Abstract
Background
India has 66 million people with diabetes, of which a large proportion do not receive adequate care. The primary health centres can serve as platforms for early detection of diabetes and continuum of care.
Objectives
This project evaluates a community-level technology-enabled system-level intervention based around the community health workers and primary-care physicians. We hypothesize that incorporation of a mobile clinical decision support system, with other process-level changes will improve identification and management of individuals with diabetes in primary care settings.
Methods
A cluster-randomized trial in sixteen villages/peri-urban areas in Andhra Pradesh and Haryana will test the feasibility and preliminary effectiveness of this intervention. The effectiveness of the extended care intervention will be evaluated by the difference in HbA1c (glycosylated hemoglobin) measured at baseline and end-line between the two study arms. Qualitative interviews of physicians, ASHA, and community members will ascertain the intervention acceptability and feasibility.
Results
A total of 1785 adults (females: 53.2%; median age: 50 years) were screened. ASHAs achieved 100% completeness of data for anthropometric, blood-pressure, and blood-glucose measures. At baseline, 63% of the participants were overweight/obese, 27.8% had elevated blood pressure, 20.3% were at high-risk for cardiovascular disease (CVD), and 21.3% had elevated blood glucose. Half of the individuals with diabetes were newly diagnosed.
Conclusion
Technology enabled transfer of simple clinical procedures from physicians to nonphysician health workers can support the provision of healthcare in under-served communities. Community health workers can successfully screen and refer patients with diabetes and/or CVD to physicians in primary healthcare system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
India currently has 66 million people with diabetes, and this number is projected to rise to 101 million by 2030 [1]. In 2016, diabetes accounted for 10.4 million disability-adjusted life years (DALYs) in India, an increase of 175% over 1990. Diabetes also contributes to DALYs from other conditions. For example, diabetes accounted for over 50% of the global increase in chronic kidney disease DALYs during this period [2]. The largest increase in diabetes DALYs was noted in rural communities in India, where 65% of the population currently resides, [3] posing challenges for India’s fragile health system [4].
A shortage of primary care workforce, lack of an efficient community-based screening program, and provision of guideline-based clinical care undermine the efforts to manage diabetes in India. Health and wellness centres (HWCs) are central to the Indian National Health Policy’s provision of comprehensive primary health care (PHC) [5]. The government plans to establish 150,000 HWCs by 2022. Innovative delivery methods, such as task-shifting and use of technology, along with improved access to medicines and diagnostics, have the potential to increase access to quality guideline-based healthcare. Task-shifting, where front-line, non-physician health workers (such as the accredited social health activists [ASHA], selected from amongst the local female residents of the village) are delegated some of the tasks traditionally performed by physicians, has been shown to improve health outcomes and processes of care [6]. Deployed primarily for maternal and child health services, they are being increasingly used to identify and manage other health conditions, including non-communicable diseases (NCDs) [7]. The potential of digital technology to improve the performance of the health workforce by providing them prompt access to job-aids and clinical decision support system (CDSS) has been increasingly recognized [8]. Various systematic reviews have outlined the role of CDSS to deliver appropriate healthcare [9, 10], but the evidence for their effectiveness and feasibility in primary healthcare is still fragmented [11].
Methods
Objectives
The IMPACT diabetes is a proof of concept randomised trial that aims to develop and evaluate a bespoke diabetes management program that empowers ASHA and PHC doctors through a mobile platform–based CDSS embedded in the public healthcare system to improve the identification and management of diabetes. The primary objective of the study is to evaluate the effectiveness of the extended care intervention by observing the difference in HbA1c (glycosylated hemoglobin) measured at baseline and end-line. The main secondary objective is to ascertain the acceptability and feasibility of the proposed intervention by conducting qualitative interviews of physicians, ASHAs, and community members.
Overall design and methods
Development of the CDSS
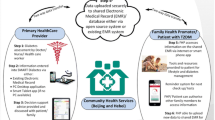
The George Institute for Global Health has developed Systematic Medical Appraisal Referral, and Treatment (SMARThealth), a mobile-based cardiovascular disease (CVD) referral and management platform for use in the primary healthcare system. The process of development and field-testing of the SMARThealth CVD has been published elsewhere [12]. Briefly, the package consists of a 7-inch tablet running android operating system with the CDSS application, training and resource support for ASHA and primary care physicians, shared electronic record functionality using open medical record system (open MRS), a prompt system for referral and follow-up, ensuring medication supply, and remuneration for the ASHAs. Data uploads occur whenever a network connection is available. ASHAs make electronic referrals to the PHC physicians and get alerts after the physician has confirmed the diagnosis and prepared a management plan.
