Abstract
The microstructural evolution and shear strength of Ti50Ni50 SMA and 316L SS infrared brazed with a gold-based filler metal Au-22Ni-8Pd (BAu-6) at 1050 °C for 180 and 300 s were investigated. The results of dynamic wetting angle measurement indicate that Au-22Ni-8Pd filler metal exhibits rather good wettability on Ti50Ni50 and 316L SS substrates if the specimens are brazed at 1050 °C for longer than 100 s. Experimental results of SEM BEIs and EDS/WDS show that the joint brazed for 300 s is composed of a layer of Ni3Ti + TiNiAu lamellae, a layer of TiNiAu and Ti(Au,Ni)4 intermetallics, and a layer of TiNi2Au one at Ti50Ni50 substrate side, and is a layer of the mixture of (Ni, Fe) + (Au) solid solutions and a layer of (Ni, Fe) solid solution with no interfacial reaction compound formed at 316L SS substrate side. All the formed lamellae/layers increase in thicknesses as the brazing time is prolonged, which prevents the brittle Ni3Ti lamellae from contacting the layer of TiNi2Au. Accordingly, the joint shear strength improves as the brazing time increases. The shear strength of a joint brazed for 300 s can reach 204 MPa, where the fracture crack begins around the interface of the TiNi2Au and layer TiNiAu/Ti(Ni,Au)4 layers. Cleavage-dominated fracture is observed in SEI fractographs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Equiatomic Ti50Ni50 shape memory alloy (SMA) has an excellent shape memory effect and pseudoelasticity [1, 2], and it also has good biocompatibility [3, 4]. The nominal composition of AISI 316L stainless steel (316 L SS) is C ∼0.03, S ∼0.03, P ∼0.020, Si ∼0.04, Mn ∼2.0, Ni ∼10 to 14, Cr ∼16 to 18, and Mo ∼2 to 3 % and the balance Fe in wt% [5]. It is well-known that stainless steels are covered by protective oxide layers, which give them high resistance to oxidation and great corrosion resistance in many environments. Because 316L SS exhibits excellent mechanical properties and good oxidation/corrosion resistance, the successful joining of Ti50Ni50 SMA and 316L SS would be highly applicable in many engineering applications. However, brazing dissimilar metals causes two major problems. First, the thermal expansion between the joined alloys has a high degree of mismatch. Second, the intermetallic compounds that form are brittle, so the quality of the joint is low, especially at the brazed interface between the brazing alloy and the substrate. Several studies have examined the problem of joining titanium alloy and SS [6, 7].
The oxide films on 316L SS are identified as multilayers mixed with iron–nickel oxide and chromium oxide [8]. Though they provide high corrosion resistance, these oxide films also act as a barrier to wettability between 316L SS and general Ag–Cu filler metals [9]. To improve the wettability with 316L SS, a gold-based filler metal could be used. Gold-based filler metals have been used to braze iron-, nickel-, and cobalt-based alloys where oxidation and/or corrosion resistance is required. They are commonly used in thin sections due to their low interaction rates with many base metals [10]. Au-22Ni-8Pd in wt% belongs to the category of Au-based braze alloys. The addition of Ni and Pd into Au matrix can significantly improve both corrosion and creep resistance of the braze alloy. It has been used in many severe corrosive environments. In a previous study, gold-based filler metals of pure Au and Au-20Cu in wt% were used in successful infrared brazing of Ti50Ni50 SMA, and the device made with the Au-bearing filler was shown to have a superior shape memory effect [11]. The dissimilar joining of Ti50Ni50 SMA and 316L SS can be applied in manufacturing golf club head. The striking plate is made by Ti50Ni50 SMA for better performance, and the rest part of the golf club head is made by the cost-effective 316L SS.
Infrared brazing is characterized by a fast heating rate of up to 50 °C/s, which is much higher than that of the traditional furnace brazing [12]. With the aid of precise thermal cycle control, infrared brazing is highly suitable for evaluating the mechanism of early-stage reaction kinetics in a brazed joint due to its very rapid thermal history. In this study, infrared brazing is applied to join Ti50Ni50 SMA and 316L SS with a gold-based filler metal, Au-22Ni-8Pd in wt% (AWS classification: BAu-6). The microstructures and shear strengths of the brazed joints were evaluated in the experiment.
