Abstract
Monodisperse, monocrystalline AuFe optical–magnetic multifunctional nanocrystals were prepared by the nanoemulsion method using a copolymer surfactant. The structure and properties of the monocrystalline nanocrystals were analyzed by X-ray diffraction, transmission electron microscopy (TEM, including high-resolution TEM), selected-area electron diffraction, UV-vis spectroscopy, and vibration sampling magnetometry. The characterization shows the outstanding monodispersity and high monocrystallinity of the nanocrystals, which possess both well-defined optical and magnetic properties revealing broadband absorption with the surface plasmon resonance, peaking at ~600 nm, and clear soft ferromagnetic behavior at room temperature. Such long-term stable, monocrystalline AuFe nanocrystals have novel optical and magnetic properties, which may offer exciting opportunities in fundamental studies and tremendous applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanocrystals of multiple constituents bestow multifunctionality to accomplish multiple purposes, most promisingly with enhanced performance in a single nanovehicle, and have gained momentum in the interdisciplinary converging exploit of physical and life sciences with nanotechnology, in prospective of practical relevance and basic studies. Of vast awareness, gold–iron (AuFe) intermetallic or alloy nanocrystals and other relevant forms are elegantly nanostructured candidates because of their marvelous structures and properties [1–3]. Gold is a precious, versatile element and has valuable, diverse applications in the fields of catalysis, electronics, and optoelectronics [3–6]. There has been a surging interest for its benign biocompatibility, superb surface chemistries, and particularly, biosensing potentiality as a result of the strong visible absorption bands ensuing from the surface plasmon resonance due to the nanostructuring of the element [3–6]. Ascribing to its marvelous magnetic properties and readiness to be converted to bio-friendly oxides, the element iron is an excellent candidate for a wide range of applications such as magnetic recording, magnetic seals, printing, magnetic resonance imaging, drug delivery, biodetection, and cell tagging and separation [7–10]. Appreciably, amalgamation of Au and Fe into one AuFe alloy or intermetallic nanostructure can be more fascinating than the corresponding monoelements, offering potential functions in both magnetic and optical properties in addition to the biological compatibility and easy linkage to biomolecules endowed by the constituents [1, 2, 11–13]. In such nanostructures, particle size and distribution, shape, composition, crystallinity, microstructure, surface properties, and aqueous solubility are vital. Facile synthesis, fine tuning of the surface properties, and precise control of the particle size and distribution are the intensive efforts of current research programs [1, 11–13].
A variety of polymers have been investigated for the formation of micelles to construct tailored nanomaterials [14–17]. The triblock copolymer, poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEO-PPO-PEO) shows biocompatibility, non-charging trait, non-toxicity, and aqueous solubility and has been used in emulsion processes as a surfactant, besides the stabilization effect and the role of a reducing agent [17–21]. Making use of the copolymer as the surfactant, we have generated long-term stable, highly crystalline Fe3O4 and Fe3O4/Au nanocrystals [20]. In this work, we report the one-pot nanoemulsion synthesis of long-term stable, monodisperse AuFe optical–magnetic nanocrystals employing the polymer. The resulting AuFe nanocrystals show high monocrystallinity, in sharp contrast to the polycrystalline state of the similar nanoparticles obtained previously [11, 12]. In our experiments, the polymer dominantly plays the role of a surfactant, whereas the functioning of reduction is taken by the much stronger reductant of 1,2-hexadecanediol [15, 20].
Experimental
The monocrystalline, monodisperse AuFe optical–magnetic nanocrystals with a nominal ratio of 1:1 were synthesized by a polymer nanoemulsion method [15, 20]. In a typical experiment, 0.375 mmol of iron (III) acetylacetonate (Fe(acac)3, 99.9%) in 10 ml octyl ether (C8H17OC8H17, 99%) was mingled with 0.375 mmol of gold (III) acetate (Au(OOCCH3)3, 99.9%), 3.75 mmol of the reduction reagent 1,2-hexadecanediol (C14H29CH(OH)CH2(OH), 90%), and the surfactant PEO-PPO-PEO (0.5272 g) in a 250-ml flask. All chemicals were purchased from Aldrich, except for gold (III) acetate from Alfa Aesar. Under vigorous magnetic stirring, the reaction mixture was first heated to 80°C and homogenized at the temperature for 1 h, then rapidly raised to 280°C and refluxed at the temperature for 1 h. After cooling down to room temperature, ethanol was added to the reacted mixture to precipitate the nanocrystals, and the ensuing dark-red product was separated from the supernatant by centrifugation, which was washed with the mixed solvents of ethanol/hexane (1:2) several times and redispersed in hexane for further use. The crystal structure of the AuFe nanocrystals was studied by powder X-ray diffraction (XRD, X’Pert Pro), whereas the morphology, crystal size, size distribution, and nanostructure of the nanocrystals were analyzed by transmission electron microscopy (TEM), including high-resolution imaging (HRTEM) and selected-area electron diffraction (SAED, JEM-100II and JEOL 2010F). The optical properties were subsequently characterized by a UV-visible spectrophotometer (UV-vis, Hitachi U4100), and vibrating sample magnetometry (VSM, Lakeshore 7300) was applied to perform magnetic measurements on the dried nanocrystals to evaluate the corresponding magnetic properties at room temperature.
