Abstract
Sulfonylureas (SU) continue to be a vital therapeutic category of oral hypoglycemic agents (OHAs) for the management of type 2 diabetes mellitus (T2DM). Physicians consider modern SU (gliclazide and glimepiride) as “safe and smart” choices for T2DM management. The presence of multiple international guidelines and scarcity of a national guideline may contribute to the challenges faced by few physicians in choosing the right therapeutic strategy. The role of SU in diabetes management is explicit, and the present consensus aims to emphasize the benefits and reposition SU in India. This pragmatic, practical approach aims to define expert recommendations for the physicians to improve caregivers’ knowledge of the management of T2DM, leading to superior patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Summary of expert recommendations
Sr. No | Recommendations | Grade of recommendation | Level of evidence | |
|---|---|---|---|---|
1 | Modern SUs are effective and safe in T2DM management when used as initial therapy combined with lifestyle modification and metformin, when target HbA1c reduction is ≥ 1.5% * * | A | 1 | |
a) Glycemic target (HbA1c) should be based on patient factors (age, life expectancy, comorbidity, risk of hypoglycemia, etc.) | ||||
b) In the absence of specific indications for cardio-renal protection | ||||
2 | Modern SU may be used in combination with other oral antidiabetic drugs (OAD) except for glinides* * | A | 1 | |
With due precaution for hypoglycemia risk in patients that are elderly or have CKD | ||||
3 | Modern SU can be beneficial as second-line options in patients that were not able to attain glycemic targets with metformin monotherapy* * | A | 1 | |
a) In the absence of specific indications for cardio-renal protection | ||||
b) With due precaution for hypoglycemia risk in patients that are elderly or have CKD | ||||
4 | Modern SU may be used as third-line agents* for managing uncontrolled T2DM with dual combination therapy * | A | 1 | |
a) In the absence of specific indications for cardio-renal protection | ||||
b) With due precaution for hypoglycemia risk in patients that are elderly or have CKD | ||||
5 | Modern SU combined with other OADs can be used with due care in elderly patients * * | A | 1 | |
a) In the absence of specific indications for cardio-renal protection | ||||
6 | Glibenclamide may be used in neonatal diabetes and maturity-onset diabetes mellitus of the young-3 (MODY-3) | A | 3 | |
7 | During long-term fast periods like Ramadan and Navratri, modern SU may be cautiously used in combination with other OADs | A | 3 | |
8 | SU (gliclazide) can be used for the management of steroid-induced mild-moderate hyperglycemia or steroid-associated worsening of T2DM | C | 4 | |
9 | Modern SU can be used in patients with renal impairment * * | A | 2 | |
Gliclazide and glipizide can be used without dose adjustment in CKD stages 3, 4, and 5, whereas glimepiride can be started conservatively at 1 mg daily in similar conditions as per KDOQI-(CKD) 2012 | ||||
Stages of CKD | ||||
Stage | eGFR (mL/min/m2) | |||
I | ≥ 90 | |||
II | 60–89 | |||
II | 30–59 | |||
IV | 15–29 | |||
V | < 15 (or dialysis) | |||
Introduction
The overall incidence and prevalence of type 2 diabetes mellitus (T2DM) cases are increasing worldwide at an alarming rate. The International Diabetes Federation (IDF) atlas predicted a rise to 643 million by 2030 [1]. The worldwide expenditure on diabetes management is projected to $845 billion (2045) from $760 billion (2019) [2]. The prevalence of T2DM is rising in India, both in rural (15%) and urban areas (19.0%) and independent of gender [3]. The risk of diabetes in young men and women is approximately 55.5 and 64.6%, respectively [4]. While most international guidelines recommend metformin and lifestyle changes in newly diagnosed T2DM [5, 6], variations are found mainly in the choice of second- and third-line oral hypoglycemia agents (OHA). Globally, few patients are achieving glycemic targets and guideline-recommended goals [7,8,9], which is disturbing considering the availability of many drugs. A more intense individualized approach is required to treat T2DM [10]. Even physicians were found to be unfamiliar with T2DM-related knowledge. Both guideline and non-guideline users had incomplete knowledge about diabetes [11]. There are barriers to implementing guidelines, such as time constraints and low availability [12]. Development of inconsistent guidelines may be one of the reasons [13]. GPs in Australia identified difficulties while providing best-practice diabetes care, such as clinical practice challenges and patient and healthcare system-related problems [14]. Sulfonylureas (SU), among the oldest oral glucose-lowering antidiabetic agent, are still commonly prescribed in many countries (Table 1).
SU accounts for approximately 20% of newly initiated OHAs, combined with other OHAs or monotherapy [21]. In India, SUs were the second most prescribed drugs (35.6%) after metformin [22,23,24]. SUs have a long-standing track record of good efficacy, adherence, and tolerability. SUs are effective insulin secretagogues and, as an add-on to metformin, could be a good choice for Indians without atherosclerotic cardiovascular disease (ASCVD). Epidemiology data revealed that low-income countries have poor availability of essential antidiabetic drugs compared to middle- and high-income countries. Furthermore, metformin was available in 65% of pharmacies and insulin only in 10% [25].
In this article, SUs are classified based on the chronology of development, including conventional SUs (glipizide, glibenclamide, and tolbutamide) and modern SU (gliclazide, gliclazide MR, glimepiride, and glipizide MR). They can also be classified based on their duration of action.
Need of consensus
Modern SUs are prescribed across pan India and are one of the mainstays of therapy. Hence, defining the role of SU in modern diabetes management is pivotal. The present consensus aims to emphasize the immense benefits of SUs and highlight their role and position in managing T2DM alone or in combination.
Methodology
One hundred and fifty endocrinologists from India convened eight advisory boards (June 2021–July 2021) to discuss the use of SUs in current practice. All meetings were conducted on a virtual platform. Specific objectives related to SU were discussed, and every expert shared their views, which led to group discussion. This document is the outcome of aspects presented and discussed in the advisory board meetings. The American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/AACE) protocol for standardized development of clinical practice guidelines was followed in forming the present consensus document [26].
Heterogeneity in type 2 DM: insulin deficiency and insulin resistance
Studies have shown south Asians (SA) to be less insulin resistant and more insulin-deficient than ethnic Black and White populations. In contrast, lower insulin secretion at baseline was a stronger predictor of T2DM in south Asians [27]. Higher diabetes incidence in SA due to poor insulin secretion indicates the need to improve insulin secretion in such a population. Though the racial difference in the incidence of T2DM has been established, insulin resistance and deficiency must be studied to develop strategies for screening, prevention, or treatment of diabetes [28]. A recent study identified four clusters among diabetes patients from India. Insulin-deficient diabetes (SIDD), insulin-resistant obese diabetes (IROD), and combined insulin-resistant and -deficient diabetes (CIRDD) were present in 26.2, 25.9, and 12.1% of the study population, while mild age-related diabetes (MARD) was present in 35.8% of the patients. The latter two, IROD and CIRDD, are unique in Indian patients [29]. The same study identified insulin deficiency in 64.2% of the population, indicating the need for sulfonylureas for optimal glycemic control in T2DM patients.