Materials and methods
The base metals used in the experiment were Ti50Ni50 and 316L SS specimens with dimensions of 15 × 7 × 4 mm3. Ti50Ni50 ingots of about 120 g were prepared by a vacuum arc remelter (VAR) from the raw materials of titanium (99.7 wt% purity) and nickel (99.99 wt% purity) and remelted six times in an argon atmosphere. The ingots were homogenized at 900 °C for 1 h and then cut with a low-speed diamond saw to the specimen size. The specimens used for the substrate were polished with SiC papers up to 1200 grit and subsequently cleaned in an ultrasonic bath with acetone as the fluid before brazing. The Au-22Ni-8Pd filler metal, produced by Wesgo Metals, Morgan Advanced Materials, USA, has a thickness of 50 μm and a liquidus temperature of 1045 °C [10].
The dynamic wetting angle of Au-22Ni-8Pd filler metal on Ti50Ni50 and 316L SS substrates was performed by the sessile drop test using a filler ball of approximately 0.15 g in vacuum during the measurement. The schematic diagrams of the wetting angle measurement facility and the specimen holder during infrared brazing can be found in previous studies [12]. An infrared heating furnace (ULVAC SINKO-RIKO RHL-P816C, Japan) was used as the main body of the vacuum furnace, which maintained a vacuum of 5 × 10−5 mbar throughout the experiment. Infrared rays were generated by the infrared lamps, transmitted via a transparent quartz tube, and focused on the specimen’s holder. One end of the quartz tube was attached to the specimen holder, and the other end transmitted the signal into the image analysis system. The filler metal was located on the substrate, and a thermocouple was simultaneously in contact with the substrate. A graphite holder below the substrate was used to enhance the absorptivity of the infrared rays. All specimens were preheated at 700 °C for 300 s prior to brazing in order to equilibrate the temperature profile of the specimen. The brazing conditions used in the experiment were 1050 °C for 180 and 300 s.
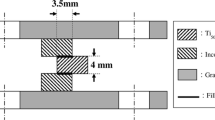
Shear tests were performed to evaluate the bonding strength of the brazed joint. Tests were carried out on three specimens for each brazing condition. A symmetrical double lap joint was employed to perform shear testing of the brazed joint, as shown in previous studies [12]. Figure 1 presents schematic diagram of the shear test sample [12, 13]. The shaded areas are the two base metals, and the outer part of the layout is the graphite fixture used in infrared brazing. Two bold black lines, 3.5 mm wide, in the middle of the graph indicate the brazing filler metal, and the specimen is sandwiched between two graphite plates. The specimen was compressed by a Shimadzu (Shimadzu Corp., Nakagyo-ku, Japan) AG-10 universal testing machine with a constant crosshead speed of 0.0167 mm/s. Cross-sections of the infrared brazed joints were cut with a low-speed diamond saw and subsequently examined using an FEI NOVA 450 scanning electron microscope (SEM). Quantitative chemical analyses of various phases in the joints were performed using an energy-dispersive X-ray spectrometer (EDS) mounted on an SEM and a wavelength dispersive spectrometer (WDS) mounted on a JEOL 8600SX electron probe microanalyzer (EPMA). The operation voltage was 15 kV with a minimum spot size of 1 μm.
Results and discussion
Dynamic wetting angle measurement
Figure 2 shows the results of dynamic wetting angle measurement for brazed specimens of Au-22Ni-8Pd filler metal on Ti50Ni50 and 316L SS substrates tested at 1050 °C. As shown in Fig. 2, for Ti50Ni50 substrate, the wetting angle of Ti50Ni50 substrate is below 30° after 100 s. However, that of 316L SS substrate decreases rapidly to 105° in the first ∼10 s, and then remains at that value from ∼10 to ∼40 s before dropping dramatically to 20° after ∼45 s. Capillarity and spreading are related to the balance of surface tensions, which determine the wetting and spreading behavior of a liquid on a solid. Interfacial reaction(s) enhance the wetting of braze melt on the substrate [10]. From the wetting angle shown for 316L SS substrate, as shown in Fig. 2, one can find that the braze melt reacts rapidly with the chromium oxide in the first ∼10 s. Thereafter, the braze melt has to overcome the barrier of the multilayered oxides on the surface, and at ∼40 s, the braze metal begins to react with the substrate. The iron and nickel dissolve into the braze melt significantly to form a layer of (Ni, Fe) solid solution at the interface, as discussed in the previous paragraph. For both substrates, the results of Fig. 2 indicate that the Au-22Ni-8Pd filler metal exhibits rather good wettability if the specimen is tested at 1050 °C for more than 100 s.