Results and discussion
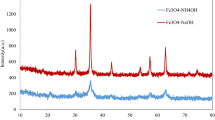
The formation of the long-term stable, monocrystalline, monodisperse AuFe optical–magnetic nanocrystals is first studied by the X-ray crystal structural analysis. As shown in Fig. 1a, the diffraction pattern of the AuFe nanocrystals has the peaks at 38.20°, 44.38°, 64.78°, and 77.74°, which are appropriately indexed to the AuFe positions of (111), (200), (220), and (311) planes [12]. The calculation of the relevant crystallographic parameter gives a value of 4.068 Å for the AuFe nanocrystals, which clearly lies between the equivalent crystallographic parameters of the corresponding constituent elements (JCPDS nos. 87-0721 and 04-0784), most probably indicating an intermetallic or a disordered face-centered cubic phase [11–13, 15, 20, 22]. Plausibly, the spacing of the AuFe nanocrystals supports yet the alloy formation if the Vegard's rule is invoked [23]. In terms of the diffraction pattern, the nanocrystals show no sign of oxidation, consistent with the previous findings for the same composition [12]. In the meantime, the single-phased diffraction pattern, suggestive of single crystallinity or monocrystallinity which is much analyzed below, is distinct from the biphased pattern of a previous product obtained from the use of the small organic surfactants, i.e., oleic acid and oleylamine [12]. More experiments were conducted to substantiate the AuFe nanostructure from the synthesis process. For instance, only Fe3O4 nanoparticles rather than Fe nanoparticles were obtained if prepared in the same way, starting from Fe(acac)3 alone, as shown in Fig. 1c which matches the inverse spinel structure of magnetite [20], whereas pure Au nanoparticles were likewise gained with the single precursor of Au(OOCCH3)3, as confirmed by the corresponding diffraction pattern in Fig. 1b which shows the cubic structure of gold [20].
The formation of the AuFe nanocrystals is further validated by the fact that all nanocrystals, as prepared, could be collected by a magnet, leaving no free Au crystals observable and thus indicating no cosedimentation of separate Fe and Au nanocrystals [12]. Possible nanostructures from sticking Au and Fe or Fe3O4 jointly, such as dendrite or dumbbell, have been decisively ruled out in the TEM analysis below.
Figure 2a shows the morphology and particle sizes of the AuFe multifunctional nanocrystals recorded by TEM. Obviously, the nanocrystals are monodisperse, nearly spherical in shape, and provide no evidence for nanostructures, such as dendrite or dumbbell, from simply joining discrete Au and Fe or Fe3O4 nanoparticles [11–13]. The size distribution of the nanocrystals is given in Fig. 2b, which is well described by the curve of a Gaussian function, showing a tight size distribution with an average particle size of ~9.3 nm in diameter. As already manifested by the XRD analysis above, the high crystallinity of the monodisperse AuFe nanocrystals is once more sustained by the HRTEM imaging, as shown in Fig. 2c. The lattices are distinct, with the spacing of 2.0 Å indicating the projection of the AuFe (200) plane. Markedly, the fringe lattices run almost uniformly over the whole nanocrystal, indicative of the single crystallinity of the nanocrystal. The inset is the result of the corresponding fast Fourier transform of the lattice image, upholding yet again the monocrystallinity of the individual nanocrystal. The direct visualization of the monocrystalline AuFe nanocrystals by HRTEM (Fig. 2c) confirms the expectation from the XRD observation above, and the high single crystallinity is in stark distinction to the polycrystalline state of the similar nanoparticles obtained previously [11, 12]. Mechanistically, the resulting monocrystallinity in this work is most probably due to the use of the polymer surfactant, in addition to the tuning of the experimental conditions such as the lowering of the heating temperature at the final reaction step from 300°C to 280°C. As the aperture is large to encompass multiple nanocrystals, however, the SAED results in, as shown in Fig. 2d, a powder pattern illustrating the superposition of diffractions of the disoriented AuFe nanocrystals. The sharpness and multiple diffraction spots observed in the pattern corroborate the high crystallinity of the monocrystalline AuFe nanocrystals as addressed above.