Oral antidiabetic drug (OAD) failure
Earlier, primary OAD failure indicated the lack of early glycemic control after starting treatment. It was typically observed in thin, low-beta-cell-reserve patients who may have been categorized with late-onset autoimmune diabetes (LADA). Earlier, patients who stopped responding to OADs after years of good management were thought to be experiencing secondary OAD failure, which indicates gradual β-cell fatigue continuing through different phases of β-cell insufficiency. Significant β-cell functional mass is lost when T2DM is diagnosed [30]. The continuous loss of beta-cell function causes the progression of T2D. Both higher HbA1C levels and a longer duration of T2DM are known to be associated with declining beta-cell function. Insidious fall of beta-cell function is one of the main causes of failure of many therapeutic classes of OHAs [31].
The pathophysiology of OAD failure includes beta (β)-cell insufficiency that happens gradually and progresses over a period of time. Usually, in a newly diagnosed patient with T2DM, about half of the beta cell functional mass is already lost. With time, the beta-cell mass further reduces by 4–7% each year, leading to progressive deposition of amyloid protein in the islets of Langerhans of the pancreas. This gradual fall in beta-cell mass and function, together with insulin resistance, worsens with weight gain. Presence of comorbidity such as sleep apnea, infections, stress, or poor lifestyle further deteriorates beta-cell mass and function. As per data, treatment failure with OHA monotherapy and dual therapy is very common. Guidelines recommend addition of either a third OHA or insulin.[30].
Now, OAD failure is characterized by high HbA1c despite concomitant use of an ideal dose of three OHAs of different classes, in which one is metformin and the second is sulfonylurea (preferred), as long as other conditions that causing hyperglycemia and poor compliance are ruled out. OAD failure can also be described in patients who are receiving triple OAD therapy but still exhibit signs of insulinopenia (osmotic symptoms, loss of weight), as well as uncontrolled hyperglycemia. Metformin and sulfonylureas have a greater impact on lowering HbA1c compared to other OADs. Currently, modern SUs are regarded as “safe and smart” options for managing type 2 diabetes.[30]. The β-cells in the pancreas can still be stimulated by SUs to secrete insulin, which helps in glycemic control. Therefore, if other OHAs or insulin is being considered to lower blood sugar levels, SUs can still be used, but caution should be taken to prevent hypoglycemia [32].
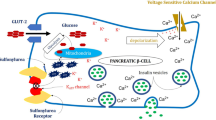
Mechanism of action
Sulfonylureas (SU) act by stimulating the β-cells located in the pancreases. SU act by closing the adenosine-triphosphate-sensitive potassium (KATP) transport channel, resulting in insulin release [33, 34]. Gliclazide has a lower risk of hypoglycemia via reduced pancreatic stimulation. First, SU restores the early insulin peak after glucose intake; second, gliclazide MR strongly binds to SUR1 receptor located in the β cells [35]. Single low-dose gliclazide (20 mg) significantly reduced plasma glucose during the oral glucose tolerance test (OGTT) along with augmentation of the classical incretin effect. Gliclazide increases β glucose sensitivity (46%) and potentiates late-phase insulin secretion at low plasma concentrations [36]. Glimepiride blocks both SUR1 and sarcolemmal SUR2 receptors. Furthermore, glimepiride has a lower rate of hypoglycemia compared to conventional SUs, attributed due to its lower binding affinity (two to three times) and fast association and dissociation with SUR protein [37].
Clinical evidence
Legacy effect
Reduction in the risk of microvascular disease by long-term SU treatment was observed in the UK Prospective Diabetes Study (UKPDS 33) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Released Controlled Evaluation (ADVANCE) [38,39,40]. The ADVANCE observational study (ADVANCE ON) indicated that intensive glucose control with gliclazide MR led to a 46% reduction in end-stage kidney disease (ESKD), suggesting that early intensive glucose control reduced the risk of ESKD development in patients with T2DM [41]. The STENO-2 study concluded that intensified therapy with multiple OHAs in patients with T2DM and microalbuminuria delays the progression to nephropathy, autonomic neuropathy, and retinopathy [42].
The phenomenon of glycemic memory has been defined as the persistence of T2DM complications even after achieving glycemic control. It is part of a broader concept, metabolic memory. Research indicates an association between poor metabolic control and subsequent worsening ability of endogenous vasoreparative systems, leading to metabolic memory or vascular glycemic memory. Thus, the need for early aggressive treatment arises that aims to prevent T2DM-related complications. Drugs that target free radicals and glycation that normalizes glucose levels may reduce long-term complications beyond the duration of intervention and despite glycemic targets being met [43].
Modern SU (glimepiride and gliclazide MR) should be initiated early in T2DM to achieve maximum glycemic benefits and obtain the benefits of metabolic memory [21].They reduce endothelial cell migration by blocking SU receptor 1-regulated NC (Ca-ATP) channels, thereby attenuating metabolic memory. Legacy effect or metabolic legacy highlights the benefits of early implementation of an intervention to safely reduce blood glucose levels with a multifactorial cardiovascular (CV) approach. In simple terms, there may be long-term benefits persisting for many years after cessation of the treatment [43].
According to the “legacy effect” concept, the cells’ DNA can store information about poor metabolic control, such as the adverse effects of hyperglycemia. This information may decrease the benefit of future improvement in glycemic control and thus cause vascular complications in target organs. Hence, early achievement of good glycemic control is critical in preventing long-term complications. The legacy effect may be more significant in patients with moderate-high CV risk but without cardiovascular disease (CVD), especially those with recent-onset diabetes [44].
Data from a meta-analysis indicates that intense treatment to achieve normoglycemia reduces the risk of macrovascular event in patients suffering from diabetes at a short duration and with no prevalent CVD and good life expectancy. Following are factors associated with worse outcomes: newly diagnosed T2DM and only 10 years of survival and elevated HbA1c levels for the first year after diagnosis [45].
The legacy effect of SU (glibenclamide) intensive therapy was shown in the UKPDS; those patients with 5 years of better glycemic control may have had fewer advanced glycation metabolites that conferred protection in the long term. Reduction in renal-disease progression, reported in ADVANCE and UKPDS, could be another possible reason for long-term protection. Results from these trials indicate that tighter glycemic control prevented microvascular complications [38], with the ADVANCE trial demonstrating sustained glycemic control up to 5 years of SU therapy. At the end of the study, HbA1C dropped to 6.5% in the intensive group compared with 7.3% in the group that received standard therapy [40].