Microstructural observations of infrared brazed Ti50Ni50/Au-22Ni-8Pd/316 L SS joints
The Ti50Ni50/Au-22Ni-8Pd/316L SS joints infrared brazed at 1050 °C for 180 and 300 s have the cross-sectional widths of about 60 μm. Figure 3a, b shows the back-scattered electron images’ (BEIs) cross-sections and WDS chemical analysis results of Ti50Ni50/Au-22Ni-8Pd/316L SS specimens infrared brazed at 1050 °C for 180 and 300 s respectively. The chemical composition of 70Au-22Ni-8Pd filler metal is 44.1 % Au, 46.5 % Ni, and 9.4 % Pd in at%. According to the SEM BEIs and WDS results shown in Fig. 3, the infrared braze joints are dominated by a (Au,Ni) solid solution (marked by F) and have interfacial areas on the Ti50Ni50 side and on the 316L SS side.
Figure 3a shows that the area on the Ti50Ni50 substrate side primarily consists of a layer of mixed intermetallics, Ni3Ti (marked by Ba) and TiNiAu (marked by Ca) lamellae (abbrev. as layer B + C), and a layer of intermetallic TiNi2Au (marked by Da) (abbrev. as layer D). Layer E is found to be a mixture of (Au,Ni) solid solution and Ni3Ti intermetallic compound. As shown in Fig. 3b, the area on the Ti50Ni50 substrate side also primarily consists of layers (B + C) and D, but the thicknesses of these two layers increased. At the same time, the Ni3Ti lamellae (marked by Bb) is also thickened and becomes enclosed in intermetallic compounds (marked by Cb1 and Cb2) which grows into a continuous layer (abbrev. as layer C) between the former layer (B + C) and layer D. The WDS results in Fig. 3 indicate that the composition of the Cb1 alloy is 32.9 % Ti, 22.9 % Ni, 35.9 % Au, and 7.7 % Pd in at%, and that of the Cb2 one is 20.1 % Ti, 39.4 % Ni, 38.1 % Au, and 2.2 % Pd in at%. These composition results suggest that the Cb1 alloy is still intermetallic TiNiAu, and that the Cb2 alloy is intermetallic Ti(Ni,Au)4. Also in Fig. 3a, b, it can be seen that the Ti50Ni50 substrate next to the layer (B + C) becomes a layer of Ni-rich TiNi solid solution (marked by Aa and Ab) because Ti atoms dissolve into the molten braze. Figure 3a, b also shows that the thicknesses of layers C and D increase as layer E is consumed with increased brazing time. In the specimen brazed at 300 s, layer E is almost eliminated.
As shown in Fig. 3a, b, the area on the 316L SS side is composed of a layer of the mixed (Ni,Fe) and (Au,Ni) solid solutions (marked by G) and a layer of (Ni,Fe) solid solution (marked by H), and no interfacial reaction compound is observed. The difference in microstructure after brazing for 180 and 300 s is that layers G and H become thicker as the brazing time increased, especially for layer G, as shown in Fig. 3b.
Microstructural evolution and the related reaction mechanism
From Fig. 3, the observed microstructural evolution and the related reaction mechanisms on the two substrate sides can be elucidated as follows. For the Ti50Ni50 substrate side, to identify the formed intermetallics observed in Fig. 3, related phase diagrams are important in order to elucidate these phases. According to Au–Ni binary alloy phase diagram, Au is completely miscible with Ni above 810.3 °C [14]. In contrast, Ti is highly reactive to Au and Ni, and there are many binary intermetallic compounds observed from Au–Ti and Au–Ni binary alloy phase diagrams [14]. Based on the WDS chemical analysis results, Ti–Ni–Au ternary alloy phase diagram is necessary to be referred, but it is still unavailable now. However, the diagram of Ti–Ni–Cu is available, and that phase diagram shows that TiNiCu and TiNi2Cu intermetallics form in this ternary alloy system [15]. Comparing the Ni–Cu binary phase diagram with that of Ni–Au, one can find that both diagrams indicate complete solid solubility at high temperature [16]. In addition, the Ti–Cu and Ti–Au binary phase diagrams show that in the range of Ti ≤ 50 at%, TiCu, TiCu2, and TiCu4 intermetallics will form in the former, and TiAu, TiAu2, and TiAu4 will form in the latter [17]. The similarities exhibited in the above two binary phase diagrams in the range of Ti ≤ 50 at% suggest a high likelihood that TiNiAu and TiNi2Au intermetallics would be indicated in a Ti–Ni–Au ternary alloy phase diagram. Therefore, from the chemical compositions indicated in Fig. 3, the layers Da and Db are identified as a TiNi2Au intermetallic compound. The lamellae Ca in layer (B + C) and the Cb1 in layer C are TiNiAu compound, and the Cb2 in layer C is Ti(Ni,Au)4 compound, as discussed in the previous paragraph. The brazing temperature of Au-22Ni-8Pd filler metal is rather high, which can cause the molten braze to be over-saturated, which in turn thickens the reacted layers C and D. Both C and D are alloyed with Pd. Au-22Ni-8Pd filler metal contains 46.5 % Ni and 9.4 Pd% in at%. The addition of Pd in the Au-based braze alloy can increase the antioxidation ability and enhance the wettability to base metals [18]. These changes would lead to more intensive reaction and, thus, a better quality of joint between substrate and braze.
