Abstract
Background
Previously, nitric oxide (NO) has been found to affect the metastatic behavior of various types of cancer. In addition, it has been found that alterations in integrin expression may have profound effects on cancer cell survival and migration. Here, we aimed at assessing the effects of non-toxic concentrations of NO on human non-small cell lung cancer (NSCLC) cells, including the expression of integrins and the migration of these cells.
Methods
The cytotoxic and proliferative effects of NO on human NSCLC-derived H460, H292 and H23 cells were tested by MTT assay. The migration capacities of these cells was evaluated by wound healing and transwell migration assays. The expression of integrins and migration-associated proteins was determined by Western blot analyses.
Results
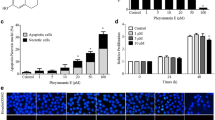
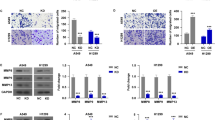
We found that NO treatment caused a significant increase in the expression of integrin αv and β1 in all three NSCLC-derived cell lines tested. Known migration-associated proteins acting downstream of these integrins, including focal adhesion kinase (FAK), active RhoA (Rho-GTP) and active cell division control 42 (Cdc42-GTP), were found to be significantly activated in response to NO. In addition, we found that NO-treated cells showed an increased motility and that this motility was associated with a significant increase in the number of filopodia per cell. We also found that NO-treated cells exhibited increased active protein kinase G (PKG), protein kinase B (AKT) and FAK expression levels. Using a pharmacological approach, we found that the integrin-modulating effect of NO is most likely brought about by a PKG/AKT-dependent mechanism, since the observed changes in integrin expression were abolished by AKT inhibitors, but not by FAK inhibitors.
Conclusion
Our data suggest a novel role of NO in the regulation of integrin expression and, concomitantly, the migratory capacity of NSCLC cells.








Similar content being viewed by others
References
A. Khosravi, S. Shahrabi, M. Shahjahani, N. Saki, The bone marrow metastasis niche in retinoblastoma. Cell. Oncol. 38, 253–263 (2015)
J. A. Joyce, J. W. Pollard, Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009)
S. Wan, Y. Liu, Y. Weng, W. Wang, W. Ren, C. Fei, Y. Chen, Z. Zhang, T. Wang, J. Wang, BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells. Cell. Oncol. 37, 363–375 (2014)
A. Koren, E. Sodja, M. Rijavec, M. Jez, V. Kovac, P. Korosec, T. Cufer, Prognostic value of cytokeratin-7 mRNA expression in peripheral whole blood of advanced lung adenocarcinoma patients. Cell. Oncol. 38, 387–395 (2015)
E. Prodromaki, A. Korpetinou, E. Giannopoulou, E. Vlotinou, μ. Chatziathanasiadou, N. Papachristou, C. Scopa, H. Papadaki, H. Kalofonos, D. Papachristou, expression of the microRNA regulators Drosha, dicer and Ago2 in non-small cell lung carcinomas. Cell. Oncol. 38, 307–317 (2015)
C. Zeng, W. Fan, X. Zhang, RRM1 expression is associated with the outcome of gemcitabine-based treatment of non-small cell lung cancer patients–a short report. Cell. Oncol. 38, 319–325 (2015)
Z. B. Cincin, M. Unlu, B. Kiran, E. S. Bireller, Y. Baran, B. Cakmakoglu, Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 38, 195–204 (2015)
R. L. Siegel, K. D. Miller, A. Jemal, Cancer statistics. CA Cancer J. Clin. 65, 5–29 (2015)
L. C. Jadeski, K. O. Hum, C. Chakraborty, P. K. Lala, Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int. J. Cancer 86, 30–39 (2000)
D. Fukumura, S. Kashiwagi, R. K. Jain, The role of nitric oxide in tumour progression. Nat. Rev. Cancer 6, 521–534 (2006)
C. Liu, C. Wang, T. Chen, H. Lin, C. Yu, H. Kuo, Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. Brit. J. Cancer 78, 534 (1998)
P. Chanvorachote, U. Nimmannit, Y. Lu, S. Talbott, B.-H. Jiang, Y. Rojanasakul, Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J. Biol. Chem. 284, 28476–28484 (2009)
N. Yongsanguanchai, V. Pongrakhananon, A. Mutirangura, Y. Rojanasakul, P. Chanvorachote, Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am. J. Physiol. - Cell Ph 308, C89–C100 (2015)
P. Chanvorachote, U. Nimmannit, C. Stehlik, L. Wang, B.-H. Jiang, B. Ongpipatanakul, Y. Rojanasakul, Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 66, 6353–6360 (2006)
X. Weiming, L. Z. Liu, M. Loizidou, M. Ahmed, I. G. Charles, The role of nitric oxide in cancer. Cell Res. 12, 311–320 (2002)
A. Huttenlocher, A. R. Horwitz, Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 3, a005074 (2011)
S. P. Holly, M. K. Larson, L. V. Parise, Multiple roles of integrins in cell motility. Exp. Cell Res. 261, 69–74 (2000)
D. G. Stupack, D. A. Cheresh, Get a ligand, get a life: integrins, signaling and cell survival. J. Cell Sci. 115, 3729–3738 (2002)