As glyburide use initially led to a more rapid decrease in glucose, it is used in newly diagnosed persons. In those who cannot use metformin, SU may be started over other OHAs in patients with newly diagnosed T2DM because glyburide led to a more initial rapid reduction of glycemia and the legacy effect of SU. The initial dose may be low and can be escalated to submaximal doses for better overall efficacy [46]. The clinical trials supporting the legacy effect of SU are summarized in Table 2.
Glycemic control
Glimepiride has been considered as potent antiglycemic drug. The glucose control in type 2 diabetes: Gliclazide MR vs. Glimepiride (GUIDE) study revealed that gliclazide MR significantly lowered the rate of confirmed hypoglycemia compared to glimepiride. The GUIDE study revealed that gliclazide MR is at least as effective compared to glimepiride in combination or as monotherapy. Regarding safety, gliclazide MR had approximately 50% fewer confirmed hypoglycemia compared to glimepiride [48]. The SU, namely, glimepiride has undergone CVOT trial as per the FDA guideline. The CAROLINA study found that using linagliptin for a median of 6.3 years, compared to glimepiride, led to a non-inferior risk of a composite cardiovascular outcome in patients with early T2DM and elevated cardiovascular risk. Blood pressure, lipid levels, fasting plasma glucose, and overall reduction in HbA1c did not differ substantially across groups [49]. In the real-world UK CPRD research, second-line gliclazide MR showed that it was superior to sitagliptin in terms of achieving the target HbA1c (less than 7.0%) quickly, with a 35% higher probability of doing so in just 3 months [HR = 1.35, p = 0.001]; gliclazide MR and sitagliptin both lasted longer than 2.5 years in this research, with similar durability and persistence [50].
The glycemia reduction approaches in diabetes: a comparative effectiveness (GRADE) study compared glycemic durability of a SU (glimepiride), GLP-1 RA (liraglutide), DPP4 inhibitor (sitagliptin), or insulin glargine in T2DM patients that continued metformin. The primary results of the GRADE study demonstrated greater glycemic durability of glimepiride compared to sitagliptin. Fewer patients receiving glimepiride (72%) developed HbA1c above 7% compared to sitagliptin group (77%) [51]. The above clinical trials are summarized in Table 3.
SU + metformin
Sulfonylureas added to metformin vs. metformin only
Metformin and SU combination has higher glycemic efficacy than metformin monotherapy (pooled between-group difference of 0.9%). Addition of a SU to OHA reduces HbA1C by 1.62% [52].
SU + metformin vs. thiazolidinedione + metformin
Patients treated with SU showed more HbA1c reduction compared to thiazolidinedione (TZD) when added to metformin [53].Treatment with glimepiride (added to metformin) compared to pioglitazone resulted in a faster decline of HbA1c levels (P = 0.024) [54].
SU + metformin vs. DPP4 inhibitor + metformin
According to a meta-analysis, glimepiride had a 12% greater glycemic efficacy compared to DPP4 inhibitors [54].
SU + metformin vs. sodium-glucose co-transporter-2 (SGLT-2) inhibitor + metformin
Glimepiride and empagliflozin showed similar HbA1c reduction at 104 weeks [55].
SU + metformin vs. glucagon-like peptide-1 (GLP1) analogs + metformin
One study showed the non-inferiority of liraglutide compared to glimepiride added to metformin [56].
SU + other oral antidiabetic drugs
The combination therapy of glimepiride added to rosiglitazone showed greater reductions in HbA1C and fasting blood glucose compared to monotherapy [56]. SU monotherapy shows better HbA1c reduction compared to other OADs (Table 4).
SU as the third line
In triple therapy, oral medications from three classes of antidiabetes agents and various insulin regimens are given in combination with two OHAs [57]. More than five meta-analysis indicate that adding a third drug to metformin and SU combination therapy is clinically more effective in reducing HbA1c than only dual therapy [58]. In one study, 74% of the patients (26/35) had well-controlled blood glucose levels (mean HbA1c of 6.9 ± 0.3%) after a mean follow-up of 37 months on triple oral therapy with a SU, metformin, and a thiazolidinedione [59]. Evidence suggests that in new-onset T2DM, triple therapy with antidiabetic drugs that enhance insulin sensitivity and beta-cell function led to a higher and more long-lasting reduction in HbA1c than treatment that only lowered glucose levels without addressing the underlying metabolic issues [60].
Glimepiride significantly improved HbA1C level (P < 0.001) from baseline when added to metformin and TZD. The same study showed that more patients on such triple therapy achieved target HbA1c(≤ 7%) compared with only metformin and TZD (62.2% vs. 26.0%, p < 0.001) [61]. Glimepiride, as part of triple OHA with sitagliptin and metformin, is known to improve the glucose-lowering effect on T2DM [62].
SU and insulin
Even with dual insulin, 50% of the patients do not achieve HbA1c < 7%. In a study, only 30% of the patients on insulin monotherapy attained glycemic targets. A Cochrane systematic review showed that SU-insulin combination therapy reduced HbA1c by 1%. Other SU-OAD combinations (metformin, AGIs, and DPP4Is) had a lower change in A1C. A meta-analysis showed that SU and insulin combination had better glycemic outcomes than insulin monotherapy (P < 0.0001). The combination of basal insulin and glimepiride reduced the requirement of total daily insulin by almost 30%. In another study, SU and once-daily basal insulin were better than premixed insulin monotherapy in reducing total insulin requirement. A Korean study concluded that glimepiride add-on to insulin glargine and metformin, reduced the HbA1c by 0.49% compared to only metformin and insulin (p < 0.01) [63].
Bedtime insulin daytime SU (BIDS)
SUs can be prescribed as part of the BIDS regimen [21]. Combination therapy is an alternative when starting insulin treatment in T2DM patients [64]. Significant and practically applicable improvements in glycemic control were achieved with flexible dosing of glimepiride/glargine regimens [65]. Table 5 summarizes the clinical evidence supporting the role of SUs in BIDS therapy.
A meta-analysis of five studies with low heterogeneity (16%) showed that bedtime neutral protamine Hagedorn (NPH) insulin plus SU (gliclazide, glimepiride, glipizide, and glibenclamide) was associated with lower HbA1c compared with once-daily NPH insulin alone (mean difference: 0.33%]; when the comparison included twice-daily insulin monotherapy regimens, this difference did not persist [67], suggesting that BIDS can lower HbA1c. The following clinical benefits are observed with BIDS: a) lesser requirement of insulin, b) reduced hypoglycemia risk (associated with insulin), c) lower the risk of weight gain, d) lowers the risk of a rise in blood pressure, and e) 24-h glycemic control.
SU vs newer OADs
During the subgroup analysis of the CAROLINA trial, linagliptin exhibited significantly less risk of hypoglycemia and falls or fractures in comparison to glimepiride. Nevertheless, both drugs were similarly effective in terms of cardiovascular and mortality outcomes across different age groups [68]. Dapagliflozin and glipizide had similar reductions in HbA1c levels (0.52%) in patients with type 2 diabetes uncontrolled on metformin. Although dapagliflozin demonstrated several advantages over glipizide, including a lower risk of hypoglycemia and weight reduction, patients on dapagliflozin experienced higher rates of genital and lower urinary tract infections than those on glipizide [56]. When SGLT-2 inhibitors are not well-tolerated or contraindicated, SU may be considered as an alternative treatment option. The practicing clinician must identify the right patient profile for SU therapy [46].
