox.2015.11.003
Hadisi Z, Nourmohammadi J, Nassiri SM (2018) The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol 107:2008–2019. https://doi.org/10.1016/j.ijbiomac.2017.10.061
Al-Snafi A (2019) A review on Lawsonia inermis: a potential medicinal plant. Int J Curr Pharm 11(5):1–13. https://doi.org/10.22159/ijcpr.2019v11i5.35695
Chaudhary G, Goyal S, Poonia P (2010) Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharm Sci Drug Res 2(2):91–98. https://doi.org/10.22159/ijcpr.2019v11i5.35695
Selvanayaki R, Ananthi T (2012) Hepatoprotective activity of aqueous extract of Lawsonia inermis against paracetamol induced rats. AJPS 2(2):75–77
Olawuyi T, Oladipo G, Alabi A, Oyewopo A, Omotoso G (2018) Lawsonia inermis leaf extract mitigates aluminium-induced testicular toxicity in wistar rats: immunohistochemical study. J Malaria & Phytomedicine 2(2). https://www.researchgate.net/publication/330005813
Kaur M, Dangi C, Singhai A, Singh M, Kosta S, Singh H, Peter J, Jain S (2014) Toxicity profile of ethanolic extract of Lawsonia inermis leaves in albino Wistar rats. WJPPS 3:835–848
Alferah MA (2012) Toxicity induced histological changes in selected organs of male (Wistar) rats by Lawsonia inermis leaf extract. European J Med Plants 2(2):151–158. https://doi.org/10.9734/EJMP/2012/1061
Huang H, Liu T, Rose JL, Stevens RL, Hoyt DG (2007) Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J Inflamm 4(1):1–11. https://doi.org/10.1186/1476-9255-4-22
Nesa L, Munira S, Mollika S, Islam M, Chouduri AU, Naher N (2014) Evaluation of analgesic, anti-inflammatory and CNS depressant activities of methanolic extract of Lawsonia inermis barks in mice. AJP 4(4):287–296
Anvari M, Talebi AR, Mangoli E, Shahedi A, Ghasemi MR, Pourentezari M (2020) Effects of acrylamide in the presence of vitamin E on sperm parameters, chromatin quality, and testosterone levels in mice. CERM 47(2):101–107. https://doi.org/10.5653/cerm.2019.03230
Shahrokhi Raeini A, Hafizibarjin Z, Rezvani ME, Safari F, AfkhamiAghda F, ZareMehrjeri F (2020) Carvacrol suppresses learning and memory dysfunction and hippocampal damages caused by chronic cerebral hypoperfusion. Naunyn-Schmiedeb Arch Pharmacol 393(4):581–589. https://doi.org/10.1007/s00210-019-01754-8
Direkvand-Moghadam A, Sayehmiri K, Delpisheh A (2014) The global trend of infertility: an original review and meta-analysis. Int J Epidemiol 1(1):35–43
Metukuri MR, Reddy CMT, Reddy P, Reddanna P (2010) Bacterial LPS mediated acute inflammation-induced spermatogenic failure in rats: role of stress response proteins and mitochondrial dysfunction. Inflamm 33(4):235–243. https://doi.org/10.1007/s10753-009-9177-4
Ginsberg Y, Lotan P, Khatib N, Awad N, Errison S, Weiner Z, Maravi N, Ross MG, Itskovitz-Eldor J, Beloosesky R (2012) Maternal lipopolysaccharide alters the newborn oxidative stress and C-reactive protein levels in response to an inflammatory stress. J Dev Orig Health Dis 3(5):358–363. https://doi.org/10.1017/s204017441200027x
Herman AP, Krawczyńska A, Bochenek J, Dobek E, Herman A, Tomaszewska-Zaremba D (2013) LPS-induced inflammation potentiates the IL-1-mediated reduction of LH secretion from the anterior pituitary explants. Clin Dev Immuno. https://doi.org/10.1155/2013/926937
Dutta S, Sengupta P, Slama P, Roychoudhury S (2021) Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci 22(18):10043. https://doi.org/10.3390/ijms221810043
Solomon OT, Sunday OG, Stephen AA, Oyetunji OA, Olaiya OG (2018) Lawsonia inermis leaf extract mitigates aluminium-induced testicular toxicity in wistar rats: immunohistochemical study. J Malaria Phytomed 2(2):1–27
Olawuyi TS, Ukwenya VO, Jimoh AGA, Akinola KB (2019) Histomorphometric evaluation of seminiferous tubules and stereological assessment of germ cells in testes following administration of aqueous leaf-extract of Lawsonia inermis on aluminium-induced oxidative stress in adult Wistar rats. JBRA Assist Reprod 23(1):24–32. https://doi.org/10.5935/1518-0557.20180080
Hsouna AB, Trigui M, Culioli G, Blache Y, Jaoua S (2011) Antioxidant constituents from Lawsonia inermis leaves: isolation, structure elucidation and antioxidative capacity. Food Chem 125(1):193–200. https://doi.org/10.1016/j.foodchem.2010.08.060
Pasandi Pour A, Farahbakhsh H (2020) Lawsonia inermis L leaves aqueous extract as a natural antioxidant and antibacterial product. Nat Prod Res 34(23):3399–3403. https://doi.org/10.1080/14786419.2019.1569006
Zheng Y, Hu Y, Liu K, Lu Y, Zhou X (2019) Therapeutic effect of Impatiens balsamina, Lawsonia inermis L. and Henna on androgenetic alopecia in mice. J South Med Univ 39(11):1376–1380. https://doi.org/10.12122/j.issn.1673-4254.2019.11.17
Acknowledgements
The authors would like to thank the Department of Physiology of Shahid Sadoughi University of Medical Sciences for using the laboratory equipment of the department.
Funding
This research supported by Yazd Neuroendocrine Research Center which Shahid Sadoughi University of Medical Sciences and Health Services (Grant No.: 7169).
Author information
Authors and Affiliations
Contributions
FZM and FSP participated in the design, planning and management of the project. FSP and MPE did the research work. SMH actively participated in data analysis, and FZM contributed to interpretation and conclusion. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Fatemeh Shahidpour, Majid Pourentezari, Fatemeh Zare Mehrjerdi, and Mahmoud Hosseini declare that we have no conflict of interest.
Ethics approval
Ethical approval of all stages of this experiment was obtained from ethical committee of Shahid Sadoughi University of Medical Sciences and Health Services with ethical code: IR.SSU.MEDICINE.REC.1399.161.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahidpour, F., Pourentezari, M., Mehrjerdi, F.Z. et al. Lawsonia inermis improves sperm parameters and testicular tissue changes caused by lipopolysaccharide. Toxicol. Environ. Health Sci. 15, 267–273 (2023). https://doi.org/10.1007/s13530-023-00180-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00180-x