The UV-vis spectrum of the monocrystalline AuFe nanocrystals (~9.3 nm) in hexane is shown in the curve II of Fig. 3a, together with that of ~8 nm Au (curve I) and ~8.0-nm Fe3O4 nanocrystals (curve III). Clearly, the AuFe nanocrystals show a broadband absorption shape with the maximum absorption at ~600 nm, confirming the surface plasmon resonance of the monocrystalline, monodisperse AuFe nanostructure [3–6, 11–13]. As shown in Fig. 3b, nonetheless, the Au nanocrystals present a tight plasmon resonance and the maximum absorption at the wavelength of ~525 nm, strongly blue shifted relative to the AuFe nanocrystals in consideration of the nanocrystal sizes [3–6, 11–13]. As expected, the spectrum of the Fe3O4 nanoparticles gives no characteristic absorption in the range of wavelength measured. Technically, the peak position and band shape of the surface plasmon resonance may be subject to factors of composition, dimension, shape, dielectric medium, and nanostructuring of the nanoparticle system, including the monocrystallinity in particular in the present case [3–6, 11–15, 24]. Moreover, the presence of the Au atoms sitting on the surface of the AuFe nanocrystals facilitates thiolation linkage to other molecules [20].
The magnetic properties of the AuFe multifunctional nanocrystals were determined by VSM at room temperature. As shown in Fig. 3b, the AuFe nanocrystals reveal well-defined soft ferromagnetic behavior with a small coercivity of ~10 Oe and a magnetization of about 8.0 emu/g at ~1 T, comparable to or better than the same composition prepared from other routes [11, 12]. The finding could be a manifestation of the monocrystallinity of this nanosystem, in opposition to the polycrystallinity of other relevant nanoparticle ensembles [11, 12]. We remark that the optical–magnetic properties and functioning of the AuFe nanocrystals can be visually demonstrated in a separation and redispersion process of the nanoparticles in hexane. Under the influence of an external magnetic field, the optical–magnetic nanocrystals were capable of being collected by a magnetic disk, and the solution changes from a homogeneous, yellowish-violet dispersion to a clear, transparent solution. The collected nanoparticles could be reversibly dispersed by agitation after removal of the magnetic field, and the above process can be repeated as many times as preferred. The finding, hence, leans on another evidence for nanostructuring of the AuFe nanocrystals and excluding the likelihood of separate Au and Fe or Fe3O4 nanoparticles which could be generated from the AuFe preparation process.
Conclusions
We have reported the synthesis and characterization of the long-term stable, monodisperse AuFe optical–magnetic bimetallic nanocrystals by the polymer nanoemulsion. The analyses show the monodispersity and high monocrystallinity of the nanocrystals, which manifest both well-defined optical and magnetic properties, revealing a broadband surface plasmon resonance peaking at ~600 nm and clear soft ferromagnetic behavior at room temperature. Such monocrystalline, biocompatible AuFe multifunctional nanocrystals may offer exciting opportunities in fundamental studies and are further explored for DNA transfection, biosensing, drug delivery, optical detection, photonic therapy, magnetic separation, manipulation, and actuation, in addition to the traditional functioning in catalysis, electronics, optoelectronics, and opto-magnetics.
