Abstract
Background
Time in range is a reliable measure of the risk of diabetes complications. High percentage of patients with diabetes fail to achieve the recommended time in range (TIR) target of 70–180 mg/dl (3.9–10 mmol/l) >70%.
Objective
This study aimed to identify factors influencing TIR prolongation.
Methods
Children aged 1–17 years with >1-year type 1 diabetes (T1D) duration, treated with continuous subcutaneous insulin infusion (CSII) ≥3 months, using continuous glucose monitoring (CGM) or intermittently scanned CGM (is-CGM) ≥1 month, and with a registration time >70% were included. Data were collected during routine diabetology visits at an outpatient clinic. Insulin pump and CGM or is-CGM reports in the most recent 14 days were recorded using a dedicated software. Legal caregivers were also asked to complete a questionnaire on how the patients use the insulin pump functions and eating habits.
Results
A sample of 110 patients was categorized into two groups: those with TIR >70% and TIR ≤70%. TIR ≤70% group presented with repeated hyperglycemia and a high glycemic variability coefficient of variation. We noted an acceptable hypoglycemia rate (3%), regardless of the TIR value. Patients with TIR >70% predominantly used predictive low glucose suspend system, maintained adequate intervals between insulin delivery and meal consumption, used the “bolus calculator” function, and more frequently created electronic reports.
Conclusions
Hyperglycemia and high glycemic variability prevent patients from achieving the target TIR. Advanced features in the CGM systems, premeal insulin bolus, and patients’ involvement in diabetes treatment are the main factors contributing to TIR prolongation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the US Type 1 Diabetes (T1D) Exchange Registry, continuous glucose monitoring (CGM) systems are currently the fastest growing technology for diabetes treatment [1]. Numerous benefits of CGM have been demonstrated; it has led to a reduction in the incidence of hypoglycemia and a decrease in glycated hemoglobin (HbA1c) levels up to 2.05% and reduce glycemic variability [2,3,4]. The increasing accuracy and advancement of CGM systems and their widespread availability have resulted in new indicators of proper glycemic control [5, 6].
In 2019, Battelino et al. published an international consensus on glycemic targets for patients using the CGM systems (Table 1), which has been accepted by many international societies, including the Polish Diabetes Association [6, 7]. The concept of the time spent in the target range of 70–180 mg/dl (3.9–10.0 mmol/l) or the time in range (TIR) warrants special attention. TIR was validated as an outcome measure in clinical trials complementing other components of glycemic control [8]. TIR is strongly associated with the occurrence of vascular complications and peripheral neuropathy [8,9,10,11]. Each 10% increase in TIR is associated with a 64% reduction in the risk of retinopathy and a 40% reduction in the risk of microalbuminuria [8]. TIR and HbA1c levels are correlated; each 10% increase in TIR is associated with a 0.6–0.8% decrease in HbA1c levels [8, 12, 13]. However, HbA1c levels may be affected by many conditions that influence the survival of the red blood cells independent of glycemia, including the glycation rates, uremia, pregnancy, smoking, and ethnicity [8]. Therefore, TIR is a more reliable measure of the risk of diabetes complications [13]. High percentage of patients with diabetes fail to achieve the recommended TIR target of >70%. Diabetes Control and Complications Trial (DCCT) obtained data from 1440 participants and demonstrated that a TIR is relatively low among patients with diabetes (52% vs. 31% for intensive vs. conventional treatment, respectively) [9]. Data from the Swedish Childhood Diabetes Registry revealed a mean TIR of 60.8% (±13.1%) [14].
This study aimed to identify factors that influence TIR prolongation, using CGM data among the pediatric population with T1D, on continuous subcutaneous insulin infusion (CSII).
Materials and Methods
The patients were recruited from the Department of Pediatric Diabetology and the Diabetic Outpatient Clinic at the Clinical Hospital. The study group were children aged 1–17 years with a >1-year T1D duration, treated with CSII ≥3 months, used CGM or intermittently scanned CGM (is-CGM) ≥1 month, and with registration time >70% (the percentage of time CGM is active, from the last 14 days) [6]. No restrictions were imposed on participation with respect to the type of CGM and is-CGM. Participants used the following devices: Medtronic Minimed: Guardian™ Sensor 3 with Guardian™ Link 3 transmitter (GL3); Sensor Enlite™ with Guardian™ 2 Link transmitter (GL2) or Guardian™ Connect (GC); Dexcom Inc: Dexcom G6 and Dexcom G5; and Abbott Diabetes Care: Free Style Libre (FSL). The records from the CGM system were registered using the dedicated software.