The platform was expanded to integrate assessment and management protocols for diabetes and its complications. The process of CDSS development was as follows: we developed a plain language algorithm for screening and management of diabetes based on the review of the current Indian [13] and international guidelines [14, 15], followed by a three-step process for the validation of the algorithm. Firstly, a group of expert physicians reviewed the plain language algorithm to assess the appropriateness of pharmacological and lifestyle recommendations. The algorithm was then converted into a statistical and programming code. In the second step, a researcher not involved with the algorithm development was given the plain language algorithm summary along with mock input data from 200 patients. Programming modifications in the statistical code were made, where necessary. Finally, the plain language rules were built as a java-based application. De-identified data from a large cross-sectional study (approximately 10,000 patients) was run using both the statistical and the programming codes. Correlation for each of the calculated variables between those generated from the programming code and the statistical code was assessed. Changes were made in the application until 100% consistency was obtained between both the outputs. The final programme code was integrated with the existing SMARThealth platform. The English language strings were translated into local languages (Telugu and Hindi) for field-testing the integrated platform.
Study design and sites
The study is a population-based, pragmatic cluster–randomized trial conducted in the public healthcare systems in two locations: rural areas of Guntur district of Andhra Pradesh and peri-urban centres in Rohtak district in Haryana. A total of 8 PHC facilities were selected in the two study areas (Fig. 1) in consultation with respective district health authorities. Within each PHC, two villages (in case of Guntur) or peri-urban areas (in case of Rohtak) were randomly selected from the list of 35 villages/areas.
Eligibility and recruitment
Within each village/area, one ASHA was assigned to screen approximately 100 community members over the age of 30 years. Participants were excluded if they had any physical illness that prevented follow-up, any intellectual disability that prevents them from following instructions or responding to the questionnaire, or if the participant is unlikely to stay in the community for the duration of the study. Pregnant women were also excluded. ASHAs screened the participants using capillary blood glucose testing (Fig. 2) in their households during their routine health promotion work. Glycosylated hemoglobin (HbA1c) test was performed on participants with random blood glucose (RBG) ≥ 200 mg/dL or fasting blood glucose (FBG) >126 mg/dL), as per the Research Society for the Study of Diabetes in India (RSSDI) clinical practice guidelines [13]. The HbA1c testing was performed by an accredited laboratory in compliance with National Glycohemoglobin Standardization Programme.
Intervention
Study intervention, information dissemination strategies, and recruitment methods were developed in consultation with the local community members, ASHAs, and healthcare professionals. Subjects with elevated blood glucose levels were referred to the participating PHC physicians. Using the SMARThealth CDSS, the physicians prescribe guideline-based medications from the essential medicine list and provide evidence-based recommendations on blood glucose and blood pressure (BP) monitoring, and lifestyle changes (Fig. 3). After physician consultation, ASHAs receive system–generated alerts on their devices about the follow-up schedules. ASHAs repeat the lifestyle recommendation and emphasize the need for treatment adherence and regular follow-up during household follow-up visits. Table 1 highlights the difference between the care facilities provided to the participants in the control and intervention areas. Participants with normal HbA1c received lifestyle modification recommendations from ASHAs and were advised a follow-up blood glucose testing after 12 months. Those found to have normal capillary blood glucose values were advised to get follow-up testing after 3 years.
Training
A training program was developed and provided to ASHAs and PHC physicians on the use of SMARThealth platform. The training manual included modules on screening and management for diabetes, its complications and CVD risk; the use of the mobile application, screening methodology, interpretation of the decision support output, context-specific management advice, and blood pressure and capillary blood sugar measurement. The training material was developed in Hindi and Telugu for use in Rohtak and Guntur respectively. The duration of the first spell of training was 5 days, followed by a minimum of 3 days of field practice. Booster training was provided after 2 weeks. PHC physician training involved one-on-one guidance in the use of the electronic data collected by the ASHAs, and interpretation of the CDSS output for management decisions.
Measurements and confirmation of diagnosis
The ASHA collected demographic and clinical data and measured height, weight, BP, and capillary blood glucose for each participant. We used A&D UA-767 Plus Bluetooth-enabled digital BP monitors (A&D Limited, Tokyo, Japan). Three readings were taken 2 to 3 min apart, and the average of the second and the third reading was taken as the final BP for each participant [16]. Abbott FreeStyle Optium Neo (Abbott Diabetes Care Inc, CA, USA) monitors were used for the measurement of capillary blood glucose.