End-organ benefits
Cardiac
The ADVANCE clinical trial and the CV outcome data from the Cardiovascular Outcome Study of Linagliptin vs. Glimepiride in Type 2 Diabetes (CAROLINA) trial provided the cardiac safety profile of SUs. The modern SUs that include glimepiride and gliclazide MR are cardiovascular (CV) safe, i.e., both are not associated with increased CV risk [40, 49].
The incidence of cardiovascular events was similar with pioglitazone and SU such as gliclazide and glimepiride (HR 0.96, p 0.79) when they were added to metformin in the thiazolidinediones or sulfonylureas and cardiovascular accidents intervention trial (TOSCAT) [47]. The CAROLINA trial indicated that glimepiride was non-inferior to linagliptin (12% vs. 11.8%, P < 0.001 for non-inferiority) in terms of composite CV outcomes in patients with early T2DM and elevated cardiovascular risk over a median of 6.3 years [49].
Results of a meta-analysis concluded that gliclazide is the only medication that significantly reduces left ventricular mass (LVM) compared to placebo and other OADs (glyburide, voglibose, metformin, sitagliptin, pioglitazone, and rosiglitazone). Though SUs are known to have extrapancreatic effects, gliclazide shows higher SUR selectivity in pancreas, so its effect on SUR protein in the heart is minimal [69]. Despite both gliclazide and glyburide being sulfonylureas, only gliclazide can significantly lower LVM. Further research is required to determine whether gliclazide reduces mortality by reducing LVM.
Although the UKCPRD study found that using SU as a second-line treatment increased the risk of heart attack, death from any cause, and severe hypoglycemia compared to continuing to use metformin alone, the GRADE study found that there were no statistically significant differences between the groups taking liraglutide, sitagliptin, glimepiride, and glargine in terms of microvascular complications, major adverse cardiovascular events (MACE), hospitalizations for heart failure, or deaths from cardiovascular causes. However, as predicted, the risk of cardiovascular disease was significantly lower in the group taking liraglutide compared to those taking sitagliptin and glimepiride [70, 71]. A prospective cohort study involving 21,451 inpatients with T2DM and chronic heart failure found that long-term treatment with glimepiride resulted in a reduced number of hospitalizations and emergency visits due to heart failure, as well as fewer hospitalizations for acute myocardial infarction or stroke [72].
Renal
The 10-year long UK Prospective Diabetes Study (UKPDS 33) demonstrated a 25% reduction in risk (P 0.0099) of microvascular complications with glibenclamide (intensive treatment group) (UKPDS 33). Data from the ADVANCE ON trial showed that long-term gliclazide (intensive treatment) prevented end-stage kidney disease (ESKD) in patients with T2DM (HR: 0.54, p = 0.007) [41, 73]. Furthermore, gliclazide, compared to glimepiride, conferred protection from renal complications in certain patients even after 6 years of follow-up [41].
SU and risk of hypoglycemia
With judicious use and appropriate monitoring, hypoglycemia can be avoided in the majority of patients. The risk for major hypoglycemia is lowest with gliclazide compared to other SUs. A study compared SU (glimepiride, glibenclamide, or gliclazide) and sitagliptin in T2DM patients during Ramadan. The results indicated a lower incidence of hypoglycemia with gliclazide (6.6%) relative to the other SU [glibenclamide (19.7%) and glimepiride (12.4%)] and similar to that observed with sitagliptin (6.7%) [74]. Another randomized multicentric study showed similar hypoglycemic events among uncontrolled T2DM Indian patients that received glimepiride or sitagliptin in combination with metformin [75].
SU and weight gain
A major side effect of using SU is weight gain. Patients taking gliclazide in the ADVANCE trial gained 0.7 kg over 5 years [40]. Gradual up titration of gliclazide did not show any weight gain [76]. Patients in the DIA-RAMADAN trial had a mean weight loss of 0.5 kg/m2 [77].
Modern sulfonylureas
Among the modern SUs, glimepiride has undergone CVOT trial and showed to be cardio-safe. Gliclazide has a good CV safety profile, prevents renal complications, and has the lowest rate of hypoglycemic events [50]. Gliclazide’s antioxidant properties are attributed to it being an ROS scavenger, inhibiting LDL oxidation, and being antiatherogenic.
Where to position SUs?
For clinical success with SU, patient characteristics and medication adherence are important factors. SU can be the preferred choice for patients with newly diagnosed T2DM, especially in those who cannot tolerate metformin. Combination therapy has advantages compared to stepwise/sequential therapy. The concept of metabolic memory or legacy effect indicates the need for early good glycemic control to reduce the risk of long-term complications. Modern SUs, when combined with lifestyle changes and metformin, are a safe and effective initial treatment for patients whose HbA1c level ≥ 7.5% [63]. Thus, early implementation of metformin and SU in T2DM patients with high baseline HbA1c and low risk of hypoglycemia would be more beneficial than a stepwise approach of metformin first followed by add-on SU.
Table 6 summarizes different patient characteristics and clinical indications for SUs.
Concept of metabolic fulcrum
Choosing the right OHA becomes challenging with new drugs, indications, laboratory tests, and algorithms. In certain middle- and low-income countries, there is limited access to resources. Hence, the physician makes diagnostic and therapeutic decisions based on years of experience. Such challenges impair glycemic and other therapeutic goals. The metabolic fulcrum-based approach (Fig. 1) is a novel concept that categorizes patients with T2DM based on their characteristics. The categories are eubolic, predominantly catabolic, or “maladaptive anabolism” [83].
Metabolic fulcrum-based approach for T2DM patients. AGIs, alpha-glucosidase inhibitors; DPP4 inhibitor, dipeptidyl peptidase inhibitors; GLP1 RA, glucagon-like peptide-1 receptor agonist; SGLT2 inhibitors, sodium-glucose cotransporter-2 inhibitor. Sulfonylurea may be placed in the center of the fulcrum and can be used along with other OHAs to the left or right
This metabolic triage can be performed using just phenotypic features. Furthermore, there is no need for sophisticated investigations. Thus, physicians and other healthcare professionals may be able to rationalize OHA therapy. This method can be practiced by HCPs with minimal investigations as well. The metabolic fulcrum is proposed to help both patients and physicians to choose a rational therapy, thus facilitating a faster attainment of optimum glycemic targets [83].
Hypothetical clinical cases/patient phenotype/patient profile
The patient profiling for the use of SU in patients with T2DM is mentioned in Table 7.