Polish citizens have equal access to healthcare services provided by the National Health Insurance System and managed by the National Health Fund. Treatment with insulin pumps is available and unpaid for, for children up to 26 years of age with T1D. The patients had access to insulin pumps Medtronic Minimed: Paradigm VEO, G640 and Roche Diabetes Care Accu-Chek Combo, free of charge.
The patients were under constant care at the outpatient clinic and had permanent access to medical assistance. Data were collected during routine clinical visit from January to April 2021. The study flow diagram is presented in Fig. 1. Insulin pump and CGM or is-CGM data were recorded using a dedicated software. CGM metrics were analyzed in the most recent 14 days, as per the recommendations of International Consensus on Time in Range [6, 8]. Moreover, legal caregivers were asked to complete a questionnaire (Appendix 1) on how the patients use the insulin pump functions and eating habits. Severe hypoglycemia was defined as an event with severe cognitive impairment (including coma and convulsions) requiring assistance by another person.
Anthropometric measurements (weight and height) were taken to calculate the body mass index standard deviation score (BMI-SDS), which was calculated using the World Health Organization child growth standards. A blood sample was taken for determining HbA1c levels. The test was performed at the hospital laboratory using a high-performance liquid chromatography (D-10 Hemoglobin Testing System, Bio-Rad Laboratories, USA) at a nondiabetic range of 4.1–6.4% (21–46 mmol/mol).
Statistical analysis
The sample of 110 patients was grouped into two categories: those with TIR >70% (study group, n = 50) and those with TIR ≤70% (control group, n = 60). Nominal variables were presented as frequencies and percentages. Numeric variables were described using basic descriptive statistics, depending on the distribution (for those with normally distributed variables, mean ± standard deviation were reported; for other distributions, the median along with the first and third quartiles are reported). The normality of variable distribution as well as skewness and kurtosis were verified using Shapiro-Wilk’s test. Homogeneity of variance was checked using Levene’s test.
Groups were compared using Pearson’s chi-square test, or Fisher’s exact test in case of categorical variables. For assessing group similarity with numeric variables, the independent Student t-test, independent Welch’s t-test, or Mann-Whitney U test were used, as appropriate. Assessment of group differences was additionally described using mean/median difference or risk ratio, with 95% confidence intervals (CIs).
Univariate logistic regression analyses were run for all variables to assess the impact of each factor on the risk of not exceeding 70% TIR. An additional multivariate logistic regression analysis was executed and presented to describe the simultaneous impact of selected variables. The variables were selected based on the significance level reached in the univariate model as well as the relationship between variables. Model quality assessment included chi-square test, Hosmer and Lemeshow goodness of fit (GOF) test, and R2 Nagelkerke as well as variance inflation factor (VIF) measures. All calculations were conducted assuming 0.05 significance level and run using R software, version 4.1.2 [15].
Univariate logistic regression analyses were performed for all independent variables separately. Odds ratios (ORs) and their 95% CIs were presented to determine the odds of patients’ TIR not exceeding the level of 70%. In the second step, a multivariate model including selected variables was built. Variables included in the second step were selected based on the significance level in the univariate models and on the relationships between the variables.
Results
Characteristic of the group
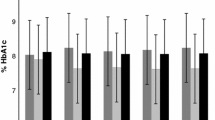
The sample of 110 patients was grouped into study and control groups with TIR >70% (study group, n = 50) and TIR ≤70% (control group, n = 60), respectively; both the groups had an equal sex distribution (50.0% each) and all participants were of Caucasian descent. Average age at diagnosis in the cohort was 7.06 ± 3.74 years, with no statistical difference between groups (p = 0.057). Significant difference occurred in the duration of the disease (p = 0.002), which was 3 years (median = 3.19) in the study group and 4 years (median = 4.34) in control group (median difference, MD = −1.15, 95% CIs [−2.58; −0.15], p = 0.002). Characteristic of the analyzed groups is presented in Table 2.
