Abstract
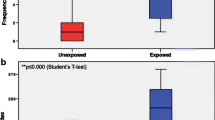
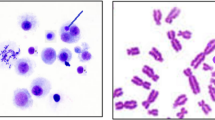
Chemical hazard evaluations are important for work-ers’ health and working environments. tert-Butyl methacrylate (CAS No. 585-07-9) is used in many in-dustries, leading to concerns about the possibility of threats to the health of workers. Since only insufficient or controversial information is available about potential related hazards, Ames assay and in vitro mammalian chromosomal aberration (CA) assay were conducted in order to gain additional information concerning any such hazards. Moreover, toxicologi- cal information from this study could be applied for workers’ rights as background knowledge, and for the preparation or update of the Materials Safety Data Sheet (MSDS) for a number of industries. In this Ames reverse mutation study, treatment of 1,250, 625, 312.5, 156.25, 78.125 pg/plate did not induce mutagenicity in Salmonella typhimurium TA98, TA100, TA1535, TA1537, or in Escherichia coli WP2uvrA with and without metabolic activation. In vitro chromosomal aberration assay was performed using the Chinese hamster lung fibroblast cell (ATCC, CRL-1935) by the direct method (− S9) and by the metabolic activated method (+S9 mix) at a dose of 0.039, 0.078, 0.156, 0.312, 0.625, 1.25, 2.5 mg/mL. Using the direct method, the seven dosages in the 48-hour treatment group did not show that the frequency of CA was proportional to the dosage. The frequency of CA is not proportional to the dosage addition for a six-hour treatment using the metabolic activated method. From these findings, the chemical does not have mutagenic potential under the conditions of the Ames assay, but also does not induce chromosomal aberrations under the tested conditions. However, further investigations are necessary because it may be harmful to workers after ingestion, inhalation, skin or eye contact. It is also desirable to prepare a local exhaust system and to wear personal protection equipment.
Similar content being viewed by others
References
European Chemicals Agency (ECHA), http://echa. europa.eu/registration-dossier/-/registered-dossier/2073/1 (2016).
Toxic Substance Control Act Test Submissions (EPA TSCATS), http://yosemite.epa.gov/oppts/epatscat8.nsf/ReportSearch?OpenForm (2016).
United States Environmental Protection Agency (EPA), Aggregated Computational Toxicology Resource (ACToR), http://actor.epa.gov/actor/faces/ACToRHome. jsp (2016).
Waegemaekers, T. H. J. M. & Bensink, M. P. M. Nonmutagenicity of 27 aliphatic acrylate esters in the Salmonella microsome test. Mutat. Res. 137, 95–102 (1984).
European Chemicals Agency (ECHA), http://echa. europa.eu/registration-dossier/-/registered-dossier/2073/7/7/2 (2016).
Organization for Economic Co-operation and Development (OECD), Guideline for the testing of chemicals. Test guideline 487 OECD 487 In Vitro Mammalian Cell Micronucleus Test (2010).
Erickson, R. P. Somatic gene mutation and human disease other than cancer: an update. Mutat. Res. 705, 96–106 (2010).
Frank, S. A. Evolution in health and medicine Sackler colloquium: somatic evolutionary genomics: mutations during development cause highly variable genetic mosaicism with risk of cancer and neurodegeneration. Proc. Natl. Acad. Sci. USA 107, 1725–1730 (2010).
Slatter, M. A. & Gennery, A. R. Primary immunodeficiencies associated with DNA-repair disorders. Expert. Rev. Mol. Med. 685, 146–165 (2010).
Bender, M. A., Griggs, H. G. & Bedford, J. S. Mechanisms of chromosomal aberration production. III. Chemicals and ionising radiation. Mutat. Res. 23, 197–212 (1974).
Evans, H. J. Chromatid aberrations induced by gamma irradiation. I. The structure and frequency of chromatid interchanges in diploid and tetraploid cells of Vicia faba. Genetics 46, 257–275 (1961).
Kirkland, D., Aardema, M., Henderson, L. & Muller, L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity. specificity and relative predictivity. Mutat. Res. 584, 1–256 (2005).
Benigni, R., Bossa, C. & Worth, A. Structural analysis and predictive value of the rodent in vivo micronucleus assay results. Mutagenesis 25, 335–341 (2010).
European Chemicals Agency (ECHA), http://echa. europa.eu/registration-dossier/-/registered-dossier/2073/9 (2016).
Organization for Economic Co-operation and Development (OECD), Guideline for testing of chemicals. Proposal for updating test guideline 473 In vitro Mammalian Chromosome Aberration Test. Adopted: 21st July 1997 (2012).
Ishidate, M. & Sofuni, T. The in vitro chromosomal aberration test using Chinese Hamster Lung (CHL) fibroblast cells in culture. In: Ashby J. editor. Progress in mutation research. Vol 5. New York: Elsevier Science Publishers, 427–432 (1985).
Chaudhary, M. & Payasi, A. Evaluation of genotoxicity of CVA1020 through Ames and in vitro chromosomal aberration tests. Br. J. Pharmacol. Toxicol. 4, 95–100 (2013).
Dusinska, M. et al. Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis 18, 371–376 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rim, KT., Kim, SJ. Genotoxic evaluation of tert-butyl methacrylate for workers’ health with bacterial reverse mutation (Ames) and in vitro mammalian chromosomal aberration assay. Toxicol. Environ. Health Sci. 8, 168–180 (2016). https://doi.org/10.1007/s13530-016-0274-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-016-0274-0