Hypertension is defined as systolic blood pressure (SBP) of ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg (20) and/or self-reported use of antihypertensive drugs. As per the RSSDI clinical practice guidelines, diabetes is defined as capillary RBG levels of ≥200 mg/dL or self-reported use of glucose-lowering drugs [13]. For those with RBG of 140–199 mg/dL, a capillary FBG test was done at home within 1 week of their RBG test, and a value >126 mg/dL was required for the diagnosis of diabetes. Participants with raised blood glucose levels underwent HbA1c testing. World Health Organization/International Society of Hypertension (WHO/ISH) low information prediction chart are used to measure the 10-year risk of a cardiovascular event (myocardial infarction or stroke) [17].
PHC visit and ASHA follow-up
In the intervention arm, a laboratory technician measured the BP and RBG of the participants at the PHC. The physicians review lifestyle advice given by ASHAs, and provide pharmacological advice with the aid of the SMARThealth CDSS. The ASHAs can track the visit and do follow-up visits to record BP, blood glucose, and adherence to prescribed medications using the Morisky medication adherence scale [18]. If any medical care is received outside the PHC (due to prevalent practice), the ASHA records the details of the type of health facility visited and reasons for not visiting PHC.
End of the study
HbA1C test will be repeated after 9 months. Focus group discussions (FGD) and semi-structured interviews of physicians, ASHAs, and community members will be conducted to ascertain the acceptability and implementation feasibility of the intervention. Semi-structured interviews and FGD guidelines will be developed based on the review of the literature and research questions. Interviews will be conducted by researchers experienced in these field settings.
Outcomes measures and analyses
Quantitative measures
The primary outcome measure will be the difference in the proportion of participants with diabetes showing a 0.5% reduction in HBA1c [19]. An a priori power calculation indicated that 10 participants with diabetes are needed per cluster to detect a 20% additional reduction in the intervention group as compared with the control group. Using two-sided tests at a significance level of 5%, and intraclass correlation coefficient (ICC) of 0.03 (based on our previous work [20], we can achieve a statistical power of 80%. Secondary outcome measures include proportions of individuals visiting a physician and adherence to blood glucose–lowering medications.
Qualitative measures
The acceptability and feasibility of the intervention will be ascertained through the understanding of (i) ASHAs’ and physicians’ experience with the intervention, (ii) impact of CDSS on staff’s usual work routines and knowledge, and (iii) patient satisfaction with the treatment and management recommendations.
Analysis
Inferential statistical analysis will be performed on an intention-to-treat basis. Student’s t test and one-way analysis of variance (ANOVA) will be applied to compare continuous variables, and the chi-squared test will be used to compare categorical variables. Due to the clustered study design, the primary outcome will be assessed using a generalized linear mixed regression model. p values of less than 0.05 will be considered statistically significant. Within-trial economic evaluation will be conducted to evaluate the incremental costs per quality-adjusted life-years gained and cost per 0.5% reduction in HbA1c. The intervention cost will be based on salaries, training, equipment, and other costs incurred with the implementation of the intervention.
All interviews and FGDs will be digitally recorded, professionally transcribed, and translated to English. We will verify 10% of the randomly selected transcriptions against the recordings for quality control. Thematic content analysis of the transcriptions and field notes will be performed using NVivo software (version 11). Two members from the research team will code transcripts based on the following deductive (a priori) codes: (i) facilitators and barriers for quality NCDs and diabetes management in resource-poor settings, (ii) satisfaction with the training, (iii) perceived usefulness and difficulties of the CDSS, (iv) perception on the behaviour change intervention package, (v) impact on work routines, and (vi) ASHAs’, doctors’, and participants’ satisfaction. Significant inductive (emerging) codes will also be identified. Coded items will be grouped into distinct themes, drawing on the methods outlined by Patton [21]. The inter-coder reliability will be checked, and a Kappa-statistic will be calculated. A third qualitative researcher will adjudicate discrepancies.
Ethical considerations
Ethical approvals were obtained from the Institutional Ethics Committees of Centre for Chronic Disease Control, New Delhi (FWA00012746), and PGIMS, Rohtak, Haryana (IEC/18/524). All the study participants provided written informed consent.
Results
The recruitment was carried out between January and August 2019 in Guntur and between August 2019 and January 2020 in Rohtak. Table 2 present the baseline characteristics of the participants. A total of 1785 participants, 53.2% females and median, (inter-quartile range, (IQR)) age of 50 (40–61) years were screened. We achieved 100% completeness of data for all anthropometric, BP, and blood glucose measures (Table 2). ASHA screenings revealed that 27.8% of the screened participants had an elevated BP, 21.3% had elevated blood glucose, and 20.3% had a high 10-year CVD risk (Table 2).
