Abstract
Background
Lifestyle modification is an integral aspect for the management of type 2 diabetes (T2D). However, it is difficult to ensure the accuracy of personalized lifestyle advice. The study aims to analyse the real-world effectiveness of personalized glycemic response based Diabefly-Pro digital therapeutics for better glycemic control.
Methods
Data from continuous glucose monitoring (CGM) of 64 participants with T2D was analysed. All participants were provided with modified lifestyle plan based on their personalized glycemic response. The CGM data was analysed for a period of 7 days, before and after the introduction of modified lifestyle plan. Primary outcome of the study was change in time in range (TIR). Secondary outcomes of the study were change in mean blood glucose, time above range (TAR), time below range (TBR) and glucose management indicator (GMI).
Results
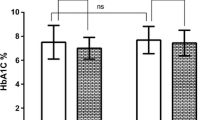
Significant improvement in glycemic control was observed after the introduction of personalized lifestyle plan. Median reduction in mean blood glucose was from 139.5 (118.3 to 169.3) mg/dL to 122.0 (101.5 to 148.8) mg/dL (p < 0.0001). TIR and GMI improved from 70.50 (50.75 to 83.50) % to 75.00 (58.25 to 89.00) % (p = 0.0001) and 6.64 (6.13 to 7.35) % to 6.23 (5.74 to 6.86) % (p < 0.0001) respectively. TAR reduced significantly from 17.00 (4.25 to 38.0) % to 6.00 (1.25 to 26.0) % (p < 0.0001). No significant increase in TBR was observed (p = 0.198).
Conclusion
Personalized glycemic response-based Diabefly-Pro digital therapeutics program was effective in achieving better glycemic control in people with T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the fastest growing health challenges with an increasing prevalence rate across the world. The global prevalence of diabetes was estimated as 463 million people which is expected to rise to 578 million by 2030 [1]. India ranks second in the world with 77 million people with diabetes in 2019, which is expected to increase to 101 million by 2030 [1]. The rising prevalence of diabetes has been attributed mainly to increasing cases of type 2 diabetes (accounting for around 90% of total cases) due to ageing, rapid urbanisation and increase in the level of risk factors like obesity, unhealthy diet and physical inactivity [2, 3]. It has been shown that adequate glycemic control among people with diabetes leads to reduction in both macrovascular and microvascular complications [4, 5]. In India, the poor level of glycemic control has been highlighted in many studies [6, 7]. The huge burden of diabetes, lack of access to trained diabetes educators, poor diabetes knowledge in patients, poor communication with healthcare teams due to pre-existing heavy loads at clinics and lack of continuous personalized interventions limits holistic management of diabetes in India [8]. The conventional mode of delivery of lifestyle management lessons using face-to-face interventions are now even more limited after the COVID-19 pandemic [9]. Thus, effective management of diabetes requires virtual patient engagement in addition to clinician’s support.
Lifestyle management and behavioral modification is an integral aspect for the effective prevention and management of T2D [10,11,12]. It includes diabetes self-management education and support (DSMES), medical nutrition therapy (MNT), physical activity, weight loss, smoking cessation counselling, and psychological support [13]. DSMES is aimed at developing the skill and ability of the patients to make informed decisions, problem solving; while the support assists patients to continue the implementation of learned skill and behaviors [14]. MNT is a demanding part of lifestyle management aimed at promoting healthy eating patterns where each individual requires a personalized solution. MNT has been recommended for patients with diabetes and has been shown to improve glycemic control in people with T2D [15, 16].
Last three decades have witnessed rapid development in digital and wireless technologies for the management of diabetes in the field of lifestyle modification, better medical devices (blood glucose meters, continuous glucose monitoring (CGM) devices) [17, 18]. Digital therapeutics have emerged as a new modality for the prevention and management of disease based on evidence-based intervention driven by use of software. Application of digital therapeutics enables large-scale deployment of treatments in a cost-effective manner wherein people can engage with the platform several times a day leading to improved outcomes as compared to conventional treatment [19, 20]. Globally, digital therapeutics technology has shown immense potential to help in the management of diabetes [21, 22]. To our knowledge, there is no personalized glycemic response-based digital therapeutics program currently present in India. The increasing burden of diabetes and relative lack of healthcare resources underline an urgent need for personalized digital therapeutics programs.
