Abstract
Objective
The relationships between carotid intima-media thickness (C-IMT) and β cell function and insulin resistance in patients with type 2 diabetes (T2D) have not been fully elucidated. This study is to investigate whether impaired glucose metabolism is etiologically associated with C-IMT in patients with T2D.
Methods
The study group consisted of 490 (284 men, 206 women) participants. Venous blood specimens were obtained from all subjects for biochemical profiles after an >8-h overnight fast. C-IMT was measured as the distance between the luminal-intimal leading edge (I-line) and the medial-adventitial leading edge (M-line) on the far wall. Insulin resistance was estimated with the homeostasis model assessment of insulin resistance (HOMA-IR). The acute insulin response to arginine was calculated as the mean of the three plasma insulin levels obtained within 2, 4, and 6 min after the arginine bolus minus the pre-stimulus plasma insulin levels.
Results
There was a graded increase in C-IMT values according to tertiles of HOMA-IR in men; the values of C-IMT were significantly decreased across the tertiles of acute insulin and C-peptide responses in women. Multivariate analysis revealed that HOMA-IR and age were positively associated with C-IMT among men participants, and acute insulin response and current smoking were the independent determinants of C-IMT in women.
Conclusion
Early insulin response stimulated by arginine is independently associated with C-IMT in women T2D individuals, whereas insulin resistance is positively correlated with C-IMT in men T2D subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2D) characterized by pathological progressive hyperglycemia is caused by insulin resistance and pancreatic β cell dysfunction. There is evidence that T2D is associated with early atherosclerosis, and is a well-established risk factor for cardiovascular diseases [1, 2]. Carotid intima-media thickness (C-IMT) is currently a widely used marker for cardiovascular conditions, in particular for atherosclerotic disease [3]. It has been addressed to be directly associated with the development of cardiovascular disease [4, 5]. On the other hand, there is growing evidence that inflammation represents as a key pathophysiological etiology in the development and progress of both atherosclerotic cardiovascular disease and T2D [6, 7]. Notably, patients with T2D were reported to have a marked increment in C-IMT compared to the age-matched non-diabetic individuals [8]. Some atherosclerosis-promoting factors may be influenced by insulin resistance alone or together with hyperinsulinemia and hyperglycemia [9,10,11]. However, previous studies have shown contradictory results regarding the relationship between C-IMT and T2D. In non-diabetic individuals, C-IMT was shown to be negatively associated with insulin secretion independently of other risk factors such as insulin sensitivity [12]. Interestingly, another study identified a gender difference suggesting that C-IMT is inversely correlated with insulin sensitivity in men, but is independently associated with fasting plasma glucose levels in women [13]. In this respect, both insulin resistance and insulin secretion should be accurately measured. Defects in insulin action and β cell function are known as hallmarks of T2D with resultant relative or absolute inadequacy of insulin secretion in response to hyperglycemia. However, the relationship between C-IMT and β cell function in T2D individuals has not been fully elucidated.
There is a need to establish novel methods that can appropriately assess disease progression and β cell mass in clinical and epidemiological studies. Currently, HOMA-β (homeostasis model assessment of β cell function) is commonly used to assess β cell function. However, this score is calculated by using fasting plasma glucose and insulin levels, which only indicates β cell function under fasting non-dynamic conditions. Furthermore, arginine-induced insulin secretion is also able to evaluate β cell function in T2D patients as well as in islet auto-transplantation studies [14, 15]. In a clinical setting, this stimulus is applied intravenously to elicit an acute insulin response (AIR) or an acute C-peptide response (ACR). The aim of the present study is to investigate the association between C-IMT and β-cell function in individuals with T2D. Physiological approaches, e.g., arginine-stimulated test (AST), were applied to participants to determine insulin resistance and early insulin secretion.
Materials and methods
Study design
The study was designed as a cross-sectional case-control study, and was conducted at the Fudan University, Zhongshan Hospital, Xiamen Branch, over a period of 2 years.