References
Kharisov BI, Kharissova OV, Yacaman MJ, Mendez UO (2009) State of the Art of the Bi- and Trimetallic Nanoparticles on the Basis of Gold and Iron. Recent Pat Nanotechnol 3:81–98
Cuenya BR, Ono LK, Croy JR, Naitabdi A, Heinrich H, Zhao J, Alp EE, Sturhahn W, Keune W (2009) Structure and phonon density of states of supported size-selected 57FeAu nanoclusters: A nuclear resonant inelastic x-ray scattering study. Appl Phys Lett. 95(143103):1–3
Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38:1759–1782
Fendler JH (ed) (1998) Nanoparticles and nanostructured films. Wiley-VCH, Weinheim
Daniel M-C, Astruc D (2004) Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem Rev 104:293–346
Nie Z, Petukhova A, Kumacheva E (2010) Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat Nanotech 5:15–25
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem Rev 108:2064–2110
Ross C (2001) Patterned magnetic recording media. Annu Rev Mater Res 31:203–235
Niemeyer CM (2001) Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew Chem Int Ed 40:4128–4158
Kim DK, Zhang Y, Kehr J, Klason T, Bjelke B, Muhammed M (2001) Characterization and MRI study of surfactant-coated superparamagnetic nanoparticles administered into the rat brain. J Magn Magn Mater 225:256–261
Chiang I-C, Chen D-H (2007) Synthesis of Monodisperse FeAu Nanoparticles with Tunable Magnetic and Optical Properties. Adv Funct Mater 17:1311–1316
Liu HL, Wu JH, Min JH, Kim YK (2008) Synthesis of monosized magnetic-optical AuFe alloy nanoparticles. J Appl Phys 103(07D529):1–3
Dahal N, Chikan V (2008) Synthesis of Water-Soluble Iron-Gold Alloy Nanoparticles. Chem Mater 20:6389–6395
Bakshi MS, Kaura A, Bhandari P, Kaur G, Torigoe K, Esumi K (2006) Synthesis of Colloidal Gold Nanoparticles of Different Morphologies in the Presence of Triblock Polymer Micelles. J Nanosci Nanotech 6:1405–1410
Liu HL, Hou P, Zhang WX, Wu JH (2010) Synthesis of monosized core–shell Fe3O4/Au multifunctional nanoparticles by PVP-assisted nanoemulsion process. Colloids Surf A Physicochem Eng Aspects 356:21–27
Sakai T, Alexandridis P (2005) Size- and shape-controlled synthesis of colloidal gold through autoreduction of the auric cation by poly(ethylene oxide)–poly(propylene oxide) block copolymers in aqueous solutions at ambient conditions. Nanotechnology 16:S344–S353
Mao C, Chen XB, Hou XM, Shen J, Zhu JJ, Zhao WB (2009) Synthesis of rambutan-like hybrid nanospheres of Au-P123. Gold Bull 42:215–218
Chen S, Li Y, Guo C, Wang J, Ma J, Liang X, Yang L-R, Liu H-Z (2007) Temperature-Responsive Magnetite/PEO-PPO-PEO Block Copolymer Nanoparticles for Controlled Drug Targeting Delivery. Langmuir 23:12669–12676
Yang J, Zhai Y, Deng Y, Gu D, Li Q, Wu Q, Huang Y, Tu B, Zhao D (2010) Direct triblock-copolymer-templating synthesis of ordered nitrogen-containing mesoporous polymers. J Colloid Interface Sci 342:579–585
Liu HL, Sonn CH, Wu JH, Lee KM, Kim YK (2008) Synthesis of streptavidin-FITC-conjugated core–shell Fe3O4-Au nanocrystals and their application for the purification of CD4+ lymphocytes. Biomaterials 29:4003–4011
Jain TK, Foy SP, Erokwu B, Dimitrijevic S, Flask CA, Labhasetwar V (2009) Magnetic resonance imaging of multifunctional pluronic stabilized iron-oxide nanoparticles in tumor-bearing mice. Biomaterials 30:6748–6756
Ko YH, Kim KJ, Han CK, Petrovic C, Hu R, Lee HH, Lee Y (2009) Pressure–volume equation of state of FeAu and FePt. High Pressure Res 29:800–805
Denton AR, Ashcroft NW (1991) Vegard’s law. Phys Rev A 43:3161–3164
Willets KA, Duyne RPV (2007) Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu Rev Phys Chem 58:267–297
Acknowledgments
This work was supported in part by the Scientific and Technological Development Projects, Science and Technology Department of Henan Province, China (nos. 092102210004 and 092300410031); by the Natural Science Research Foundation Grants, Education Department of Henan Province, China (nos. 2009A150004 and 2009A150005); the Pioneer Research Center Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, South Korea (no. 2010-0002190); and the Industrial Core Technology Development Program funded by the Ministry of Knowledge Economy, South Korea (no. 10033183).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Liu, H., Zhang, W., Hou, P. et al. Facile growth of monocrystalline gold–iron nanocrystals by polymer nanoemulsion. Gold Bull 44, 21–25 (2011). https://doi.org/10.1007/s13404-010-0003-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-010-0003-4