*TyG index may be a reliable biomarker of insulin resistance. Lipid profile can determine if the patient can respond to SU or is insulin resistant (IR) [84, 85]
SU in recent Diabetes Guidelines
Sulfonylureas have the potential to be utilized in all stages of T2DM, ranging from an early stage as monotherapy along with lifestyle adjustments to a later stage as an add-on therapy to basal insulin, with intermediate steps involving dual oral therapy (add-on to metformin) or triple therapy (add-on to metformin plus a gliptin or a gliflozin). However, in each step, other pharmacological options may offer certain benefits. Opting for SUs in place of newer antidiabetic drugs like SGLT2 inhibitors, DPP4 inhibitors and GLP-1RAs could potentially deprive patients of important benefits like cardiorenal protection. The treatment regime should be individualized for each patient and tailored to the patient’s clinical profile and preferences while also considering cost and availability of the drugs, especially in low-resource countries like India [86] (Fig. 2).
SU in recent Diabetes Guideline: Country-wise diabetes guidelines (data from Refs. [6, 21, 86,87,88,89,90,91,92]). DPP4, dipeptidyl peptidase 4; GLP-1 RA, glucagon-like peptide-1 receptor agonist; ICMR, Indian Council of Medical Research; IDF, International Diabetes Federation; MODY,,maturity-onset diabetes of the young (MODY); NICE, National Institute for Health and Care Excellence; OHA, oral hypoglycemic agent; RSSDI-ESI—Research Society for Study of Diabetes in India–Endocrine Society of India; SAFES, South Asian Federation of Endocrine Societies; SGLT2, sodium-glucose cotransporter-2; SU, sulfonylurea; TZD, thiazolidinediones; WHO, World Health Organization
Specific clinical situations
Maturity-onset diabetes of the young (MODY)
MODY is a genetic type of diabetes described as early onset and a primary pancreatic β-cell dysfunction. Less than 2% of T2DM cases are actually MODY, but it is usually misdiagnosed as type 1 or 2 diabetes mellitus. SUs are recommended as a first-line treatment for MODY since patients with HNF1A-MODY are sensitive to SU therapy. An observational study indicated that patients with HNF1A-MODY could be switched safely from insulin to a SU, maintaining excellent glycemic control [79]. SU monotherapy is the recommended first-line treatment option in patients with MODY according to SAFES guidelines [21].
Renal impairment
Though real-world evidence suggests that glimepiride and gliclazide have similar clinical outcomes in patients with renal impairment and T2DM, gliclazide reduces renal complications and progression in specific patients. Among all SUs, gliclazide has a lower risk of doubling creatinine compared to glimepiride in patients that have preserved renal function (GFR ≥ 60 mL/min/1.73 m2) and good glycemic control (HbA1c < 7%), and in older subjects (≥ 62 years, HR: 0.52) [73, 93].
Dose adjustment for modern SU such as gliclazide and glipizide is not required in patients with CKD stages 3, 4, and 5. In contrast, glimepiride can be started conservatively at 1 mg daily in similar conditions, as per the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guideline for Diabetes and Chronic Kidney Disease (CKD) [93]. The RSSDI guidelines recommend glipizide and gliclazide as the preferable drug in the SU class of OHAs in patients with moderate-severe renal impairment. Low-dose gliclazide MR and glimepiride can be used in patients with mild to moderate renal impairment [87]. The preferred SU for patients with moderate to severe renal impairment should be a shorter-acting SU, metabolized in the liver like glipizide. Low-dose gliclazide and glimepiride may be used in mild to moderate renal impairment cases [21].
Older people
Geriatric patients are at an increased risk of hypoglycemia due to age-related fall in renal and hepatic functions, concomitant illnesses, poor nutrition, and polypharmacy. Thus, while prescribing SU to the elderly, physicians may prescribe low- or short-acting SU [21]. The IDF guideline for older people recommends SU as first-line when metformin is contraindicated and as second-line to metformin if glycemic targets are not achieved [94].
During Ramadan
While fasting, patients with T2DM on gliclazide MR had a lower risk of hypoglycemia and maintained good glycemic control and weight. No severe hypoglycemic events (HEs) were reported in the DIA-RAMADAN study. Furthermore, there was significant reduction in HbA1c (− 0.3%), fasting blood glucose (− 9.7 mg/dL), and BMI (− 0.2 kg/m2) after 4–6 weeks post Ramadan (P < 0.001) [77].
Steroid-induced diabetes
Glucocorticoid-induced hyperglycemia has a high incidence of 12%. Almost all medical subspecialties use glucocorticoids extensively. It is well established that glucocorticoid therapy may elicit new-onset T2DM and worsen hypoglycemia in patients with preexisting T2DM.
The incidence of glucocorticoid-induced hyperglycemia is high at 12%. Glucocorticoids are extensively used in almost every subspecialty of medicine. Glucocorticoid therapy is well known to provoke new-onset T2DM and invariably worsens hyperglycemia in patients with preexisting diabetes mellitus. Glucocorticoid-induced diabetes mellitus (GIDM) is characterized by an abnormal rise in blood glucose due to glucocorticoid therapy in a patient with or without a prior history of diabetes mellitus. Adverse effects of glucocorticoids are believed to be caused by increased insulin resistance, glucose intolerance, and reduced β-cell mass from β-cell dysfunction [95].
The approach to management steroid-induced hyperglycemia is usually individualized, and insulin is the drug of choice in most clinical situations of persistent hyperglycemia. According to a guideline from the Joint British Diabetes Societies (JBDS) for the inpatient care group, “a once-daily morning SU (e.g., gliclazide) may best manage the glucose excursion associated with a once-daily oral steroid” [96]. Insulin or SU doses should be titrated according to capillary blood glucose (CBG) readings to avoid hypoglycemia (Table 8). Sulfonylureas, such as gliclazide, glipizide, or glimepiride, are the first choice of OHA for the management of hyperglycemia due to use of steroids, since their blood-glucose-lowering action is immediate and postprandial hyperglycemia also improves. If patients are on OADs, single-dose gliclazide 40 mg for every 5 mg of prednisolone (or an equivalent steroid) taken before breakfast may be the best way to manage hyperglycemia in patients on once-daily steroids. The maximum one-time dose of gliclazide is 240 mg in such patients; according to CBG, readings need to be taken several times daily to identify hypo- or hyperglycemia. An additional dose of gliclazide (SU) or intermediate-acting insulin can be given to the patients in the evening that are uncontrolled on intermediate-acting insulin (NPH or detemir) [88, 89].
Sulfonylureas such as gliclazide, glipizide, or glimepiride, are the first choice of OHA for the management of hyperglycemia due to use of steroids, since their blood-glucose-lowering action is immediate and postprandial hyperglycemia also improves. If patients are on OADs, single-dose gliclazide 40 mg for every 5 mg of prednisolone (or an equivalent steroid) taken before breakfast may be the best way to manage hyperglycemia in patients on once-daily steroids. The maximum one-time dose of gliclazide is 240 mg in such patients; according to CBG, and readings need to be taken several times daily to identify hypo- or hyperglycemia. An additional dose of gliclazide (SU) or intermediate-acting insulin can be given to the patients in the evening that are uncontrolled on intermediate-acting insulin (NPH or detemir) [97, 98].