Patients had CSII implemented just after diabetes diagnosis without differences between groups (p = 0.324). Medtronic Paradigm VEO (49.1%) was the most common type of insulin pump used. There was no difference in insulin types (p = 0.224) but infusion set type differed significantly between groups (p = 0.014). Ninety-degree teflon cannulas were used by majority of the participants in the study group (72.0%). Majority of the patients (78.2%) reported exchanging the infusion set every 3 days. There was no statistical difference between the frequency of replacement of infusion sets between the two groups (p = 0.559). Median time of CGM system usage was 763.50 days and did not differ between the groups (p = 0.318). However, the groups used different types of CGM (p = 0.022). In the study group, most of the patients used GL3 (32.0%); in the control group, more than half (60.0%) the patients used FSL.
There was a significant difference (p = 0.018) between the study group (74.0%) and the control group (50%) in the number of patients who generated electronic reports based on CGM and insulin pump data in a domestic environment. Those who were unable to create electronic reports at home had 56% higher chance of being in TIR ≤70% group (RR = 1.56, 95% CIs = [1.12; 2.17]). The study group patients were more likely to use the “bolus calculator” function (56.0% vs. 33.3%, respectively). The lack of function usage tended to increase the chances of lower TIR by 55% (RR = 1.55, 95% CIs = [1.06; 2.27]). There was no difference in the proportion of patients counting their carbohydrate and fat-protein units (FPU) between the two groups (p = 0.374 and p = 0.371, respectively). Carbohydrate exchanges were calculated by almost all patients (95.5%), while FPU were considered by more than half of them (56.4%).
The patients in the study group were more disciplined with regard to the latency period between insulin delivery and meal consumption (72.0%), when compared to the control group (41.7%, p < 0.001). Not maintaining a latency period between insulin delivery and meal consumption was correlated with an 88% higher chance of not reaching the 70% TIR level (RR = 1.88, 95% CIs = [1.33; 2.66], p = 0.001). Diabetic ketoacidosis (DKA) and severe hypoglycemic episodes were rare in both groups with no differences.
Data from CGM, except for time below the <70 mg/dl range, differed significantly between groups. Average glucose was higher in the control group and the difference ranged from MD = −1.00, 95% CIs [−1.00; −0.50], p = 0.036 for time spent below target glycemia (TBR, time below range) <54 mg/dl to MD = −13.78, 95% CIs [−16.36; −11.20], p < 0.001 for time spent above the target blood glucose level (TAR) >180 mg/dl. The total daily dose of insulin per kilogram (TDD) was significantly different between the two groups (MD = −0.12, 95% CIs [−0.20; −0.03], p = 0.007). Also, the level of basal insulin rate (BIR) per kilogram differed between the groups (MD = −0.07, 95% CIs [−0.11; −0.02], p = 0.005).
Logistic regression results
The longer disease duration is correlated with the worse TIR and one additional year increased the risk of not reaching 70% TIR by 20% (OR = 1.20, 95% CIs [1.06; 1.37], p = 0.007). Use of metal cannulas increased the risk of TIR ≤70% by three times, compared to usage of 90° teflon infusion sets (OR = 3.23, 95% CIs [1.37; 8.04], p = 0.009). The lack of both domestic report generation and usage of “bolus calculator” function almost tripled the risk of TIR ≤70% (OR = 2.85, 95% CIs [1.29; 6.55], p = 0.011 and OR = 2.55, 95% CIs [1.18; 5.60], p = 0.018, respectively). Lack of keeping a latency period between insulin delivery and meal consumption increased the risk by four times (OR = 4.43, 95% CIs [1.98; 10.45], p < 0.001). The HbA1c levels had a strong impact on the risk, with a 1% increase in HbA1c levels relating to a 161 times higher risk (OR = 161.93, 95% CIs [27.14; 1852.76], p < 0.001). Analyzing the CGM data, the strongest impact on the risk was noted for TAR >250 mg/dl; for every 1 pp rise, the risk increased four times (OR = 3.89, 95% CIs [2.31; 9.20], p < 0.001). Additionally, there was a significant risk of having a TIR ≤70% when both TDD and BIR increased with an impact of 11 and 101 times, respectively (TDD: OR = 11.75, 95% CIs [2.00 to 82.70], p = 0.009, BIR: OR = 101.08, 95% CIs [3.93; 3443.02], p = 0.007).