Figure 4 presents the self-reported diagnosis and treatment history of the participants with hypertension and diabetes. Half of the participants with hypertension and 43% of the participants with diabetes were newly diagnosed.
Discussion
The encouraging completion rates of anthropometric, BP, and blood glucose measures in this multicomponent health-system intervention embedded in routine primary care practice settings reiterates that with training and retraining, the ASHA are able to identify individuals with hypertension and diabetes, and can contribute to improving efficiency of service delivery [22]. While the ASHAs are used to completing a paper-based assessment checklist and motivate the community members to visit the community-based screening camps, the current intervention goes beyond and allows them to accurately identify those in need for referral using an automated electronic decision support system.
The effectiveness of the SMARThealth platform in linking community-based assessments to doctor level care has been tested in the areas of CVD and mental health [23, 24]. The addition of BP and blood glucose testing allowed expansion of the capability of the platform but also challenges the argument that ASHA cannot acquire such skills. The role of technological innovations is important in resource-poor settings of India, where the health system has been criticized for its unacceptably low quality and poor effectiveness [25]. Recent systematic reviews has shown that integration of mobile technology within the health system can overcome challenges related to service accessibility and treatment quality in primary care settings in India [10, 26, 27]. In a recent cluster randomised trial, Prabhakaran et al. found that electronic decision support for healthcare providers was not better than ‘enhanced usual care’ in management of blood pressure or diabetes in community [28]. Despite the null result, the trial showed that implementation of an mHealth intervention tool was feasible.
The baseline data provides us information about anthropometric and metabolic characteristics of the study subjects. Of note is the high proportion of undiagnosed cases of hypertension and diabetes in the community. Several national and state-level community-based studies have reported similar findings. A cross-sectional study of 5127 individuals in the state of Punjab revealed that 70% of the people with hypertension were undiagnosed or untreated [29]. Similarly, the Indian Council of Medical Research India Diabetes Study (n=57,117 in 14 states) found that 47.3% of participants with diabetes were previously undiagnosed [30]. These studies were done 6 to 10 years ago, and the proportion of untreated cases has remained unchanged despite the increasing awareness of NCDs, reinforcing the need to bring in a new approach.
A strength of the study is its pragmatic design that allows the utilization of existing care pathways in the Indian primary care systems. The existing workforce will implement this multicomponent intervention in the context of their current roles and responsibilities and during their routine health promotion work, obviating the need for additional household visits. The combination of quantitative and qualitative findings would help identify key components of the health system that might need strengthening for prevention and management of NCDs. Implementation in two geographies and health systems will provide information around the generalisability of the findings.
The study also has some limitations. The main purpose of this feasibility study is to obtain preliminary estimates (such as the variance of treatment effect) that can be used for planning a larger trial. Due to the limited sample size, our study may be underpowered to detect a true effect of the intervention. Secondly, because of the non-random selection of the participants, we would not be able to estimate the true prevalence of diabetes. However, based on the study objectives, we have chosen outcomes that are meaningful for routine practice.
In conclusion, the IMPACT diabetes study will provide evidence whether transfer of simple clinical procedures from physicians to non-physician health workers and judicious use of technology can be effectively deployed for managing diabetes and its complications in underserved communities. Findings of this study would feed into a larger trial that would provide information on process measures and the cost-effectiveness of this multicomponent intervention.
References
International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium. International Diabetes Federation. 2019. Available from: https://diabetesatlas.org/atlas/ninth-edition/ [Accessed 27th June 2021].
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–81.
The World Bank. Rural Population - India. 2018. Available from: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS [Accessed 27th June 2021].
Tripathy JP. Burden and risk factors of diabetes and hyperglycemia in India: findings from the Global Burden of Disease Study 2016. Diabetes Metab Syndr Obes. 2018;11:381–7.
Ministry of Health and Family Welfare Government of India. National Health Policy. 2017. Available from: https://www.nhp.gov.in/nhpfiles/national_health_policy_2017.pdf [Accessed 27th June 2021].
Seidman G, Atun R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Hum Resour Health. 2017;15(1):29.
Bassi A, John O, Praveen D, Maulik PK, Panda R, Jha V. Current status and future directions of mHealth interventions for health system strengthening in India: systematic review. JMIR mHealth uHealth. 2018;6(10):e11440.
Jindal D, Gupta P, Jha D, Ajay VS, Goenka S, Jacob P, Mehrotra K, Perel P, Nyong J, Roy ATN. Development of mWellcare: an mHealth intervention for integrated management of hypertension and diabetes in low-resource settings. Glob Health Action. 2018;11(1):1517930.