The current study is aimed at exploring the real-world effectiveness of Diabefly-Pro digital therapeutics program designed for providing lifestyle, nutritional and behavioral coaching to people with T2D with the use of connected medical device (like CGM) based on their personalized glycemic response for better glycemic control. We analysed the real-world data on glycemic parameters collected from CGM for 14 days. The effectiveness of the program was analysed on the basis of parameters like mean glucose, time in range (TIR), time above range (TAR), time below range (TBR) and glucose management indicator (GMI). The correlation of TIR with diabetes complications has been established in various studies [23, 24].
During the program, a personalized lifestyle plan was created based on the individual glycemic response during the first 7 days of the study while following their usual lifestyle. The variation in glycemic parameters for 7 days, pre and post the introduction of the personalized lifestyle plan was analysed to understand the change in glycemic control. Thus, the study was aimed at the analysis of effectiveness of the personalized lifestyle plan provided to participants based on CGM monitoring using the digital therapeutics platform.
Materials and methods
Study design
The study involved the analysis of de-identified data from 64 participants with T2D using the Diabefly-Pro program (Fitterfly Healthtech Pvt Ltd, Mumbai, India) who continued using the platform for 14 days after program initiation. The participants were recruited based on referrals by clinicians and through social media advertisements. The participants self-declared their T2D status and were contacted through telephone before joining the program wherein the program details were explained to them. The screening was based on the inclusion and exclusion criteria for the study. Eligible candidates who provided written or electronic consent to participate and to provide the de-identified data for research purpose were enrolled in the study. In case consent was not given for use of data for research, the program participation was provided without any changes in the quality of care. The study was aimed at analysis of de-identified data; no investigational products were used and standard clinical treatment was followed throughout the study.
The inclusion criteria for the study were (1) age ≥ 18 years, (2) owns a smartphone and is willing to utilize the mobile application and (3) has a minimum level of literacy to read and understand in English language. The exclusion criteria of the study included (1) presence of physical, cognitive and psychiatric impairment which can prevent participation in the program; (2) pregnancy; (3) severe complications (end stage chronic kidney failure, chronic liver disease); (4) history of unstable angina pectoris or stroke within the past 6 months; and (5) any recent surgical procedure that causes major physical, cognitive or psychiatric impairment that will prevent participation in program as determined by registered medical practitioner were excluded. Additionally, recent history of gastro-intestinal tract surgery including bariatric surgery which affects the ability to follow dietary regimen led to exclusion of patients.
The primary outcomes of the study were changes in TIR. The secondary outcomes of the study included the change in TAR, TBR, mean glucose and GMI. The study involved the comparison of outcomes for 7 days pre and post the introduction of the personalized lifestyle plan.
Sample size calculation
Minimum sample size for pre- and post-test comparison was calculated using University of California San Francisco online calculator [25]. A sample size of 64 was required to achieve 80% power to detect an estimated standard deviation of the post-over-pre change of 15.88% [26] with an effect size of 0.35 at 5% level of significance using a two-sided Wilcoxon test with Gaussian approximation.
Program
Figure 1 shows the schematic of Diabefly-Pro program which is an evidence-based digital therapeutics program based on personalized glycemic response. The program provides access to the Fitterfly mobile application and helps the participants by creating a personalized lifestyle plan (diet, activity and lifestyle) based on an individual’s glycemic characteristics. The digital therapeutics program has been designed to incorporate various features like tools to record and track medical and anthropometric parameters; in-app based extensive Indian food database for tracking calories, macro and micronutrient in meals; digital tracking tools for meals, exercise; in-app access to evidence-based educational and motivational content for diabetes management; support from health coaches (diabetes educators) to manage stress and sleep quality; regular feedback and support from health coaches for building lifelong habits through behavioural modifications. The Diabefly-Pro program also provides access to nutritionists, clinical psychologists and physiotherapists for providing personalized care to people with diabetes.