Sulfonylureas and Covid-19
With regard to Covid-19, few retrospective studies showed no significant difference for intensive care unit (ICU) admissions (RR = 1.35, p = 0.56), mechanical ventilation (RR 3.55, P 0.22), severe disease (OR 1.16, P 0.74), and death (OR 0.84, P 0.79). Furthermore, a prospective clinical study on diabetes patients with Covid-19, that received SU and glinides found no benefits or harm on primary or secondary endpoints on days 7 and 28. Thus, no evidence of harm suggests that SUs may not be stopped in Covid-19 patients unless very sick, hospitalized, or contraindicated [99]. The nationwide National Diabetes Audit database in England (February 2020 to August 2020) found a negative association between metformin, sulfonylureas, and SGLT2 inhibitors and the risk of COVID-19-related death [100]. Thus, the data suggest a protective effect of sulfonylureas on Covid-19 patients with T2DM.
Modern SU that selectively binds to the pancreatic SUR may be safer than non-selective drugs in hospitalized patients with severe Covid-19. Although a RCT is not done, the preliminary data from a nationwide French registry of MI showed improvement in outcomes in patients that received gliclazide/glimepiride compared to glibenclamide. Unexpected alterations in meal intake occur in ICU patients; SU may increase the risk for severe hypoglycemia in ICU patients [101].
This document can help clinicians and policymakers to reduce the gap between scientific evidence of SU and recommended clinical practice in T2DM management. Furthermore, this expert consensus document gives recommendations for clinicians who are uncertain about different patient profiles that can benefit from treatment with SU. The main objective is to improve quality of clinical decisions and patient outcomes.
Conclusion
SUs are one of the most commonly prescribed antidiabetic drugs, and their efficacy and safety are well-established, making them an integral part of T2D pharmacotherapy. The choice of SU should be highly individualized, along with careful monitoring of patients with a high risk of hypoglycemia. Based on the available evidence, modern SUs should be utilized instead of traditional SUs for T2DM management because of their enhanced efficacy, cardiovascular safety, lower risk of hypoglycemia, and weight-neutralizing effects. These advantages position modern SUs as a front-line agent in the early management of T2DM. For effective and vigilant use of SUs, appropriate patient selection, proper patient education, physician training, and careful drug and dose selection are essential.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Released Controlled Evaluation; ADVANCE ON – ADVANCE Observational study
- CAROLINA:
-
Cardiovascular Outcome Study of Linagliptin vs Glimepiride in Type 2 Diabetes
- GRADE:
-
Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness
- GUIDE:
-
GlUcose control in type 2 diabetes: Diamicron MR vs. Glimepiride; MR – modified release
- TOSCA-IT:
-
Thiazolidinediones or Sulfonylureas and Cardiovascular Accidents Intervention Trial;
- UK CPRD:
-
UK clinical practice research datalink
- UKPDS:
-
UK Prospective Diabetes Study
References
Home, Resources, diabetes L with et al. IDF Diabetes atlas | tenth edition. https://diabetesatlas.org/ (19 November 2021, date last accessed).
Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072.
Ranasinghe P, Jayawardena R, Gamage N, et al. Prevalence and trends of the diabetes epidemic in urban and rural India: a pooled systematic review and meta-analysis of 17 million adults. Ann Epidemiol. 2021;58:128–48.
Luhar S, Kondal D, Jones R, et al. Lifetime risk of diabetes in metropolitan cities in India. Diabetologia. 2021;64:521–9.
World Health Organization. Continuity of essential health services: facility assessment tool: a module from the suite of health service capacity assessments in the context of the COVID-19 pandemic: interim guidance 20 October 2020. Geneva: World Health Organization; 2020.
Recommendations | Type 2 diabetes in adults: management | Guidance | NICE. https://www.nice.org.uk/guidance/ng28/chapter/Recommendations#drug-treatment-2 (14 September 2021, date last accessed)
Ashraf H, Faraz A, Ahmad J. Achievement of guideline targets of glycemic and non-glycemic parameters in north Indian type 2 diabetes mellitus patients: a retrospective analysis. Diabetes Metab Syndr. 2021;15:425–31.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2009;15:540–59.
Pérez CM, Febo-Vázquez I, Guzmán M, et al. Are adults diagnosed with diabetes achieving the American Diabetes Association clinical practice recommendations? P R Health Sci J. 2012;31:18–23.
Miñambres I, Mediavilla JJ, Sarroca J, et al. Meeting individualized glycemic targets in primary care patients with type 2 diabetes in Spain. BMC Endocr Disord. 2016;16:10.
Corriere MD, Minang LB, Sisson SD, et al. The use of clinical guidelines highlights ongoing educational gaps in physicians’ knowledge and decision making related to diabetes. BMC Med Educ. 2014;14:186.
Larme AC, Pugh JA. Evidence-based guidelines meet the real world: the case of diabetes care. Diabetes Care. 2001;24:1728–33.
Seidu S, Khunti K. Non-adherence to diabetes guidelines in primary care - the enemy of evidence-based practice. Diabetes Res Clin Pract. 2012;95:301–2.
Thepwongsa I, Kirby C, Paul C, et al. Management of type 2 diabetes: Australian rural and remote general practitioners’ knowledge, attitudes, and practices. Rural Remote Health. 2014;14:2499.
Chu W-M, Ho H-E, Huang K-H, et al. The prescribing trend of oral antidiabetic agents for type 2 diabetes in Taiwan. Medicine (Baltimore). 2017;96: e8257.
Tan YZ, Cheen MHH, Goh S-Y, et al. Trends in medication utilization, glycemic control and outcomes among type 2 diabetes patients in a tertiary referral center in Singapore from 2007 to 2017. J Diabetes. 2019;11:573–81.
Ji L, Lu J, Weng J, et al. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. J Diabetes. 2015;7:166–73.
Kitten AK, Kamath M, Ryan L, et al. National ambulatory care non-insulin antidiabetic medication prescribing trends in the United States from 2009 to 2015. PLoS ONE. 2019;14: e0221174.
Liatis S, Dafoulas GE, Kani C, et al. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nationwide prescription database. Diabetes Res Clin Pract. 2016;118:162–7.
Wilkinson S, Douglas I, Stirnadel-Farrant H, et al. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000–2017. BMJ Open. 2018;8: e022768.
Kalra S, Aamir AH, Raza A, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19:577–96.
Sultana G, Kapur P, Aqil M, et al. Drug utilization of oral hypoglycemic agents in a university teaching hospital in India. J Clin Pharm Ther. 2010;35:267–77.