J. D. Hood, D. A. Cheresh, Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91–100 (2002)
H. Truong, E. H. Danen, Integrin switching modulates adhesion dynamics and cell migration. Cell Adhes. Migr. 3, 179–181 (2009)
N. C. Wong, B. M. Mueller, C. F. Barbas, P. Ruminski, V. Quaranta, E. C. Lin, J. W. Smith, αv integrins mediate adhesion and migration of breast carcinoma cell lines. Clin. Exp. Metastasis 16, 50–61 (1998)
R. Hosotani, M. Kawaguchi, T. Masui, T. Koshiba, J. Ida, K. Fujimoto, M. Wada, R. Doi, M. Imamura, Expression of integrin αvβ3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas 25, e30–e35 (2002)
P. T. Caswell, H. J. Spence, M. Parsons, D. P. White, K. Clark, K. W. Cheng, G. B. Mills, M. J. Humphries, A. J. Messent, K. I. Anderson, Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510 (2007)
D. Wang, S. Müller, A. R. Amin, D. Huang, L. Su, Z. Hu, M. A. Rahman, S. Nannapaneni, L. Koenig, Z. Chen, The pivotal role of integrin β1 in metastasis of head and neck squamous cell carcinoma. Clin. Cancer Res. 18, 4589–4599 (2012)
H. Guillou, A. Depraz-Depland, E. Planus, B. Vianay, J. Chaussy, A. Grichine, C. Albiges-Rizo, M. R. Block, Lamellipodia nucleation by filopodia depends on integrin occupancy and downstream Rac1 signaling. Exp. Cell Res. 314, 478–488 (2008)
R. Mayor, C. Carmona-Fontaine, Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 20, 319–328 (2010)
W. Guo, F. G. Giancotti, Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 (2004)
S. H. Francis, J. L. Busch, J. D. Corbin, cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010)
S. H. Lee, J. S. Byun, P. J. Kong, H. J. Lee, D. K. Kim, H. S. Kim, J.-H. Sohn, J. J. Lee, S. Y. Lim, W. Chun, Inhibition of eNOS/sGC/PKG pathway decreases Akt phosphorylation induced by Kainic acid in mouse hippocampus. Korean J. Physiol. Pharmacol. 14, 37–43 (2010)
D. J. Sieg, C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, D. D. Schlaepfer, FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249–256 (2000)
S. K. Mitra, D. D. Schlaepfer, Integrin-regulated FAK–Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 (2006)
M. S. Roberts, A. J. Woods, T. C. Dale, P. van der Sluijs, J. C. Norman, Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of αvβ3 and α5β1 integrins. Mol. Cell Biol. 24, 1505–1515 (2004)
P. K. Lala, C. Chakraborty, Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2, 149–156 (2001)
P. Allavena, A. Sica, G. Solinas, C. Porta, A. Mantovani, The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 66, 1–9 (2008)
M. Valko, C. Rhodes, J. Moncol, M. Izakovic, M. Mazur, Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 (2006)
A. Sanuphan, P. Chunhacha, V. Pongrakhananon, P. Chanvorachote, Long-term nitric oxide exposure enhances lung cancer cell migration. Biomed. Res. Int. 2013, 186972 (2013). doi:10.1155/2013/186972
P. Wongvaranon, V. Pongrakhananon, P. Chunhacha, P. Chanvorachote, Acquired resistance to chemotherapy in lung cancer cells mediated by prolonged nitric oxide exposure. Anticancer Res. 33, 5433–5444 (2013)
P. Chanvorachote, U. Nimmannit, L. Wang, C. Stehlik, B. Lu, N. Azad, Y. Rojanasakul, Nitric oxide negatively regulates Fas CD95-induced apoptosis through inhibition of ubiquitin-proteasome-mediated degradation of FLICE inhibitory protein. J. Biol. Chem. 280, 42044–42050 (2005)
P. Chunhacha, P. Chanvorachote, Roles of caveolin-1 on anoikis resistance in non small cell lung cancer. Int. J. Physiol. Pathophysiol. Pharmacol. 4, 149–155 (2012)
A. Maiuthed, P. Chanvorachote, Cisplatin at sub-toxic levels mediates integrin switch in lung cancer cells. Anticancer Res. 34, 7111–7117 (2014)
E. G. Sarris, M. W. Saif, K. N. Syrigos, The biological role of PI3K pathway in lung cancer. Pharmaceuticals 5, 1236–1264 (2012)
J. Luo, B. D. Manning, L. C. Cantley, Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4, 257–262 (2003)
J. Brognard, A. S. Clark, Y. Ni, P. A. Dennis, Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 61, 3986–3997 (2001)
S. K. Mitra, D. A. Hanson, D. D. Schlaepfer, Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 (2005)
Acknowledgments
This study was funded by the Thailand Research Fund (RSA5780043). The funders had no role in the study design, the data collection and analysis, the decision to publish or the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Saisongkorh, V., Maiuthed, A. & Chanvorachote, P. Nitric oxide increases the migratory activity of non-small cell lung cancer cells via AKT-mediated integrin αv and β1 upregulation. Cell Oncol. 39, 449–462 (2016). https://doi.org/10.1007/s13402-016-0287-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-016-0287-3