The Diabefly-Pro program is a 90-day program which consists of three phases; the first phase is the observation phase which involves CGM monitoring based on normal lifestyle (daily meals, activity, sleep quality and stress) of participants; the second phase is the intervention phase where nutritionist and physiotherapist provide every patient with a diet and exercise plan respectively based on their personalized glycemic response data collected from CGM monitoring. Feedback regarding stress management and sleep quality is also provided. The participants are instructed to follow the modified diet and exercise plan and are monitored again for next 7 days using CGM monitoring; the third phase of the program aimed at sustaining the lifestyle modification introduced during the second phase of the program while including regular feedback and support from health coaches to build lifelong lifestyle change for better management of diabetes.
The compliance to the program was ensured through regular video and telephonic calls by remote health coaches every 15 days, providing planned education material containing motivational content for diabetes self-management via mobile application and charging a payment fee for program participation.
Data collection
All the participants enrolled in the program received a link to download the Fitterfly mobile application from both android and ios playstore. A trained program staff performed the application of CGM sensor (FreeStyle Libre Pro, Abbott Diabetes Care) during home visit. The platform used for downloading and collection of the CGM data was FreeStyle Libre Pro Software (Abbott Diabetes care). The study was conducted for 14 days wherein in the initial 7 days the participants followed their normal lifestyle and the CGM data was collected. The data from the CGM sensor was collected at home by trained personnel. The participants also maintained their food and physical activity diary with time stamps in the Fitterfly app. The initial 7 days CGM data was analysed to understand the personalized glycemic response of individuals. After the completion of initial 7 days on the program, the participants were explained their glycemic response to various meals and physical activities. The nutritionist and physiotherapist created a personalized meal and exercise plan for participants based on their initial 7 days. The participants were instructed to follow the recommended personalized lifestyle plan for the next 7 days of the study. The CGM data for the next 7 days was collected again and analysed to understand the effectiveness of the intervention. During the entire duration of 14 days, the participants had access to the Fitterfly mobile application for creating daily log of food diary, physical activity, medication, water intake, sleep and various anthropometric (weight, height, BMI) and clinical parameters (fasting blood sugar, HbA1c). The educational content was also made available to improve the problem-solving ability and to understand the self-care activities for people with diabetes.
Baseline demographic data was provided by participants using the mobile application. The data from CGM included metrics like TIR, TAR, TBR and mean glucose levels. GMI was used as an estimate of glycated hemoglobin level, as described by Bergenstal et al. 2018 [27]. Mean glucose level (CGM) was used for calculation of GMI.
Statistical analysis
The statistical analysis was performed using R software (Version 4.0.3; The R Foundation). Categorical data was represented as number (%); continuous data was expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)) as appropriate for the data distribution. Normality of data was tested using the Shapiro-Wilk test. The comparison of glycemic control parameters pre and post the introduction of personalized lifestyle plan was evaluated using Wilcoxon signed-rank test. p ≤ 0.05 was considered statistically significant. The variation in TIR among groups with different modalities of management including only lifestyle modification (no pharmacotherapy), oral hypoglycemic agents (OHAs), insulin and combination of OHAs and insulin were compared using one-way analysis of variance. The correlation between change in TIR with the various parameters at the time of enrolment like age, duration of diabetes, weight and BMI was studied using Pearson test for parametric data and Spearman test for non-parametric data.
Results
Table 1 shows the baseline characteristics of 64 participants with T2D enrolled in the study. Overall, the participants consisted of 35.93% (23/64) females; the mean age of the participants was 51.08 ± 11.84 years with an average duration of diabetes of 9.67 ± 8.9 years. The mean weight and BMI of the participants were 72.75 ± 12.36 kg and 26.38 ± 4.0 kg/m2 respectively. The medication details of participants showed 10.94% (7/64) of participants were using insulin, 37.50% (24/64) used oral hypoglycemic agents (OHAs) and 32.81% (21/64) were using both insulin and OHAs and 18.75% (12/64) did not use any pharmacotherapy (only lifestyle modification). Further, 56.25% (36/64) of the participants were on biguanide, 45.31% (29/64) were on sulfonylurea, 28.13% (18/64) were on dipeptidyl peptidase (DPP)-4 inhibitors, 23.44% (15/64) were on Sodium-glucose cotransporter-2 (SGLT2) inhibitors, 14.06% (9/64) were on alpha-glucosidase inhibitors, 10.94% (7/64) were on thiazolidinediones and 4.68% (3/64) were on other OHAs or non-specified medications. Then, 62.5% (40/64) of patients had comorbidity other than diabetes.