On the 316L SS substrate side, Fig. 4a shows the liquidus projection of the Au–Fe–Ni ternary alloy phase diagram, and the important reaction scheme is also included in the figure [14, 18]. It is clear that the dissolution of Fe and Ni from 316L SS substrate into the molten braze significantly increases the melting point of the braze alloy. Accordingly, isothermal solidification of the molten braze can be observed, especially at the interface between braze and substrate, because the molten braze contains high contents of Fe and Ni. Figure 4b plots a pseudobinary phase diagram for Au–Fe–Ni [14, 19], which indicates both Au-rich and (Ni,Fe)-rich solid solutions. As indicated by the chemical analysis results shown in Fig. 3, (Ni,Fe) solid solution (the layer H) and the mixture of (Ni,Fe) + (Au) solid solutions (the layer G) abut the 316L SS substrate. The experimental results are consistent with the phase diagram.
Shear strengths of infrared brazed joints
Table 1 shows the shear strengths of specimens formed with different brazing conditions. The shear strength of joints brazed at 1050 °C for 300 s can reach 204 MPa. Figure 5a, b presents SEM BEI cross-sections, SEI fractographs, and EDS chemical analyses of the fractured Ti50Ni50/Au-22Ni-8Pd/316L SS joints brazed at 1050 °C for 180 and 300 s respectively. It can be seen clearly that the brazed specimen in Fig. 5a fractured alternatively along the interface of layers A and (B + C) and that of layers (B + C) and D, with the latter interface being the major source of fracture. The specimen in Fig. 5b fractured along the interface of layers C and D. From Fig. 5, no distortion of the braze can be observed, demonstrating that the cracking is dominated by brittle fracture. Cleavage dominated fracture can be observed in the SEI fractograph, and the EDS chemical analysis results are consistent with those in Fig. 3, which reveal that the fracture initiates around the interface of layers (B + C) and D for 180 s and around that of layers C and D in the specimen brazed for 300 s. This difference indicates that the TiNi2Au intermetallic may be stronger than the Ni3Ti and TiNiAu intermetallics. The difference in the shear strengths from brazing for 180 and 300 s is closely related to the increasing thicknesses of lamellae B and layers C and D, for both the Ni3Ti and TiAuNi lamellae of layer (B + C) contact layer D in the 180 s brazed specimen, but only layer C (intermetallics TiAuNi and Ti(Ni,Au)4) neighbors layer D in the 300 s one. The intrinsically brittle Ni3Ti and TiNiAu intermetallics may cause the difference in the shear strengths of these two specimens.
Conclusion
The microstructural evolution and shear strength of Ti50Ni50 SMA and 316L SS infrared brazed with gold-based filler metal Au-22Ni-8Pd (BAu-6) at 1050 °C for 180 and 300 s have been investigated. Measurements of the dynamic wetting angle indicate that Au-22Ni-8Pd filler metal has rather good wettability on Ti50Ni50 and 316L SS substrates if the specimens are brazed at 1050 °C for longer than 100 s. Experimental results of SEM BEIs and EDS/WDS show that the infrared braze joints are dominated by a (Au,Ni) solid solution and have the interfacial areas on the Ti50Ni50 side and on the 316L SS side. On the Ti50Ni50 side, the 180-s brazed joint is comprised of a layer of the mixed intermetallics Ni3Ti and TiNiAu lamellae (layer B + C), a layer of intermetallic TiNi2Au (layer D), and a layer of mixed (Au,Ni) solid solution and intermetallic Ni3Ti (layer E). In the 300-s brazed joint, the layers are rather similar to those in the 180-s joint, but the thicknesses of the Ni3Ti lamellae and TiNiAu in layer (B + C) and that of layer D were thicker at the expense of the layer E. At the same time, a layer composed of intermetallics TiNiAu and Ti(Ni,Au)4 (layer C) forms in between the original layers (B + C) and D. On the 316L SS side, the 180- and 300-s brazed joints are both a mixture of (Ni, Fe) + (Au) solid solutions and a layer of (Ni, Fe) solid solution, and no interfacial reaction compound formed on the 316L SS side. The increase in brazing time causes the formation of layer C, which keeps the brittle Ni3Ti lamellae from directly contacting the TiNi2Au layer. Therefore, the shear strength of the joint increased as the brazing time is prolonged. The shear strength of a specimen brazed for 300 s can reach 204 MPa. The fracture cracks in such specimens begin around the interface of layer (B + C) and layer D in the 180 s joint, and in the 300 s joint, around layer C and layer D. Cleavage dominated fracture is observed in SEI fractographs.