The multivariate model verified the simultaneous impact of HbA1c levels and different latency period categories on the risk of TIR ≤70%. The model identified high impact of additional 1 pp of HbA1c and the risk increased 278 times (OR = 278.81, 95% CIs [35.56 to 4915.73], p < 0.001). Further, maintaining a latency period of 5 or 10 min between insulin delivery and meal consumption reduced the risk by 95% (vs. not waiting). These showed OR = 0.05, 95% CIs [0.00 to 0.37], p = 0.007 for 5 min latency and OR = 0.05, 95% CIs [0.00 to 0.60], p = 0.036 for 10 min latency (Appendix 2).
Discussion
This study attempted to identify factors that contributed to prolonged TIR in children and adolescents using CSII. We found that the main problem of patients not achieving the >70% target range included repeated hyperglycemia and high glycemic variability defined by coefficient of variation (CV). Participants achieving TIR >70% predominantly used predictive low glucose suspend systems, maintained adequate interval between insulin delivery and meal consumption, used bolus calculator, and more frequently created electronic reports. Moreover, in this group, we observed lower daily and basal insulin requirements.
Furthermore, disease duration >3 years in our study participants lowered the probability of exceeding 70% TIR, and one additional year of diabetes duration increased the risk by 20%. The diabetes duration in the group was not long (median, 3 and 4 years for study and control groups, respectively) and the whole cohort had good metabolic control as assessed by HbA1c levels (median 7.08%; 54 mmol/mol). The possible reason for this trend is the effect of the patients’ age (median age, 12 years); when they become adolescents, the glycemic control worsens compared to childhood. Adolescents have the highest glycemic variability and poorest metabolic control (especially those aged 13–18 years) [16, 17].
We found no differences between the insulin analogs used by the study participants. There is a huge interest now on faster acting insulin analogs. As demonstrated, faster acting insulin aspart (faster aspart) used in children prolonged TIR (38% vs. 50%) and is more effective than insulin aspart in reducing postprandial hyperglycemia during the first and second hour after consuming a meal [18, 19]. We did not note in our study group that more participants used faster aspart. We also found no influence of the type of insulin pump on the TIR.
Previous studies have not reported differences between steel and teflon infusion sets in their function over 7 days [20]. In this study, we found that teflon cannulas correlated with better TIR. The possible cause of the observed trend is the predominant use of teflon cannulas in our diabetology center, thus making them the first choice for most patients.
The general rule concerning length of use is 2 and 3 days for steel and teflon infusion sets, respectively [21, 22]. Our study participants declared changing their infusion sets regularly (78.2% declared every 3-day exchange), without differences between the two groups.
We recruited participants who used both types of CGM: real-time CGM (RT-CGM) and is-CGM. Some RT-CGMs work with insulin pump and have the following additional features: predictive low glucose suspend (PLGS) or low glucose suspend (LGS), that influence metabolic control. Among our study group, patients with TIR >70% predominantly used the PLGS system (GL3). We noted that not all participants who had the opportunity of using PLGS system took advantage of it. Thirty-four participants (in both groups) used Medtronic G640 insulin pump, but only 28 used compatible CGM system (GL3) with PLGS function. The possible cause of that is dissatisfaction with the CGM system due to inaccurate blood glucose measurements, need for calibration, and/or lack of mobile phone application. We observe that parents having younger children prefer the CGM system with a mobile phone application to manage diabetes remotely as it increases their sense of security. There is high quality evidence that PLGS leads to decreased time spent in hypoglycemia and nocturnal hypoglycemia, with no increase in the mean blood glucose concentration and hyperglycemia episodes [23,24,25].
Participants in the study group were more likely to calculate the insulin dose using “bolus calculator,” an available feature of automated bolus calculation in most insulin pumps. Adult user data indicates that the use of a “bolus calculator” improved HbA1c levels, mean blood glucose levels, and glucose variability [26, 27]. On the other hand, a randomized controlled trial in a pediatric group did not reveal any additional effect of “bolus calculator” use with regard to HbA1c levels, postprandial blood glucose values, or other study outcomes [26].