Table 2 shows the changes in glycemic control metrics pre and post the intervention of personalized lifestyle modification plan. The mean glucose in participants reduced significantly from 139.50 (118.30 to 169.30) mg/dL to 122.0 (101.50 to 148.80) mg/dL (p < 0.0001). There was a significant improvement in the TIR from 70.50 (50.75 to 83.50) % to 75.00 (58.25 to 89.00) % (p = 0.0001). The TAR significantly reduced from 17.00 (4.25 to 38.0) % to 6.00 (1.25 to 26.0) % (p < 0.0001). The TBR did not change significantly pre and post the intervention (p = 0.198). GMI has been used as an approximate measure of lab HbA1c value. GMI improved significantly from 6.64 (6.13 to 7.35) % to 6.23 (5.74 to 6.86) % (p < 0.0001).
Figure 2 shows the mean change in mean blood glucose, TIR, TAR and TBR when compared pre and post the intervention. Mean blood glucose was reduced by 18.78 ± 21. 12 mg/dL. Percentage of time in TIR showed a mean improvement of 6.61 ± 13.44%. Percentage of time in TAR and TBR showed a mean reduction of 6.05 ± 11.54% and 2.07 ± 15.58% respectively. GMI showed a mean reduction of 0.45 ± 0.50%.
No significant variation in the change in TIR was observed among groups with different modality of treatment including only lifestyle modification (no pharmacotherapy), OHAs, insulin and combination of insulin and OHAs (p = 0.48). The real-world effectiveness of the lifestyle modification plan as quantified through the significant improvement in TIR showed no significant correlation with factors like age (p = 0.34) and other parameters recorded at time of enrolment like weight (p = 0.89), BMI (p = 0.71) and duration of diabetes (p = 0.32).
Discussion
The study was aimed at the analysis of real-world effectiveness of Diabefly-Pro digital therapeutics platform providing support based on personalized glycemic response to people with T2D in India. The study was aimed at the analysis of glycemic response 1-week pre and post the intervention of personalized lifestyle plan based on individualized inputs for nutrition and exercise based on CGM monitoring. The results showed that the intervention of personalized lifestyle plan for 1 week in participants led to significant improvement in their metabolic control which was shown by improvement in mean blood glucose, TIR, TAR and GMI. Then, 85.93% (55/64) participants showed reduction in mean blood glucose 7 days post the intervention. Further, 60.93% (39/64) of the participants showed improvement in TIR by ≥ 5%. Moreover, 39.06% (25/64) and 28.12% (18/ 64) showed improvement in TIR ≥ 10% and ≥ 15% respectively.
Every incremental 5% increase in TIR has been associated with significant clinical benefits in people with T2D [23]. Ten percent reduction in TIR has been shown to increase the hazard rate for retinopathy progression and microalbuminuria development by 64% and 40% respectively [24]. Time above range decreased significantly suggesting the potential of nutritional modification via intervention leading to reduced glycemic excursion in participants. Postprandial hyperglycemia has been shown to be a direct risk factor for development of cardiovascular disease in people with diabetes [28]. Reduction in mean glucose and GMI showed significant improvement in glucose control in participants using the modified lifestyle intervention for only 1 week.
The current study showed a significant mean change in mean blood glucose, TIR and TAR by 18.78 ± 21, 12 mg/dL, 6.61 ± 13.44%, and 6.05 ± 11.54% respectively. There was no significant increase in TBR post the introduction of lifestyle modification plan. This was similar to results reported for a virtual diabetes clinic for patients with T2D [29]. The study showed a mean increase in TIR by 10.2 ± 20.5%; mean reduction in TAR by 7.2 ± 15.4%; and mean reduction in mean glucose by 14.6 mg/dL at 4 months on the virtual diabetes clinic platform. No change in TBR was observed. TIR has aptly been compared to a “glycemic compass” for evaluation of the health status and to plan for future management strategy in people with diabetes [30]. Digital therapeutic program like Diabefly-Pro can leverage the benefits of TIR to navigate management of T2D towards personalized and expert-driven care based on lifestyle modification.
