Abstract
Objective
The present investigation was undertaken for determination of LD50 and studying response exhibited by selected cultivars of pigeon pea for various seedling parameters.
Methods
Five promising cultivars of pigeon pea viz., ‘Manak,’ ‘PAU 881,’ ‘Pusa 992,’ ‘PA 291’ and ‘VLA 1’ were induced with different doses of sodium azide (SA). SA was diluted to different concentrations (1 mM, 2 mM, 3 mM, 4 mM and 5 mM) in 0.1 M phosphate buffer of pH 3.0. Seeds were soaked in each concentration of mutagen for 6 h followed by germinating treated seeds in towel paper. Observations regarding various parameters were collected according to International Seed Testing Association (ISTA) guidelines. Probit analysis based on seed germination percentage was done for determination of LD50.
Results
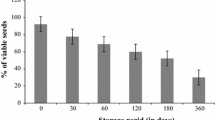
The results revealed that cultivars exhibited differential response for seedling parameters when induced with varying concentrations of SA. LD50 ranged between 1.60 mM and 2.69 mM SA. The cultivar ‘Manak’ exhibited the highest degree of tolerance against inhibitory effect of SA whereas ‘VLA 1’ was the most sensitive cultivar as it had the lowest LD50. Seed germination and other seedling parameters followed a general repressive trend with a related increase in concentrations of SA with quite a few exceptions. Estimates of cultivar ‘Pusa 992’ were not fully consistent with the repressive trend for a few seedling parameters.
Conclusion
The present study strengthened the fact that interaction between particular genotype and specific mutagen exclusively determines the rate of mutation and chances of obtaining viable, distinctive and favorable mutants to diversify the breeding material. The findings of the present investigation will serve as a benchmark for growers and plant breeders who intend to produce fruitful mutations to increase genetic variability in the primary gene pool of pigeon pea. Further, it is recommended to carry out respective field evaluation to screen out favorable mutants with higher crop value.


Similar content being viewed by others
References
Saxena KB, Singh L, Gupta MD (1990) Variation for natural out-crossing in pigeonpea. Euphytica 46:143–146
Greilhuber J, Obermayer R (1998) Genome size variation in Cajanus cajan (Fabaceae): a reconsideration. Plant Syst Evol 212:135–141
Saxena KB, Kumar RV, Rao PV (2002) Pigeonpea nutrition and its improvement. In: Basra AS, Randhawa IS (eds) Quality improvement in field crops. Food Products Press, pp 227–260
Saxena KB, Kumar RV, Sultana R (2010) Quality nutrition through pigeonpea—a review. Health 2:1335–1344
AICRP on Pigeonpea (2020). Project coordinator’s report (Kharif crops), 2019–20, All India Coordinated Research Project on Pigeonpea, ICAR-Indian Institute of Pulses Research, Kanpur, India
FAOSTAT (2019) Statistical Database, FAO, Rome. http://faostat.fao.org (Accessed on April 25, 2021)
Saxena RKV, Gowda CL, Hari D (2013) Upadhyaya, Shivali Sharma, KN Reddy, Rachit. Genetic and Genomic Resources of Grain Legume Improvement, 181
Pathirana R (2011) Plant mutation breeding in agriculture. Plant Sci Rev 6:107–126
IAEA mutant database. International Atomic Energy Agency, Vienna; 2021. Available from: URL: http://mvgs.iaea.org/Search.aspx (Accessed 9 January 2021)
Feldmann KA, Malmberg RJ, Dean C (1994) Mutagenesis in Arabidopsis. In: Meyerowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 137–172
Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M (1998) Arabidopsis thaliana: a model plant for genome analysis. Science 282:679–682
Khan S, Wani MR, Parveen K (2006) Sodium azide induced high yielding early mutant in lentil. Agricul Sci Dig 26:65–66
Sidduqui S, Meghvansi MK, Hasan Z (2007) Cytogenetic changes induced by sodium azide (NaN3) on Trigonella foenum-graecum L. seeds. S Afr J Bot 73:632–635
Kulthe MP, Kothekar VS (2011) Effects of sodium azide on yield parameters of chickpea (Cicer arietinum L.). J Phytol 3:39–42
Srivastava P, Marker S, Pandey P, Tiwari DK (2011) Mutagenic effects of sodium azide on the growth and yield characteristics in wheat (Triticum aestivum L. em. Thell.). Asian J Plant Sci 10:190–201
Elfeky S, Abo-Hamad S, Saad-Allah KM (2014) Physiological impact of sodium azide on Helianthus annuus seedlings. Int J Agron Agric Res 4:102–109
Eze JJ, Dambo A (2015) Mutagenic effects of sodium azide on the quality of maize seeds. J Adv Lab Res Biol 6:77–82
El-Kaaby EJS, Al-Ajeel SA, Al-Anny JA, Al-Aubaidy AA, Ammar K (2015) Effect of the chemical mutagen sodium azide on plant regeneration of two tomato cultivars under salinity stress condition in vitro. J Life Sci 9:27–31
Herwibawa B, Kusmiyati F (2017) Mutagenic effects of sodium azide on the germination in rice (Oryza sativa L. cv. Inpago Unsoed 1). Jurnal Agroteknologi 7:9–14
Hussain S, Khan WM, Khan MS, Akhtar N, Umar N, Ali S, Ahmed S, Shah SS (2017) Mutagenic effect of sodium azide (NaN3) on M2 generation of Brassica napus L. (variety Dunkled). Pure Appl Biol 6:226–236