From August 2018 to February 2021, a total of 490 patients with T2D (284 men, 206 women) aged between 40 and 70 year-old from the department of Endocrinology, Zhongshan Hospital (Xiamen), Fudan University, China, had been recruited into this present ongoing cohort. Patients were diagnosed as diabetes based on American Diabetes Association (ADA) 2018 criteria: (1) a self-reported history of diabetes previously diagnosed by health care professionals; (2) fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L); (3) 2-h plasma glucose (2-h PG, OGTT) ≥200 mg/dL (11.1 mmol/L); or (4) glycosylated hemoglobin A1c (HbA1c) ≥6.5% (48 mmol/mol). All patients were examined Glutamic acid decarboxylase antibody (GAD-Ab) negative to exclude type 1 diabetes. Subjects with the following conditions were also excluded: specific types of diabetes due to other causes, cancer, pregnancy, lactation, abnormal liver or/and renal function, infectious diseases, evidence of hyperthyroidism, hypothyroidism, alcoholism, viral hepatitis (type B or C), or those who underwent vascular surgery such as carotid endarterectomy or stenting. The study protocol was approved by the Human Research Ethical Committee of the Zhongshan Hospital (Xiamen), Fudan University. Written informed consent was obtained from each participant before the start of the study.
Waist circumference, heart rate, and blood pressure (BP) were measured in all participants. After an >8-h overnight fast, venous blood specimens were obtained from all subjects for biochemical profiles as well as low density lipoprotein (LDL-cholesterol), high density lipoprotein (HDL-cholesterol), triglycerides, high-sensitivity C-reactive protein (hs-CRP), uric acid, and estimated glomerular filtration rate (eGFR); the information of current smoking and body mass index (BMI) were also obtained.
Carotid intima-media thickness measurement
High resolution B-mode ultrasound of the extracranial carotid arteries was performed by trained and certified technicians following a standardized protocol [13]. A segment about 1 cm proximal to the carotid bifurcation was imaged in the longitudinal plane and C-IMT was measured as the distance between the luminal–intimal leading edge (I-line) and the medial–adventitial leading edge (M-line) on the far wall. C-IMT was assessed in three contiguous sites at 1-mm intervals and the average of the three values was used for analyses. The mean of the left and right measurements was used in this analysis.
Clinical and biochemical measurements
Clinical parameters, such as age, gender, systolic blood pressure, heart rate, body mass index (BMI), and waist circumference were collected. Blood pressure was measured using a standard sphygmomanometer in the sitting position, as the average of the last two of three consecutive measurements obtained at 3-min intervals. BMI was calculated as body weight in kilograms divided by the square of body height in meters (kg/m2). Waist circumference was taken at umbilical level to the closest centimeter. Fasting blood samples were obtained in the early morning for biochemical studies including serum creatinine, high-density lipoproteins (HDL), low-density lipoproteins (LDL) triglyceride, uric acid, glycated hemoglobin (HbA1c), and high-sensitivity C-reactive protein (hs-CRP). The glomerular filtration rate was estimated by the modified modification of diet in renal disease equation with the new Japanese coefficient [16].
Arginine stimulation test
Dynamic testing of β cell function was evaluated by AST performed according to the method of Robertson [17]. Before the test, glucose levels were measured to ensure that they were between 4 and 12 mmol/L. After baseline samples were drawn for glucose, insulin, and C-peptide at 0 min, an intravenous injection of 5 g of arginine (given as 50% arginine HCl) was administered over 30 s, with time 0 set halfway through the arginine injection. Samples for plasma glucose, insulin, and C-peptide were collected from the contralateral arm at 2, 4, and 6 min after the arginine injection.
Insulin secretion and insulin resistance
Insulin resistance was estimated with the homeostasis model assessment of insulin resistance (HOMA-IR) according to the formula HOMA-IR = fasting insulin (FINS) (μU/ml) × fasting plasma glucose (FPG) (mmol/L)/22.5 [18]. For the assessment of pancreatic β cell function, we used the homeostasis model assessment of β cell function (HOMA-β) according to the formula HOMA-β = 20×FINS (μU/ml)/(FPG mmol/L-3.5). The pancreatic β cell function was also evaluated by area under curve of insulin (INSAUC) according to the formula INSAUC = INS0+2×INS2+2×INS0+INS6, where INS0, 2, 6 is the plasma insulin levels obtained within 0, 2, and 6 min after the arginine bolus. The acute insulin response (AIR) to arginine was calculated as the mean of the three plasma insulin levels obtained within 2, 4, and 6 min after the arginine bolus minus the prestimulus plasma insulin level. In addition, the acute C-peptide response (ACR) to arginine was calculated as described for insulin.