The change in TIR for groups using different modalities of treatment (which included lifestyle modification (no pharmacotherapy), insulin, OHAs and combination of OHAs and insulin) did not vary significantly. This showed that the pharmacological treatment modality did not affect the effectiveness of the program. Also, the change in TIR did not show significant association with variables like age, duration of diabetes, weight and BMI (at the time of enrolment). Thus, the effectiveness of the program remained uniform irrespective of the variation in all the above parameters.
The study showed the real-world implementation of Diabefly-Pro digital therapeutics program. The study showed significant improvement in glycemic parameters after only 7 days on the personalized lifestyle plan. The 14-day study thus showed significant potential of the program for providing better glycemic control in patients with T2D. The strength of the study includes the remote recruitment, intervention and assessment with low level of missing data. Limitations of the present study include self-selection samples, referral bias and non-randomized design. This approximated the enrolment in real-world commercial programs. The study excluded patients who were not using CGM monitoring. The study population had less participation from women, which might be due to social and economic factors. The study analyzed short-term improvement in glycemic control. Future studies with longer duration, larger sample size and control groups will further confirm the effectiveness of the program.
Conclusion
Results of the study indicated that personalized lifestyle modification based on glycemic data from CGM had a positive impact on the glycemic control of patient with T2D. The study showed the effectiveness of Diabefly-Pro program for providing better glycemic control in patients with T2D. The program showed significant improvement in TIR, TAR, mean glucose within 7 days of introductions of personalized lifestyle plan. Thus, the Diabefly-Pro program is an effective platform for individuals with T2D for achieving clinically significant glycemic control.
Data availability
Due to contractual obligations of Fitterfly Healthtech Pvt. Ltd., the data cannot be shared.
Code availability
Fitterfly mobile application is available to download from android and iOS play store.
Change history
12 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13410-022-01127-7
References
Saeedi, P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., Shaw J.E., Bright D., Williams R., IDF Diabetes Atlas Committee, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract, 2019. 157: p. 107843, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition, DOI: https://doi.org/10.1016/j.diabres.2019.107843.
IDF Diabetes Atlas 9th Edition 2019, Brussels, Belgium, International Diabetes Federation, 2019.
Mohan V. Why are Indians more prone to diabetes? J Assoc Physicians India. 2004;52:468–74.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet, 1998. 352(9131): p. 837-53.
Leal, J.et al., Temporal validation of the UKPDS outcomes model using 10-year posttrial monitoring data. Diabetes Care, 2013. 36(6): p. 1541-6, DOI: https://doi.org/10.2337/dc12-1120
Unnikrishnan R, Anjana RM, Deepa M, Pradeepa R, Joshi SR, Bhansali A, Dhandania VK, Joshi PP, Madhu SV, Rao PV, Lakshmy R, Jayashri R, Velmurugan K, Nirmal E, Subashini R, Vijayachandrika V, Kaur T, Shukla DK, Das AK, et al. Glycemic control among individuals with self-reported diabetes in India--the ICMR-INDIAB study. Diabetes Technol Ther. 2014;16(9):596–603. https://doi.org/10.1089/dia.2014.0018.
Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Research and Care. 2019;7(1):e000654. https://doi.org/10.1136/bmjdrc-2019-000654.
Basu S, Sharma N. Diabetes self-care in primary health facilities in India—challenges and the way forward. World J Diabetes. 2019;10(6):341–9. https://doi.org/10.4239/wjd.v10.i6.341.
Ranscombe P. How diabetes management is adapting amid the COVID-19 pandemic. Lancet Diabetes Endocrinol. 2020;8(7):571. https://doi.org/10.1016/S2213-8587(20)30181-9.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. https://doi.org/10.1056/NEJMoa012512.
Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG, Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–96. https://doi.org/10.1001/jama.2012.67929.