References
Otsuka K, Shimizu K (1986) Pseudoelasticity and shape memory effects. Int Met Rev 31:93–114
Otsuka K, Ren X (2005) Physical metallurgy of Ti-Ni-based shape memory alloys. Prog Mater Sci 50:511–678
Duerig T, Pelton A, Stockel D (1999) An overview of nitinol medical applications. Mater Sci Eng A 273:149–160
Michiardi A, Aparicio C, Planell JA, Gil FJ (2007) Electrochemical behavior of oxidized NiTi shape memory alloy for biomedical applications. Surf Coat Technol 201:6484–6488
Davis JR (1990) ASM metals handbook vol. 1, ASM International, Materials Park, pp. 843-850
Tashi RS, Mousavi SA, Atabaki MM (2014) Diffusion brazing of Ti-6Al-4V and austenitic stainless steel using silver-based interlayer. Mater Des 54:161–167
Lee JG, Hong SJ, Lee MK, Rhee CK (2009) High strength bonding of titanium to stainless steel using an Ag interlayer. J Nucl Mater 395:145–149
Montemor MF, Ferreira MGS, Hakiki NE, Belo MDC (2000) Chemical composition and electronic structure of the oxide films formed on 316L stainless steel and nickel based alloys in high temperature aqueous environments. Corros Sci 42:1635–1650
Kozlova O, Voytovych R, Devismes MF, Eustathopoulos N (2008) Wetting and brazing of stainless steels by copper-silver eutectic. Mater Sci Eng A 495:96–101
Schwartz M (1993) Brazing: for the engineering technologist. ASM International, Materials Park, pp 109–111
Shiue RH, Wu SK (2006) Infrared brazing of Ti50Ni50 shape memory alloy using gold-based braze alloys. Gold Bull 39:200–204
Shiue RK, Wu SK, Chen SY (2003) Infrared brazing of TiAl intermetallic using BAg-8 braze alloy. Acta Mater 51:1991–2004
Shiue RK, Wu SK, Hung CM (2002) Infrared repair brazing of 403 stainless steel with a nickel based braze alloy. Metall Mater Trans A 33A:1765–1773
Massalski TB (1990) Binary alloy phase diagrams, ASM International, Materials Park, pp. 402-404, 442-445, 2874-2876
Villars P, Prince A, Okamoto H (1997) Handbook of ternary alloy phase diagrams vol. 8. ASM International, Materials Park, pp 9846–9861
Davis JR (1992) ASM handbook vol. 3. ASM International, Materials Park, pp. 2.173 & p. 2.73
Villars P, Prince A, Okamoto H (1997) Handbook of ternary alloy phase diagrams, ASM International, Materials Park, pp 2.78 & pp 2.180
Humpston G, Jacobson DM (2001) Principle of soldering and brazing. ASM International, Materials Park, pp 43–47
Yu YK, Liaw DW, Shiue RK (2005) Infrared brazing Inconel 601 and 422 stainless steel using the 70Au-22Ni-8Pd braze alloy. J Mater Sci 40:3445–3452
Acknowledgments
The authors gratefully acknowledge the financial support of this research by Ministry of Science and Technology (MOST), Taiwan, under grant no. MOST103-2221-E002-063.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shiue, RK., Wu, SK., Chen, CP. et al. Infrared brazing of Ti50Ni50 shape memory alloy and 316L stainless steel with Au-22Ni-8Pd filler. Gold Bull 48, 57–62 (2015). https://doi.org/10.1007/s13404-015-0160-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-015-0160-6