Statistical analysis
The statistical analyses were carried out by Statistical Package for Social Sciences 18 (SPSS). Continuous variables are presented as mean ± SD (standard deviation) or median (interquartile range) and categorical variables as percentages of patients in the study. Subjects were divided into two groups according to gender and each group was further subdivided into tertiles of fasting plasma insulin, glucose concentrations and index of insulin secretion and resistance. Comparisons were drawn by chi-square test, unpaired Student t test, and one-way analysis of variance, as appropriate. Two-sided p values of less than 0.05 were considered statistically significant. To find the parameters that explain the significance of the variance of the dependent variables, stepwise multivariate linear regression analysis was performed to correlated parameters with C-IMT and p value <0.05 was considered as indication of statistical significance. Regression models, with standardized baseline C-IMT as the dependent variable and established risk factors as independent variables, were run separately for men and women.
Results
Demographic, clinical, and laboratory data
The demographic, clinical, and laboratory data from participants were shown in Table 1. At baseline, men and women differed in age, waist circumference, systolic BP, HDL-cholesterol, current smoking, uric acid, fasting insulin, INSAUC, HOMA-IR, HOMA-β%, and C-IMT (Table 1). There were no significant differences in fasting glucose, AIR, ACR, and HbA1c.
Correlations between C-IMT and insulin secretion related factors
At baseline, C-IMT was detected to be significantly higher in men. We next examined C-IMT across the tertiles of fasting plasma insulin and glucose concentrations, and index of insulin secretion and insulin resistance in men and women. Notably, C-IMT values were positively correlated with HOMA-IR, but no significant differences in IMT values were identified across tertiles of HOMA-β, fasting glucose concentrations, INS0, AIR, INSAUC, and ACR (Table 2), suggesting that the elevated C-IMT values may be attributed by the progress of insulin resistance in men. However, completely different conclusions were drawn from the data in women. C-IMT levels were markedly decreased across the tertiles of AIR and ACR in female participants. No significant differences were found in C-IMT values across the tertiles of HOMA-IR, HOMA-β, fasting glucose concentrations, INS0, and INSAUC (Table 2). Collectively, these data indicate that changes in C-IMT are strongly associated with insulin resistance status in men, but are more prone to be controlled by β cell function in women.
Independent determinants of C-IMT in men and women
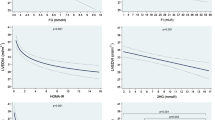
To further explore the independent determinants of C-IMT in men and women, we then assessed the roles of insulin resistance or insulin secretion in association with C-IMT in our study participants. Multivariate analysis revealed that HOMA-IR and age were positively associated with C-IMT in male participants, after adjusting for other traditional risk factors (waist circumference, systolic BP, LDL-cholesterol, and current smoking) (Table 3). Whilst, in women, AIR and current smoking were unveiled to be the independent determinants of C-IMT by multivariate analysis (Table 3), suggesting that both the risk factors and the mechanism underlying of carotid plaques differ in gender. Taken together, C-IMT is significantly higher in the highest HOMA-IR tertile in men (p<0.001), and in contrast, C-IMT is higher in the lowest tertile of AIR index in women (p<0.001; Fig. 1).
Independent gender-specific determinants of C-IMT by tertiles in T2D individuals. A C-IMT with insulin resistance (HOMA-IR) in men. B C-IMT with insulin secretion (AIR index) in women. p values indicated are adjusted for age, BMI, waist circumference, systolic BP, low-density lipoprotein-cholesterol, eGFR, and current smoking
Discussion
In the present study, we assessed C-IMT in 490 T2D participants. The arginine stimulation test (AST) method was applied as the established measurement to assess insulin secretion in patients. We for the first time demonstrated that insulin resistance and insulin secretion are independent risk factors of C-IMT in men and women with T2D, respectively.