Tigges C, Wennehorst K, Saliger B, Englert H. CHIP Germany: impact of a lifestyle coaching intervention on nutritional behaviour change in primary and secondary prevention of type 2 diabetes and the importance of social-cognitive variables. Gesundheitswesen. 2017;79(8-09):619–26. https://doi.org/10.1055/s-0035-1555785.
Lifestyle Management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S46.
Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Hess Fischl A, Maryniuk MD, Siminerio L, Vivian E. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the american diabetes association, the American Association of Diabetes Educators, and the academy of nutrition and dietetics. Diabetes Care. 2015;38(7):1372–82. https://doi.org/10.2337/dc15-0730.
Briggs Early K. And K. Stanley, position of the academy of nutrition and dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018;118(2):343–53.
Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, Reppert A, Robinson M. Academy of nutrition and dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet. 2017;117(10):1659–79.
Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. https://doi.org/10.1136/bmj.39063.689375.55.
Ramchandani N, Heptulla RA. New technologies for diabetes: a review of the present and the future. Int J Pediatr Endocrinol. 2012;2012(1):28.
Berman MA, Guthrie NL, Edwards KL, Appelbaum KJ, Njike VY, Eisenberg DM, Katz DL. Change in glycemic control with use of a digital therapeutic in adults with type 2 diabetes: cohort study. JMIR Diabetes. 2018;3(1):e4. https://doi.org/10.2196/diabetes.9591.
Nordyke RJ, Appelbaum K, Berman MA. Estimating the impact of novel digital therapeutics in type 2 diabetes and hypertension: health economic analysis. J Med Internet Res. 2019;21(10):e15814. https://doi.org/10.2196/15814.
Fleming GA, et al. Diabetes digital app technology: benefits, challenges, and recommendations. a consensus report by the european association for the study of diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care. 2020;43(1):250. https://doi.org/10.2337/dci19-0062.
Sepah SC, Jiang L, Peters AL. Long-term outcomes of a web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17(4):e92. https://doi.org/10.2196/jmir.4052.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ III, Garg S, Grunberger G, Heller S, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. https://doi.org/10.2337/dci19-0028.
Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–5. https://doi.org/10.2337/dc18-1444.
Kohn, M.A., and Senyak, J et al., Sample size calculators [Internet]. UCSF CTSI. [cited 2022 May 5]. Available from: https://sample-size.net/, DOI: https://doi.org/10.1080/10428194.2022.2086249
Verma R, et al. Evaluation of personalized glycemic response based diabefly-pro digital therapeutics program for improvement in time in range in people with type 2 diabetes. Diabetes Technol Ther. 2022;24(S1):A-1–A-235.
Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, Brown AS, Heinemann L, Aleppo G, Ryan DB, Riddlesworth TD, Cefalu WT. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–80. https://doi.org/10.2337/dc18-1581.
Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54(1):1–7. https://doi.org/10.2337/diabetes.54.1.1.
Majithia AR, Kusiak CM, Armento Lee A, Colangelo FR, Romanelli RJ, Robertson S, Miller DP, Erani DM, Layne JE, Dixon RF, Zisser H. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor-driven virtual diabetes clinic: prospective trial. J Med Internet Res. 2020;22(8):e21778. https://doi.org/10.2196/21778.
Kalra S, Saboo B. The glycaemic compass: Time in range. J Pak Med Assoc. 2021;71(2(A)):562–3.
Acknowledgment
The authors express gratitude to all the participants involved in the study. This study was funded by Fitterfly Healthtech Pvt. Ltd.
Funding
The study was funded by Fitterfly Healthtech Pvt. Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The work involved the secondary analysis of participant data which was deidentified, hence ethical approval was not taken.
Consent to participate
Informed consent to participate in the program was obtained from all the study participants.
Consent for publication
All study participants provided informed consent to publish the deidentified research data.
Conflict of interest
AS is CEO and Co-founder of Fitterfly Healthtech Pvt Ltd. RV, SJ, SM, SB and RR are paid employees at Fitterfly.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, R., Bhardwaj, S., Lathia, T. et al. Personalized glycemic response led digital therapeutics program improves time in range in a period of 14 days. Int J Diabetes Dev Ctries 43, 425–432 (2023). https://doi.org/10.1007/s13410-022-01111-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01111-1