Abstract
Background
Chemotherapeutic agents such as cisplatin are commonly used in patients with clinically unresectable or recurrent esophageal cancer (ESCA). However, patients often develop resistance to cisplatin, which in turn leads to a poor prognosis. Studies have shown that FAM111B may be involved in the development of tumors as an oncogene or tumor suppressor gene. However, the pathological role and corresponding mechanism of FAM111B in ESCA are still unclear.
Methods
The GEPIA web tool, ENCORI Pan-Cancer Analysis Platform and UALCAN-TCGA database were used to study the expression of FAM111B in ESCA. CCK-8, angiogenesis, Transwell and xenograft assays were applied to explore the biological function of FAM111B in ESCA. Western blot, RT-qPCR, and RNA-seq analyses were applied to study the FAM111B/GSDMA axis in the progression of ESCA cells. CCK-8 and xenograft assays were used to study the role of the FAM111B/GSDMA axis in determining the sensitivity of ESCA to cisplatin.
Results
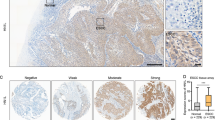
Our results demonstrated that FAM111B is highly expressed in ESCA tissues compared to normal tissues. We showed that FAM111B promotes the progression of ESCC cells by binding to GSDMA and that the trypsin protease domain is essential for the activity of FAM111B. Furthermore, we showed that the FAM111B/GSDMA axis regulates cisplatin sensitivity in ESCA.
Conclusions
Overall, we identified a novel FAM111B/GSDMA axis regulating ESCA tumorigenesis and chemosensitivity, at least in ESCC cells.








Similar content being viewed by others
Data availability
The databases used and/or analyzed during the current study are available from the corresponding authors (Xiaoxiong Xiao, E-mail: xiaoxx1988@csu.edu.cn) on reasonable request.
References
H.C. Puhr, G.W. Prager, A. Ilhan-Mutlu, How we treat esophageal squamous cell carcinoma. ESMO Open 8(1), 100789 (2023)
M. Sheikh et al., Current status and future prospects for esophageal cancer. Cancers (Basel) 15(3), 765 (2023)
J. An et al., Natural products for esophageal cancer therapy: from traditional medicine to modern drug discovery. Int J Mol Sci 23(21), 13558 (2022)
Y. Baba et al., Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci 111(9), 3132–3141 (2020)
Y. Jia et al., Long non-coding RNA NORAD/miR-224-3p/MTDH axis contributes to CDDP resistance of esophageal squamous cell carcinoma by promoting nuclear accumulation of β-catenin. Mol Cancer 20(1), 162 (2021)
E. Perland, R. Fredriksson, Classification systems of secondary active transporters. Trends Pharmacol Sci 38(3), 305–315 (2017)
A.K. Holzer, S.B. Howell, The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66(22), 10944–10952 (2006)
A. Yaneff et al., MRP4/ABCC4 As a new therapeutic target: meta-analysis to determine cAMP binding sites as a tool for drug design. Curr Med Chem 26(7), 1270–1307 (2019)
J. Zhou et al., The drug-resistance mechanisms of five platinum-based antitumor agents. Front Pharmacol 11, 343 (2020)
S. Dilruba, G.V. Kalayda, Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol 77(6), 1103–1124 (2016)
W.P. Roos, B. Kaina, DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett 332(2), 237–248 (2013)
X. Zhu et al., DNMT3B-mediated FAM111B methylation promotes papillary thyroid tumor glycolysis, growth and metastasis. Int J Biol Sci 18(11), 4372–4387 (2022)
S. Mercier et al., Mutations in FAM111B cause hereditary fibrosing poikiloderma with tendon contracture, myopathy, and pulmonary fibrosis. Am J Hum Genet 93(6), 1100–1107 (2013)
H. Sun et al., FAM111B, a direct target of p53, promotes the malignant process of lung adenocarcinoma. Onco Targets Ther 12, 2829–2842 (2019)
S. Mercier et al., FAM111B mutation is associated with pancreatic cancer predisposition. Pancreas 48(5), e41–e42 (2019)
H. Wu, C. Liang, Pan-cancer analysis of the tumorigenic effect and prognostic diagnostic value of fam111b in human carcinomas. Int J Gen Med 16, 1845–1865 (2023)
X. Xiao et al., Silencing of UAP1L1 inhibits proliferation and induces apoptosis in esophageal squamous cell carcinoma. Mol Carcinog 60(3), 179–187 (2021)
K. Kawasaki et al., FAM111B enhances proliferation of KRAS-driven lung adenocarcinoma by degrading p16. Cancer Sci 111(7), 2635–2646 (2020)
S. Wenmaekers et al., A Potential Role for HUWE1 in Modulating Cisplatin Sensitivity. Cells 10(5), 1262 (2021)
C. He et al., Elevated H3K27me3 levels sensitize osteosarcoma to cisplatin. Clin Epigenetics 11(1), 8 (2019)
I. Lakshmanan et al., MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53. Clin Cancer Res 23(14), 3906–3917 (2017)
X. Gong et al., The structure and regulation of the E3 ubiquitin ligase HUWE1 and its biological functions in cancer. Invest New Drugs 38(2), 515–524 (2020)
C. Guo et al., Integrated analysis of multi-omics alteration, immune profile, and pharmacological landscape of pyroptosis-derived lncRNA pairs in gastric cancer. Front Cell Dev Biol 10, 816153 (2022)
M. Boivin et al., CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol 115(3), 407–413 (2009)