Elevated C-IMT has been demonstrated to be associated with insulin resistance (evaluated by HOMA-IR), glucose intolerance, and higher fasting glucose levels, especially in non-diabetic individuals and even in obese children [9, 13, 17, 19], indicating that early atherosclerosis in prediabetes may be causally linked to endothelial insulin resistance. In fact, hyperinsulinemia resulted from insulin resistance has been reported to cause endothelial dysfunction and atherosclerosis [20]. More importantly, insulin supplementation showed the ability to improve several vascular functions in animal models and people with insulin resistance [21]. On the other hand, Roussel et al. demonstrated that insulin secretion (evaluated by the early insulin response index which was calculated as the ratio of insulin change over the first 30 min of the OGTT, to plasma glucose at 30 min) is also associated with early carotid atherosclerosis in non-diabetic population independently of other risk factors such as insulin resistance [12]. Similarly, β cell function (estimated by HOMA-β) was still significantly related to an increased risk of poor functional outcomes in non-diabetic ischemic stroke patients after adjusting insulin resistance [18]. Furthermore, C-IMT was addressed to be associated with impaired β cell function in non-diabetic people [11]. Together these hinted that defects in insulin resistance and/or insulin secretion may pathophysiologically be connected with the development of carotid atherosclerosis. Given the above evidence in non-diabetic population, we raised the likelihood that this association may also exist in T2D population. Indeed, our data clearly revealed that C-IMT value is significantly correlated with insulin resistance (evaluated by HOMA-IR) and insulin secretion (evaluated by ACR and AIR) in T2D patients. More interestingly, these associations are manifested in a gender-specific manner, which is in line with the previous findings that C-IMT is associated with insulin sensitivity in men, but with fasting plasma glucose in women, respectively [13]. This discrepancy might reflect a gender-specific mechanism involved in the development of atherosclerosis in T2D patients. However, the explanation behind is not fully understood, and therefore, future studies focus on exploring the causal relationship between impaired glucose metabolism and atherosclerosis in different gender groups are needed.
The progression of C-IMT is determined by a plethora of risk factors, such as age, blood pressure, lipids, smoking, obesity, and CRP [22, 23], though we found the C-IMT value is significantly correlated with insulin resistance and insulin secretion in a gender-specific manner. It is noteworthy that diet, exercise, parental history of premature death from coronary heart disease are also associated with carotid atherosclerotic plaques [24], which may confound the interpretation of our results. Therefore, in the present study, we employed stepwise multivariate linear regression analysis to explore the independent C-IMT-correlated parameters in gender-divided subgroups to adjust the potential confounding factors. After excluding the traditional risk factors (waist circumference, systolic BP, LDL-cholesterol, and current smoking), our multivariate regression model revealed the true independent determinants of C-IMT in T2D population in a gender-specific manner. Taken together, these suggest that the risk factors of carotid plaques as well as the mechanisms underlying may differ in gender in T2D individuals. Further studies are needed to explore the more comprehensive relationships between C-IMT and arginine-stimulated insulin secretion and insulin resistance after excluding all the possible variables.
HOMA-IR and HOMA-β are widely used for evaluating insulin resistance and insulin secretion, respectively [18, 25, 26]. It is noteworthy that these two measurements derived from fasting samples are limited to merely reflecting fasting nondynamic conditions in clinical settings. In addition, oral glucose tolerance test (OGTT) and euglycemic-hyperinsulinemia clamp have also been applied to evaluate insulin sensitivity [11,12,13]. In our study, AST was employed to examine the first phase insulin secretion and the reserved function of β cells. It has been established that β cell dysfunction plays a key role in the pathogenesis of diabetes development that leads to defects in glucose-stimulated insulin secretion. However, β cells still retain the act to stimuli by non-sugar substances such as arginine [27]. Arginine is more potent to trigger secretion in β cells than glucose, and hence, can be used to evaluate the reserved function of islet β cells. In fact, hyperglycemic clamp technique is a more accurate application for evaluating islet β cell secretory function [14]. Nonetheless, the high technique requirement and long operative time limit its utility in clinical settings. In contrast, AST is far less technically demanding and can bring reproducible and complementary measures of β cell function [28]. More importantly, in young T2D individuals, AST is beneficial to reflect β cell reserve regardless of disease duration and treatment [29]. At last, it has also been demonstrated that arginine is preferred to glucagon for the assessment of β cell function [30].