The International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends carbohydrate counting from the onset of diabetes, because it is correlated with improved glycemic control and quality of life among both adults and adolescents [28]. There are few methods of calculating carbohydrate, but research found no evidence to suggest that one particular method is superior to another [28]. In our diabetology center, during the first hospitalization, patients use 10 g carbohydrate portions and are introduced to carbohydrate counting and insulin dose calculations by using an individualized insulin-to-carbohydrate ratio. It is worth emphasizing that almost all study participants declared that they were counting carbohydrates (95.5%). Some patients in our center count also FPU because those macronutrients (fat and protein) lead to delayed hyperglycemia (up to 3–6 h after the meal) [29]. Usually, patients count that 1 FPU equals 100 kcal of fat or protein and requires the same amount of insulin (as an extended bolus) as 10 g of carbohydrates [29]. Over half (56.4%) of the participants declared that they counted FPU, without any differences between the two groups.
The timing of insulin bolus plays a crucial role in achieving stable glycemic values and long TIR. The recommended insulin timing is 15–20 min before meal consumption [28, 30]. Previous studies revealed that rapid-acting insulin analogs before meals as opposed to after meals reduce postprandial glycemia by almost 30% [30]. We found also that participants in the study group were more disciplined with regard to maintaining a latency period between insulin delivery and meal consumption (72.0%) than participants in the control group.
We observed significantly higher insulin doses for both total daily dose and basal insulin dose among participants with poor metabolic control (TIR ≤70% group). Previous studies clearly indicate that uncontrolled glycemia (chronic hyperglycemia) is a risk factor of insulin resistance [29]. Interestingly, the study participants’ insulin requirements are still being recommended by ISPAD at ranges of 0.7 to 1.0 IU/kg/day [29]. During puberty, the requirements may increase even up to 2 U/kg/day [29]. The study and control group patients required about 0.71 and 0.83 IU/kg/day, respectively.
Appropriate disease self-management is a crucial factor affecting good metabolic control in diabetes. Hence, it was not surprising that creating electronic reports for glycemic trends and insulin requirements using a dedicated platform in a domestic environment was related with longer TIR. Considering recent advancements in diabetes due to the use of technology such as smartphone applications and telemedicine, there is significant opportunity to achieve better patients’ involvement in diabetes self-management and subsequently improve metabolic control and possibly ease the disease burden.
Conclusions
Maintaining an adequate interval between insulin delivery and meal consumption, usage of PLGS system with CSII, usage of “bolus calculator” function, and patients’ involvement in the diabetes treatment (generating electronical reports in a domestic environment) may be the factors contributing to prolonged TIR.
Patients with shorter TIR have a higher insulin requirement.
Hyperglycemia and high glycemic variability are the main problems preventing patients from achieving the goal of treatment of TIR >70%.
Patients using CGM systems achieved an acceptable rate of hypoglycemia, regardless of the achieved TIR values.
Data Availability
Data available on request.
Change history
28 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13410-024-01314-8
References
Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72. https://doi.org/10.1089/dia.2018.0384.
De Block C, Manuel-Y-Keenoy B, Van Gaal L. A review of current evidence with continuous glucose monitoring in patients with diabetes. J Diabetes Sci Technol. 2008;2:718–27. https://doi.org/10.1177/193229680800200426.
Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:S25–37. https://doi.org/10.1089/dia.2017.0035.
Dovc K, Cargnelutti K, Sturm A, et al. Continuous glucose monitoring use and glucose variability in pre-school children with type 1 diabetes. Diabetes Res Clin Pract. 2019;147:76–80. https://doi.org/10.1016/j.diabres.2018.10.005.
Bellido V, Pinés-Corrales PJ, Villar-Taibo R, et al. Time-in-range for monitoring glucose control: is it time for a change? Diabetes Res Clin Pract. 2012;177: 108917. https://doi.org/10.1016/j.diabres.2021.108917.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603. https://doi.org/10.2337/dci19-0028.
Araszkiewicz A, Bandurska-Stankiewicz E, Budzyński A et al. Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin Diabetol. 2020;91:790:2020.
Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. https://doi.org/10.1186/s13098-020-00529-z.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–5. https://doi.org/10.2337/dc18-1444.
Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22:72–8. https://doi.org/10.1089/dia.2019.0251.
Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8:1–8. https://doi.org/10.1136/bmjdrc-2019-000991.
Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13:614–26. https://doi.org/10.1177/1932296818822496.