M. Liu et al., Predictive biomarkers of dicycloplatin resistance or susceptibility in prostate cancer. Front Genet 12, 669605 (2021)
X. Lu, T. Guo, X. Zhang, Pyroptosis in Cancer: Friend or Foe? Cancers (Basel) 13(14), 3620 (2021)
X. Ouyang et al., Pyroptosis, inflammasome, and gasdermins in tumor immunity. Innate Immun 29(1–2), 3–13 (2023)
J. Shi et al., Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575), 660–665 (2015)
K.W. Chen et al., Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. Embo J 38(10), e101638 (2019)
Q. Wang et al., A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579(7799), 421–426 (2020)
W. Deng et al., Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602(7897), 496–502 (2022)
W. Zou, J.D. Wolchok, L. Chen, PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 8(328), 328rv4 (2016)
A. Ribas, J.D. Wolchok, Cancer immunotherapy using checkpoint blockade. Science 359(6382), 1350–1355 (2018)
M. Macchiaiolo et al., Expanding phenotype of FAM111B-related disease focusing on liver involvement: Literature review, report of a case with end-stage liver disease and proposal for a new acronym. Am J Med Genet A 188(10), 2920–2931 (2022)
A. Seo et al., FAM111B mutation is associated with inherited exocrine pancreatic dysfunction. Pancreas 45(6), 858–862 (2016)
A.L. Welter, Y.J. Machida, Functions and evolution of FAM111 serine proteases. Front Mol Biosci 9, 1081166 (2022)
A. Arowolo, C. Rhoda, N. Khumalo, Mutations within the putative protease domain of the human FAM111B gene may predict disease severity and poor prognosis: A review of POIKTMP cases. Exp Dermatol 31(5), 648–654 (2022)
S. Hoffmann et al., FAM111 protease activity undermines cellular fitness and is amplified by gain-of-function mutations in human disease. EMBO Rep 21(10), e50662 (2020)
Acknowledgements
Not applicable.
Funding
This work were supported by grants from the National Natural Science Youth Foundation of China (Grant No. 82203838 (Xiaoxiong Xiao)); Natural Science Foundation General Program of Hunan Province (Grant No. 2022JJ40830 (Xiaoxiong Xiao)); Natural Science Foundation General Program of Changsha City (Grant No. kq2014290 (Xiaoxiong Xiao)); National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases (Lung Cancer, grant number: z027002 (Xiaoxiong Xiao)); National Natural Science Foundation of China (Grant No. 81772800, 82072945 (Pian Liu)).
Author information
Authors and Affiliations
Contributions
Haiqin Wang. supervised the experiments, and was responsible for bioinformatics and statistical analysis. Haohui Wang. carried out the in vitro and in vivo experiments, and wrote the original draft. Jiajing Chen. collaborated to collect specimens and record clinical information. Pian Liu. conducted the major revision work, and provided critical reviews. Xiaoxiong Xiao. generated the study design, provided the clinical specimens and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki principles. It was approved by the Animal Use and Care Committees at Xiangya hospital, Central South University.
Consent for publication
All subjects have written informed consent.
Conflict of interest
The authors have declared that no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haiqin Wang and Haohui Wang contributed equally.
Supplementary Information
Below is the link to the electronic supplementary material.
13402_2023_871_MOESM1_ESM.tif
Supplementary file1 Supplementary figure 1 A and B, The univariate and multivariate Cox regression analyses through the GEO dataset GSE53622. p values as indicated. C and D, Lymph node metastasis of KYSE410 cells with FAM111B knockdown. E, The expression of SNAI1 in RNA-seq of FAM111B. Fand G, KYSE410 and TE-1 cells were infected with shControl, shFAM111B #1, or shFAM111B #2 for 72 h. Cells were collected for RT-qPCR analysis (F), and Western blot analysis (G). H, The excised subcutaneous tumors of nude mice were subjected to IHC staining of SNAI1. Data presents as mean ± SD with six replicates. ***, p < 0.001 (TIF 35268 KB)
13402_2023_871_MOESM2_ESM.tif
Supplementary file2 Supplementary figure 2 A and B, KYSE410 cells were transfected with indicated shRNAs for 72 h. Cells were collected and subcutaneously injected into the nude mice. The tumor growth curve was shown in panel B. Data presents as mean ± SD with six replicates. ***, p < 0.001. C and D, KYSE410 and TE-1 cells were transfected with indicated shRNAs for 72 h and treated with cisplatin, cells were collected for Western blot analysis to measure the expression of cleaved caspase 3. ***, p < 0.001. E and F, The excised subcutaneous tumors of nude mice were subjected to IHC staining of cleaved caspase 3. Data presents as mean ± SD with six replicates. ***, p < 0.001 (TIF 47227 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Wang, H., Chen, J. et al. Overexpressed FAM111B degrades GSDMA to promote esophageal cancer tumorigenesis and cisplatin resistance. Cell Oncol. 47, 343–359 (2024). https://doi.org/10.1007/s13402-023-00871-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00871-0