Souza NM, Sebaldt RJ, Mackay JA, Prorok JC, Weise-Kelly L, Navarro T, et al. Computerized clinical decision support systems for primary preventive care: a decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement Sci. 2011;6:87.
Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765.
Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(1):29–43.
Praveen D, Patel A, Raghu A, Clifford GD, Maulik PK, Mohammad Abdul A, et al. SMARTHealth India: development and field evaluation of a mobile clinical decision support system for cardiovascular diseases in rural India. JMIR mHealth uHealth. 2014;2(4):e54.
Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38(Suppl 1):1–115.
American Diabetes Association. American diabetes association standards of medical care in diabetes-2017. Diabetes Care. 2017;40(1):S1–S135. https://professional.diabetes.org/files/media/dc_40_s1_final.pdf.
International Diabetes Federation. Recommendations for managing type 2 diabetes in primary care. Diabetes Research and Clinical Practice. 2017. Available from: https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html [Accessed 27th June 2021].
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
World Health Organization. WHO/ISH cardiovascular risk prediction charts. WHO. World Health Organization; 2011. Available from: https://www.who.int/ncds/management/WHO_ISH_Risk_Prediction_Charts.pdf?ua=1[Accessed 27th June 2021].
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–54.
Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63–71.
Peiris D, Praveen D, Mogulluru K, Ameer MA, Raghu A, Li Q, et al. SMARThealth India: a stepped-wedge, cluster randomised controlled trial of a community health worker managed mobile health intervention for people assessed at high cardiovascular disease risk in rural India. Liu G, editor. PLoS One. 2019;14(3):e0213708.
Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res. 1999;34(5 Pt 2):1189–208.
Abdel-All M, Abimbola S, Praveen D, Joshi R. What do accredited social health activists need to provide comprehensive care that incorporates non-communicable diseases? Findings from a qualitative study in Andhra Pradesh, India. Hum Resour Health. 2019;17(1):73.
Patel A, Praveen D, Maharani A, Oceandy D, Pilard Q, Kohli MPS, et al. Association of multifaceted mobile technology–enabled primary care intervention with cardiovascular disease risk management in rural Indonesia. JAMA Cardiol. 2019;4(10):978.
Maulik PK, Tewari A, Devarapalli S, Kallakuri S, Patel A. The systematic medical appraisal, referral and treatment (SMART) mental health project: development and testing of electronic decision support system and formative research to understand perceptions about mental health in rural India. PLoS One. 2016;11(10):e0164404.
Scott KW, Jha AK. Putting Quality on the Global Health Agenda. N Engl J Med. 2014;371(1):3–5.
Ajay VS, Jindal D, Roy A, Venugopal V, Sharma R, Pawar A, et al. Development of a smartphone-enabled hypertension and diabetes mellitus management package to facilitate evidence-based care delivery in primary healthcare facilities in India: the mPower heart project. J Am Heart Assoc. 2016;5(12)
Tian M, Ajay VS, Dunzhu D, Hameed SS, Li X, Liu Z, et al. A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard Trial) in rural Tibet, China, and Haryana. India. Circulation. 2015;132(9):815–24.
Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation. 2018;139(3):380–91.
Tripathy JP, Thakur JS, Jeet G, Chawla S, Jain S. Alarmingly high prevalence of hypertension and pre-hypertension in North India-results from a large cross-sectional STEPS survey. PLoS One. 2017;12(12):e0188619.
Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–96.
Acknowledgements
We thank the community members from the eight villages, the ASHA and the primary health care physicians for their participation in the study. We also thank Dr. Chetan Purad for his assistance in the development of the CDSS and Mr. Mohammad Ameer Abdul and Mr. Anup Rathee for facilitating field activities and data collection.
Funding
The IMPACT diabetes study is funded by European Foundation for the Study of Diabetes/Sanofi Collaborative Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The funders had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation.
Ethics approval and consent to participate
Ethical approvals were obtained from the Institutional Ethics Committees of Centre for Chronic Disease Control, New Delhi (FWA00012746), and PGIMS, Rohtak, Haryana (IEC/18/524). All the study participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bassi, A., Arfin, S., John, O. et al. Innovative mobile-health led participatory approach to comprehensive screening and treatment of diabetes (IMPACT diabetes): rationale, design, and baseline characteristics. Int J Diabetes Dev Ctries 43, 353–362 (2023). https://doi.org/10.1007/s13410-022-01082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01082-3