Hirsch IB, Welsh JB, Calhoun P, et al. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36:1637–42. https://doi.org/10.1111/dme.14065.
Petersson J, Åkesson K, Sundberg F, et al. Translating glycated hemoglobin A1c into time spent in glucose target range: a multicenter study. Pediatr Diabetes. 2019;20:339–44. https://doi.org/10.1111/pedi.12817.
Hornik K and the R Core Team, R Foundation for Statistical Computing, Vienna, Austria. https://cran.r-project.org/doc/FAQ/R-FAQ.html
Gill A, Gothard MD, Briggs Early K. Glycemic outcomes among rural patients in the type 1 diabetes T1D exchange registry, January 2016-March 2018: a cross-sectional cohort study. BMJ Open Diabetes Res Care. 2022;10:e002564. https://doi.org/10.1136/bmjdrc-2021-002564.
Beck RW, Miller KM, Foster NC. The T1D exchange clinic network and registry: 10 years of enlightenment on the state of type 1 diabetes in the United States. Diabetes Technol Ther. 2019;21:310–2. https://doi.org/10.1089/dia.2019.0129.
Costa C, Linhares MI, Bastos F et al. Effect of ultra-rapid insulin aspart on glycemic control in children with type 1 diabetes: the experience of a Portuguese tertiary centre. Diabetol Int. 2022;1-7. https://doi.org/10.1007/s13340-021-00565-8
Fath M, Danne T, Biester T, et al. Faster-acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18:903–10. https://doi.org/10.1111/pedi.12506.
Patel PJ, Benasi K, Ferrari G, et al. Randomized trial of infusion set function: steel versus teflon. Diabetes Technol Ther. 2014;16:15–9. https://doi.org/10.1089/dia.2013.0119.
Heinemann L. Insulin infusion sets: a critical reappraisal. Diabetes Technol Ther. 2016;18:327–33. https://doi.org/10.1089/dia.2016.0013.
Schmid V, Hohberg C, Borchert M, et al. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy - trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976–82. https://doi.org/10.1177/193229681000400429.
Alotaibi A, Al Khalifah R, McAssey K. The efficacy and safety of insulin pump therapy with predictive low glucose suspend feature in decreasing hypoglycemia in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Pediatr Diabetes. 2020;21:1256–67. https://doi.org/10.1111/pedi.13088.
Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41:2155–61. https://doi.org/10.2337/dc18-0771.
Biester T, Kordonouri O, Holder M, et al. ‘Let the algorithm do the work’: reduction of hypoglycemia using sensor-augmented pump therapy with predictive insulin suspension (SmartGuard) in pediatric type 1 diabetes patients. Diabetes Technol Ther. 2017;19:73–182. https://doi.org/10.1089/dia.2016.0349.
Schmidt S, Nørgaard K. Bolus calculators. J Diabetes Sci Technol. 2014;8:1035–41. https://doi.org/10.1177/1932296814532906.
Van Meijel LA, van den Heuvel-Bens SP, Zimmerman LJ, et al. Effect of automated bolus calculation on glucose variability and quality of life in patients with type 1 diabetes on CSII treatment. Clin Ther. 2018;40:862–71. https://doi.org/10.1016/j.clinthera.2018.02.004.
Smart CE, Annan F, Higgins LA, et al. ISPAD clinical practice consensus guidelines 2018: nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:136–54. https://doi.org/10.1111/pedi.12738.
Danne T, Phillip M, Buckingham BA, et al. ISPAD clinical practice consensus guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:115–35. https://doi.org/10.1111/pedi.12718.
Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35:306–16. https://doi.org/10.1111/dme.13525.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. EK-K collected, analyzed and interpreted the data, and developed the first draft of the manuscript. AS conceptualized the study, coordinated the data collection process, and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the ethical standards and principles of the Declaration of Helsinki, as revised in 2013. The Ethics Committee of the Medical University of Warsaw approved the study (KB/215/2020).
Consent to participate
Written informed consent was obtained from the legal guardians and participants >16 years of age. Verbal informed consent was obtained from all the participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the Results section of the Abstract, the statement “TIR >70%” should be “TIR ≤70%”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emilia, KK., Agnieszka, S. Factors affecting the prolongation of glycemic time in range among children with type 1 diabetes using continuous glucose monitoring systems: A case control study. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-024-01310-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-024-01310-y