Agarwal AA, Jadhav PR, Deshmukh YA. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J Basic Clin Pharm. 2014;5:79–83.
Jain J, Sharma P, Jain J, et al. Utilization pattern of oral hypoglycemic agents for diabetes mellitus type 2 patients attending out-patient department at tertiary care centre in Bhopal, Madhya Pradesh. India Int J Basic Clin Pharmacol. 2017;5:1826–30.
Chow CK, Ramasundarahettige C, Hu W, et al. Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol. 2018;6:798–808.
Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2011;17(Suppl 2):1–53.
Narayan KMV, Kondal D, Kobes S, et al. Incidence of diabetes in South Asian young adults compared to Pima Indians. BMJ Open Diabetes Res Care. 2021;9: e001988.
Garg R. Insulin resistance versus insulin deficiency: evidence of racial differences in the pathogenesis of type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9: e002261.
Anjana RM, Pradeepa R, Unnikrishnan R, Tiwaskar M, Aravind SR, Saboo B, Joshi SR, Mohan V. New and unique clusters of type 2 diabetes identified in Indians. J Assoc Physicians India. 2021;69(2):58–61.
Jindal S, Kalra S. Developing a definition for oral antidiabetic drug (OAD) failure. JPMA J Pak Med Assoc. 2020;70:547–51.
Ikegami H, Babaya N, Noso S. β-Cell failure in diabetes: common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J Diabetes Investig. 2021;12:1526–39.
Kunavisarut T, Sriussadaporn S, Lertwattanarak R. Beta-cell function in type 2 diabetic patients who failed to maintain good glycemic status with a combination of maximum dosages of metformin and sulfonylurea. Diabetes Metab Syndr Obes Targets Ther. 2019;12:761–70.
Ashcroft FM, Gribble FM. Tissue-specific effects of sulfonylureas: lessons from studies of cloned K(ATP) channels. J Diabetes Complications. 2000;14:192–6.
Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–19.
Singh AK, Singh R. Is gliclazide a sulfonylurea with difference? A review in 2016. Expert Rev Clin Pharmacol. 2016;9:839–51.
Cordiner RLM, Mari A, Tura A, et al. The impact of low-dose gliclazide on the incretin effect and indices of beta-cell function. J Clin Endocrinol Metab. 2021;106:2036–46.
Briscoe VJ, Griffith ML, Davis SN. The role of glimepiride in the treatment of type 2 diabetes mellitus. Expert Opin Drug Metab Toxicol. 2010;6:225–35.
Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med. 2008;359:1618–20.
Zoungas S. ADVANCE in context: the benefits, risks and feasibility of providing intensive glycaemic control based on gliclazide modified release. Diabetes Obes Metab. 2020;22:5–11.
Heller SR, on behalf of the ADVANCE Collaborative Group. A summary of the ADVANCE Trial. Diabetes Care. 2009;32:S357–61.
Wong MG, Perkovic V, Chalmers J, et al. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39:694–700.
Gaede P, Lund-Andersen H, Parving H-H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91.
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr Clin Res Rev. 2016;10:S176–83.
Viñas Esmel E, Naval Álvarez J, Sacanella ME. The legacy effect in the prevention of cardiovascular disease. Nutrients. 2020;12:3227.
Prattichizzo F, de Candia P, De Nigris V, et al. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110: 154308.
Abrahamson MJ. Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? Yes, they continue to serve us well!: Table 1. Diabetes Care. 2015;38:166–9.
Vaccaro O, Masulli M, Nicolucci A, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5:887–97.
Schernthaner G, Grimaldi A, Di Mario U, et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34:535–42.
Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155.
Zaccardi F, Jacquot E, Cortese V, et al. Comparative effectiveness of gliclazide modified release versus sitagliptin as second-line treatment after metformin monotherapy in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2020;22:2417–26.
Trial GRADEs effectiveness of four common second-line type 2 diabetes drugs. diabetes.medicinematters.com 2021 https://diabetes.medicinematters.com/ada-2021/medications/grade-study-metformin-add-ons/19311618 (13 October 2021, date last accessed).
Hirst JA, Farmer AJ, Dyar A, et al. Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis. Diabetologia. 2013;56:973–84.
Bolen S, Tseng E, Hutfless S, et al. Diabetes medications for adults with type 2 diabetes: an update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016.
Umpierrez G, Issa M, Vlajnic A. Glimepiride versus pioglitazone combination therapy in subjects with type 2 diabetes inadequately controlled on metformin monotherapy: results of a randomized clinical trial. Curr Med Res Opin. 2006;22:751–9.
Ridderstråle M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700.
Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15:204–12.
Davidson MB. Triple therapy: definitions, application, and treating to target. Diabetes Care. 2004;27:1834–5.
Moon MK, Hur K-Y, Ko S-H, et al. Combination therapy of oral hypoglycemic agents in patients with type 2 diabetes mellitus. Diabetes Metab J. 2017;41:357–66.
Bell DSH, Ovalle F. Long-term efficacy of triple oral therapy for type 2 diabetes mellitus. Endocr Pract. 2002;8:271–5.
Abdul-Ghani M, Puckett C, Adams J, et al. Durability of triple combination therapy versus stepwise addition therapy in patients with new-onset T2DM: 3-year follow-up of EDICT. Diabetes Care. 2021;44:433–9.
Roberts VL, Stewart J, Issa M, et al. Triple therapy with glimepiride in patients with type 2 diabetes mellitus inadequately controlled by metformin and a thiazolidinedione: results of a 30-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2005;27:1535–47.
Arai K, Maeda H, Sirabe S, et al. Glimepiride strongly enhances the glucose-lowering effect in triple oral antidiabetes therapy with sitagliptin and metformin for Japanese patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15:335–41.
Kalra S, Bahendeka S, Sahay R, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus - international task force. Indian J Endocrinol Metab. 2018;22:132–57.
Olsson PO, Lindström T. Combination-therapy with bedtime nph insulin and sulphonylureas gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes Metab. 2002;28:272–7.
Standl E, Maxeiner S, Raptis S, et al. Good glycemic control with flexibility in timing of basal insulin supply: a 24-week comparison of insulin glargine given once daily in the morning or at bedtime in combination with morning glimepiride. Diabetes Care. 2005;28:419–20.
He Y-L, Serra D, Wang Y, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2007;46:577–88.
Abdi H, Azizi F, Amouzegar A. Insulin monotherapy versus insulin combined with other glucose-lowering agents in type 2 diabetes: a narrative review. Int J Endocrinol Metab. 2018;16: e65600.
Espeland MA, Pratley RE, Rosenstock J, et al. Cardiovascular outcomes and safety with linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulphonylurea glimepiride in older people with type 2 diabetes: a subgroup analysis of the randomized CAROLINA trial. Diabetes Obes Metab. 2021;23:569–80.