T2D is suggested to be linked with atherosclerotic cardiovascular diseases through multifactorial pathways. For instance, HDL-cholesterol levels or the HDL-based makers have been established to be associated with various disorders, including hypertension [31], hepatosteatosis [32], thyroiditis [33], and in particular, diabetes [34, 35]. Importantly, these conditions are also tightly connected with high burden of inflammation that is well known to play a key role in the development of atherosclerosis in T2D patients [36]. In addition, there is evidence that women generally have higher HDL-cholesterol levels than men [37, 38], which is also confirmed by the baseline data in our study population. These together strengthen our results that atherosclerotic cardiovascular disease is gender-specifically associated with T2D.
Clinically, the gender-specific C-IMT values demonstrated by our findings provide a novel means of risk assessment for T2D patients, which may shed light on the personalized T2D treatment. Moreover, our data also highlight the significance of usage of AST-based insulin secretion in clinical settings to evaluate the relationship between T2D and development of atherosclerosis.
Limitations
The current study cannot determine the causal relationship between arginine-stimulated insulin secretion and C-IMT due to its cross-sectional and unpaired design. In addition, the relatively small sample size may result in reduced statistical power. A larger population from more clinical study sites is warranted in the future. Notably, patients with diabetes may develop various severe vascular complications, which cannot be merely reflected by C-IMT with ultrasound detection. In addition, C-IMT values may also be affected by many other factors, such as premature deaths in family, diet, and exercise, which may confound the explanation of the results. Therefore, more parameters correlated with C-IMT value and/or atherosclerosis occurrence such as percentage of stenosis and peak systolic velocity (PSV) of carotid that indicating vascular plaque formation are of necessity to be included in the analysis model to establish a more comprehensive explanation.
Conclusion
Early insulin response stimulated by arginine is independently associated with C-IMT in women T2D individuals, whereas insulin resistance is positively correlated with C-IMT in men T2D subjects. These gender-specific findings provide prognostic and therapeutic implications in the personalized management of patients with T2D. Furthermore, AST has the potential to be used as a reliable parameter in evaluating the relationship between impaired glucose metabolism and development of atherosclerosis.
Data Availability
All data collected, generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation. 1999;100(10):1132-3. https://doi.org/10.1161/01.cir.100.10.1132.
Eikendal AL, et al. Common carotid intima-media thickness relates to cardiovascular events in adults aged <45 years. Hypertension. 2015;65(4):707–13.
Lin CY, et al. Association between urine lead levels and cardiovascular disease risk factors, carotid intima-media thickness and metabolic syndrome in adolescents and young adults. Int J Hyg Environ Health. 2020;223(1):248–55.
Poredos P. Intima-media thickness: indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc Med. 2004;9(1):46–54.
Riley WA, et al. Reproducibility of noninvasive ultrasonic measurement of carotid atherosclerosis. The Asymptomatic Carotid Artery Plaque Study. Stroke. 1992;23(8):1062–8.
Sincer I, et al. Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Postepy Kardiol Interwencyjnej. 2018;14(3):263–9.
Aktas G, et al. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad Med. 2023;135(5):519–23.
Niskanen L, et al. Carotid artery intima-media thickness in elderly patients with NIDDM and in nondiabetic subjects. Stroke. 1996;27(11):1986–92.
Shinozaki K, et al. Insulin resistance as an independent risk factor for carotid artery wall intima media thickening in vasospastic angina. Arterioscler Thromb Vasc Biol. 1997;17(11):3302–10.
Hedblad B, et al. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo. Sweden Diabet Med. 2000;17(4):299–307.
Andreozzi F, et al. Increased carotid intima-media thickness in the physiologic range is associated with impaired postprandial glucose metabolism, insulin resistance and beta cell dysfunction. Atherosclerosis. 2013;229(2):277–81.
Roussel R, et al. Beta-cell function is associated with carotid intima-media thickness independently of insulin resistance in healthy individuals. J Hypertens. 2016;34(4):685–91.