Singh C, Olejniczak J (1983) Modification of mutagenic efficiency of sodium azide. Cytologia 48:437–444
Owais WM, Kleinhofs A (1988) Metabolic activation of the mutagen azide in biological systems. Mutat Res 197:313–323
Sangle SM, Mahamune SE, Kharat SN, Kothekar VS (2011) Effect of mutagenisis on germination and pollen sterility in pigeonpea. Biosci Discov J 2:127–130
Sangle SM (2018) Induction of some promising mutants in pigeon pea. Int J Res Anal Rev 5:585–589
Mathew BA, Sule HA, Toluhi OJ, Idachaba SO, Ibrahim AA, Abuh SJ (2015) Studies on protein composition of pigeon pea [Cajanus Cajan (L.) Millspaugh] treated with sodium azide and gamma radiation. J Pharm Biol Sci 10:01–04
Sangle SM (2020) Induced genetic variability for quantitative traits in Pigeonpea. Indian J Agric Res 54:139–146
Gad SC (2014) LD50/LC50 (Lethal Dosage 50/Lethal Concentration 50). In: Wexler P (ed) Encyclopedia of Toxicology, 3rd edn. Academic Press, New York, pp 58–60
Kodym A, Afza R, Forster BP, Ukai Y, Nakagawa H, Mba C (2012) Methodology for physical and chemical mutagenic treatments. In: Plant mutation breeding and biotechnology, pp 169–180
Sikder S, Biswas P, Hazra P, Akhtar S, Chattopadhyay A, Badigannavar AM, D’Souza SF (2013) Induction of mutation in tomato (Solanum lycopersicum L.) by gamma irradiation and EMS. Indian J Genet Plant Breed 73:392–399
Yadav P, Meena HS, Meena PD, Kumar A, Gupta R, Jambhulkar S, Rani R, Singh D (2016) Determination of LD50 of ethyl methanesulfonate (EMS) for induction of mutations in rapeseed-mustard. J Oilseed Brassica 1:77–82
Raina A, Khursheed S, Khan S (2018) Optimisation of mutagen doses for gamma rays and sodium azide in cowpea genotypes. Trends Biosci 11:2386–2389
Nepolian T, Jeberson MS, Singh NB, Sharma PR, Shashidhar KS (2018) Study of biological effects of Sodium Azide in M1 generation and estimation of LD50 for growth and reproduction parameters. Pharma Innova J 7:291–293
Cabahug RAM, Ha MKTT, Lim KB, Hwang YJ (2020) LD50 determination and phenotypic evaluation of three Echeveria varieties induced by chemical mutagens. Toxicol Environ Health Sci 12:1–9
Giri SP (2014) Studies of mutagenic sensitivity in pigeonpea [Cajanus cajan (L.) Mill sp.]. Biosci Discov 5:227–229
Saroj SK, Poudel PP, Singh MN (2016) Induced genetic variability with EMS and studies on frequency and spectrum of chlorophyll mutations in Pigeonpea. Electron J Plant Breed 7:209–214
Ariraman M, Gnanamurthy S, Dhanavel D, Bharathi T, Murugan S (2014) Mutagenic effect on seed germination, seedling growth and seedling survival of Pigeon pea (Cajanus cajan (L.) Millsp). Int Lett Nat Sci 21:41–49
Herwibawa B (2018) The effects of sodium azide on seed germination and seedling growth of chili pepper (Capsicum annum L. CV. Landung). In IOP Conference Series: Earth and Environmental Science, Vol 102, No 1, pp 012052
Haliloğlu K, Kaysim M (2019) Determination LD50 of Sodium Azide (NaN3) for Induction Mutations in Safflower Seed (Carthamus tinctorius L.). In: 4th International conference on advances in natural & applied sciences, pp 567
Kolhe PN, Varne MD, Deshmukh SN, Harke SN, Wagh SG (2020) Assessment of mutagenicity induced by different mutagens in coriander (Coriandrum sativum L.). Biotechnol J Int 24:12–21
Awan MA, Konzak CF, Rutger JN, Nilan RA (1980) Mutagenic effects of Sodium Azide in rice. Crop Sci 20:663–668
Mostafa GG (2011) Effect of sodium azide on the growth and variability induction in Helianthus annuus L. Int J Plant Breed Genet 5:76–85
Ali A, Yubey K, Deka UK, Tomar SMS (2014) Effect of sodium azide on seed germination and related agro-metrical traits in M1 lentil (Lens culinaris Medik.) generation. World J Agric Sci 10:95–102
Munir N, Safdar I, Naz S (2015) Effect of induced mutation for varietal improvement in some local grapevine cultivars. J Anim Plant Sci 25:234–242
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Chrispeels MJ, Varner JE (1967) Gibberellic acid enhanced synthesis and release of α-amylase and ribonuclease by isolated barley aleurone layers. Plant Physiol 42:398–406
Sato M, Gaul H (1967) Effect of EMS on fertility in barley. Radiation Bot 7:7–10
Ananthaswamy HN, Vakil UK, Sreenivasan A (1971) Biochemical and physiological changes in gamma-irradiated wheat during germination. Radiat Bot 11:1–12
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Acknowledgements
The authors duly acknowledge the Department of Genetics and Plant Breeding, CCS HAU for providing research funds and facilities for the smooth conduct of the experiment. There was no specific funding for this work. All authors contributed equally to the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lakshmi Chaudhary, Rajat Sharma, and Mukesh Kumar declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chaudhary, L., Sharma, R. & Kumar, M. Estimation of LD50 and effect of sodium azide on germination and seedling parameters of different cultivars of Cajanus cajan (L.) Millspaugh. Toxicol. Environ. Health Sci. 13, 279–285 (2021). https://doi.org/10.1007/s13530-021-00105-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-021-00105-6