Ida S, Kaneko R, Murata K. Effects of oral antidiabetic drugs on left ventricular mass in patients with type 2 diabetes mellitus: a network meta-analysis. Cardiovasc Diabetol. 2018;17:129.
Douros A, Dell'Aniello S, Yu OHY, Filion KB, Azoulay L, Suissa S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ. 2018;362:k2693. https://doi.org/10.1136/bmj.k2693.
GRADE Study Research Group; Nathan DM, Lachin JM, Bebu I, Burch HB, Buse JB, Cherrington AL, Fortmann SP, Green JB, Kahn SE, Kirkman MS, Krause-Steinrauf H, Larkin ME, Phillips LS, Pop-Busui R, Steffes M, Tiktin M, Tripputi M, Wexler DJ, Younes N. Glycemia reduction in type 2 diabetes — microvascular and cardiovascular outcomes. N Engl J Med. 2022;387(12):1075–1088. https://doi.org/10.1056/NEJMoa2200436.
He W, Yuan G, Han Y, Yan Y, Li G, Zhao C, Shen J, Jiang X, Chen C, Ni L, Wang DW. Glimepiride use is associated with reduced cardiovascular mortality in patients with type 2 diabetes and chronic heart failure: a prospective cohort study. Eur J Prev Cardiol 2022;zwac312. https://doi.org/10.1093/eurjpc/zwac312
Lee Y-h, Lee CJ, Lee HS, et al. Comparing kidney outcomes in type 2 diabetes treated with different sulphonylureas in real-life clinical practice. Diabetes Metab. 2015;41:208–15.
Al Sifri S, Basiounny A, Echtay A, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011;65:1132–40.
Devarajan TV, Venkataraman S, Kandasamy N, et al. Comparative evaluation of safety and efficacy of glimepiride and sitagliptin in combination with metformin in patients with type 2 diabetes mellitus: Indian multicentric randomized trial - START study. Indian J Endocrinol Metab. 2017;21:745–50.
Zoungas S, Chalmers J, Kengne AP, et al. The efficacy of lowering glycated haemoglobin with a gliclazide modified release-based intensive glucose lowering regimen in the ADVANCE trial. Diabetes Res Clin Pract. 2010;89:126–33.
Hassanein M, Al Sifri S, Shaikh S, et al. A real-world study in patients with type 2 diabetes mellitus treated with gliclazide modified-release during fasting: DIA-RAMADAN. Diabetes Res Clin Pract. 2020;163: 108154.
Ahrén B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–8.
Kim S-H. Maturity-onset diabetes of the young: what do clinicians need to know? Diabetes Metab J. 2015;39:468–77.
McCluskey D, Touger MS, Melis R, et al. Results of a randomized, double-blind, placebo-controlled study administering glimepiride to patients with type 2 diabetes mellitus inadequately controlled with rosiglitazone monotherapy. Clin Ther. 2004;26:1783–90.
Chou HS, Palmer JP, Jones AR, et al. Initial treatment with fixed-dose combination rosiglitazone/glimepiride in patients with previously untreated type 2 diabetes. Diabetes Obes Metab. 2008;10:626–37.
Harashima S-I, Ogura M, Tanaka D, et al. Sitagliptin add-on to low dosage sulphonylureas: efficacy and safety of combination therapy on glycaemic control and insulin secretion capacity in type 2 diabetes. Int J Clin Pract. 2012;66:465–76.
Kalra S, Department of Endocrinology, Bharti Hospital Karnal, India, Gupta Y, et al. Choice of glucose-lowering therapy— a metabolic fulcrum-based approach. US Endocrinol. 2015;11:79.
Unger G, Benozzi SF, Perruzza F, et al. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr Engl Ed. 2014;61:533–40.
Simental-Mendía LE, Guerrero-Romero F. The correct formula for the triglycerides and glucose index. Eur J Pediatr. 2020;179:1171–1171.
Scheen AJ. Sulphonylureas in the management of type 2 diabetes: to be or not to be? Diabetes Epidemiol Manag. 2021;1: 100002.
Chawla R, Madhu S, Makkar B, et al. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020;24:1.
ICMR Guidelines for management of type 2 diabetes 2018. Indian Council of Medical Research.
Guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. https://apps.who.int/iris/handle/10665/272433 (14 September 2021, date last accessed).
International Diabetes Federation. Global guideline for type 2 diabetes 2005. Brussesl: International Diabetes Federation; 2017.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11:1020–76.
Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, Zou D, Guo L, Ji Q, Chen L, Chen L, Dou J, Guo X, Kuang H, Li L, Li Q, Li X, Liu J, Ran X, Shi L, Song G, Xiao X, Yang L, Zhao Z; Chinese Diabetes Society. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. https://doi.org/10.1002/dmrr.3158.
National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis Off J Natl Kidney Found. 2012;60:850–86.
International Diabetes Federation, Sinclair A, Dunning T, et al. Managing older people with type 2 diabetes: global guideline. 2013.
Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab Seoul Korea. 2017;32:180–9.
Roberts A, James J, Dhatariya K, et al. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies ( JBDS ) for inpatient care group. Diabet Med. 2018;35:1011–7.
Morris D. Steroid-induced diabetes and hyperglycaemia. Part 2: management. 20:5.
Panikar V, Sosale A, Agarwal S, et al. RSSDI clinical practice recommendations for management of in-hospital hyperglycaemia—2016. Int J Diabetes Dev Ctries. 2016;36:1–21.
Singh AK, Singh R, Saboo B, et al. Non-insulin anti-diabetic agents in patients with type 2 diabetes and COVID-19: a critical appraisal of literature. Diabetes Metab Syndr Clin Res Rev. 2021;15:159–67.
Hadjadj S, Wargny M. Glucose-lowering treatments and COVID-19 mortality in T2DM. Nat Rev Endocrinol. 2021;17:387–8.
Hasan SS, Kow CS, Bain A, et al. Pharmacotherapeutic considerations for the management of diabetes mellitus among hospitalized COVID-19 patients. Expert Opin Pharmacother. 2021;22:229–40.
Acknowledgment
The authors acknowledge all the doctors who participated in the advisory board meetings, which were instrumental in developing the recommendations and final manuscript. The authors acknowledge IntelliMed Healthcare Solutions LLP, Mumbai, India, for medical writing support.
Funding
The publication fee was funded by Dr. Reddy’s Laboratories, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Competing interests
Authors AKD, BS, RC, SRA, RR, AKS, JJM, AJ, PS, VD, SK, SJ, and GRS are on the advisory board of Dr. Reddy`s Laboratories. Authors RD and KD are employees of Dr. Reddy`s Laboratories.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, A.K., Saboo, B., Chawla, R. et al. Time to reposition sulfonylureas in type 2 diabetes management in Indian context: A pragmatic practical approach. Int J Diabetes Dev Ctries 43, 856–874 (2023). https://doi.org/10.1007/s13410-023-01192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01192-6