Kozakova M, et al. Insulin sensitivity and carotid intima-media thickness: relationship between insulin sensitivity and cardiovascular risk study. Arterioscler Thromb Vasc Biol. 2013;33(6):1409–17.
Ward WK, et al. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74(4):1318–28.
Robertson RP. Consequences on beta-cell function and reserve after long-term pancreas transplantation. Diabetes. 2004;53(3):633–44.
Matsuo S, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Santos IS, et al. Insulin resistance is associated with carotid intima-media thickness in non-diabetic subjects. A cross-sectional analysis of the ELSA-Brasil cohort baseline. Atherosclerosis. 2017;260:34–40.
Zhou M, et al. Association between beta-cell function estimated by HOMA-beta and prognosis of non-diabetic patients with ischaemic stroke. Eur J Neurol. 2018;25(3):549–55.
Ozkan EA, et al. The evaluation of carotid intima-media thickness and mean platelet volume values and correlation with cardiac functions in obese children. Int J Clin Exp Med. 2015;8(12):22557–63.
Del Turco S, et al. Insulin resistance and endothelial dysfunction: a mutual relationship in cardiometabolic risk. Curr Pharm Des. 2013;19(13):2420–31.
Monnier L, et al. Insulin and atherosclerosis: how are they related? Diabetes Metab. 2013;39(2):111–7.
Ikezaki H, et al. Small dense low-density lipoprotein cholesterol and carotid intimal medial thickness progression. J Atheroscler Thromb. 2020;27(10):1108–22.
Pujia A, et al. Common carotid arterial wall thickness in NIDDM subjects. Diabetes Care. 1994;17(11):1330–6.
Zureik M, et al. Differential association of common carotid intima-media thickness and carotid atherosclerotic plaques with parental history of premature death from coronary heart disease: the EVA study. Arterioscler Thromb Vasc Biol. 1999;19(2):366–71.
Jensen CC, et al. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51(7):2170–8.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95.
Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28(2):187–218.
Shankar SS, et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care. 2016;39(9):1602–13.
Sjostrand M, et al. Assessment of beta-cell function in young patients with type 2 diabetes: arginine-stimulated insulin secretion may reflect beta-cell reserve. J Intern Med. 2014;275(1):39–48.
Robertson RP, et al. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab. 2014;307(8):E720-7.
Aktas G, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. 2022;134(3):297–302.
Kosekli MA, et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras (1992). 2021;67(4):549–54.
Kurtkulagi O, et al. Hashimoto’s thyroiditis is associated with elevated serum uric acid to high density lipoprotein-cholesterol ratio. Rom J Intern Med. 2021;59(4):403–8.
Kocak MZ, et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras (1992). 2019;65(1):9–15.
Aktas G, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098–102.
von Scholten BJ, et al. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J Diabetes Complications. 2016;30(2):248–55.
Pascot A, et al. HDL particle size: a marker of the gender difference in the metabolic risk profile. Atherosclerosis. 2002;160(2):399–406.
Brown SA, et al. Plasma lipid, lipoprotein cholesterol, and apoprotein distributions in selected US communities. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1993;13(8):1139–58.
Acknowledgments
We thank all the participants for their commitment to the study. The authors are also grateful to the staff for their efforts in data collection and management.
Funding
Open access funding provided by Lund University. This study was funded by the Xiamen Medical and Health Guidance Project (No. 3502Z20209050); and Fujian Provincial Natural Science Foundation Project (No. 2022J011422).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: C.L., N.C., and W.L.; collection of data: W.L., K.W, X.L., S.Z., J.Z., and S.L.; data analysis: W.L., K.W, and X.L.; manuscript writing: C.L., N.C., and W.L.; all authors revised the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethical approval
In accordance with ethical guidelines of the 1975 Declaration of Helsinki, this study was approved by the ethics committee of Zhongshan Hospital (Xiamen), Fudan University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, W., Wang, K., Luo, X. et al. β cell function and insulin resistance have gender-specific correlations with carotid intima-media thickness in type 2 diabetes. Int J Diabetes Dev Ctries 44, 409–416 (2024). https://doi.org/10.1007/s13410-023-01260-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01260-x