Abstract
This study describes the preparation of zinc oxide nanocomposite and its application as photocatalyst in the degradation of methylene blue under sunlight irradiation. Zinc oxide-coated biochar (ZnO/BC) nanocomposite was prepared using the hydrothermal method from banana peels and zinc acetate dihydrate as precursors. The biochar (BC), ZnO nanoparticles (ZnONPs), and ZnO/BC nanocomposite were characterized, and the results showed that ZnONPs had definite crystallinity and a regular surface morphology, with particles approximately 18 nm in size, and a XRD pattern corresponding to ZnONPs. In contrast, the ZnO/BC nanocomposite exhibited a more amorphous structure consistent with wurtzite (ZnO) and an irregular surface morphology with clusters of white particles measuring around 20–30 nm. Tauc’s plot was used to calculate the band gaps energy of ZnONPs (3.04 eV) and ZnO/BC nanocomposite (2.89 eV). Catalyst-free, ZnONPs, and ZnO/BC nanocomposite were utilized for the photocatalytic degradation of methylene blue (MB) under sunlight irradiation for 0 to 120 min in which ZnO/BC nanocomposite showed excellent photocatalytic degradation of MB under sunlight irradiation at 0 to 120 min due to lower band gap energy and synergetic effect between ZnO and BC. Dosages of 100 mg (ZnO/BC nanocomposite) and 10 ppm (MB) were optimized to obtain the best photocatalytic degradation efficiency (92 %) under sunlight irradiation with 0 to 120 min. The process was conducted with various parameters like dosage variation, concentration of MB, and different pH 3, 5, 8, and 10 to improve the photocatalytic degradation of MB from wastewater. The results indicated that the optimal conditions for the photocatalytic degradation of MB (92% after 120 min) were a catalyst dosage of 100 mg, an MB concentration of 10 ppm, and a pH of 10. This work demonstrates the potential of ZnO/BC nanocomposite photocatalyst for application in wastewater treatment and environment remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water pollution stands as a significant global concern, exerting far-reaching impacts on both climate and the environment, affecting various life forms [1]. Bodies of water worldwide have been compromised due to the influx of dyes from industrial processes, especially the textile sector, with MB dye among the pollutants posing a particular threat to freshwater resources [2]. Given the escalating demand, the production of dyes is slated to rise, necessitating diverse techniques such as biological, chemical, pharmaceutical, pulp, dye processing, and waste management to address the issue [3]. Traditional methods like adsorption on activated carbon, reverse osmosis, ultra-filtration, coagulation by chemical agents, and ion exchange on synthetic adsorbent resins are being replaced by novel approaches.
Methylene blue (MB) dye has found widespread use across industries like paper, paint, textiles, pharmaceuticals, cosmetics, and food [4, 5]. Regrettably, the wastewater from these sectors, when combined with drinking water, results in heavily contaminated water harboring dangerous pollutants for both humans and animals [6]. Exposure to MB triggers severe health problems, including nausea, mental confusion, vomiting, methemoglobinemia, eye irritation, and profuse sweating [2]. An array of techniques has been explored to eliminate MB from water, ranging from filtration, adsorption, and electrochemical oxidation to reverse osmosis, membrane separation, ion exchange, and catalytic reactions [7,8,9,10,11,12,13,14]. Nonetheless, these techniques have their limitations, including high costs, protracted procedures, and incomplete MB removal. Considering their affordability, ease of use, photocatalytic reaction, and post-mineralization catalyst retrieval, photocatalytic methods have emerged as a viable solution [15, 16].
In recent years, advanced oxidation processes (AOPs) have gained traction as swift, efficient, and non-toxic approaches, with the photo-Fenton reaction, ozonation, and photocatalysis playing pivotal roles in degrading various pollutants, both organic and inorganic [17,18,19]. Photocatalytic degradation serves diverse purposes, spanning wastewater treatment, air purification, sterilization, heavy metal remediation, and pollution breakdown [20]. While activated carbon is a mainstay in wastewater treatment, its high production costs necessitate alternate activation methods [21]. Biomass conversion methods like pyrolysis, combustion, liquefaction, gasification, and others have been explored as cost-effective activation strategies [22,23,24]. There has been a recent emphasis on semiconductor photocatalysts as effective instruments for environmentally friendly wastewater treatment, operating on the principle of decomposing pollutants into benign byproducts [25, 26]. Various semiconducting metal oxides, such as TiO2, CeO2, ZnO, SnO2, and CuO, have undergone thorough investigation as photocatalysts [27,28,29,30,31]. ZnO, due to its cost-effectiveness, chemical stability, and efficacy, has garnered significant attention [32, 33]. Photocatalysis offers advantages such as the utilization of clean solar energy, high efficiency, and minimal generation of hazardous waste, making it applicable in diverse fields, ranging from environmental remediation to materials science [34]. Key characteristics of photocatalysts include their extensive active surface area, effective charge separation, ability to absorb visible light, and enduring photostability. Despite the preference for ZnO nanoparticles in photocatalytic applications over bulk materials, they exhibit certain limitations, such as solubility in acidic conditions, photo-leaching in basic solutions under UV light, rapid electron-hole recombination, and insufficient absorption of visible light energy [35].
Over the last decade, biochar has been produced through thermal means from diverse biomass sources like plant waste, wood chips, banana peels, orange peels, and coconut shells [36, 37]. Biochar boasts distinct physicochemical attributes like a generous surface area, strong carbonaceous properties, and superior electrical conductivity [38]. Its exceptional adsorption capacity aids in full mineralization of organic pollutants, while also curtailing electron-hole recombination during photocatalytic degradation. Moreover, biochar is environmentally friendly, renewable, and cost-effective [39]. Bananas, rich in fibers, vitamins B6 and C, potassium, and magnesium, confer numerous health benefits like aiding digestion and weight management [40]. Banana peels are replete with oxygen, hydrogen, and carbon, forming functional groups like carboxylic and hydroxyl groups on the biochar surface, proving instrumental in organic dye and pesticide degradation [41].
Various metal oxide-coated biochar nanocomposites, like carboxymethyl cellulose-modified ZnO-biochar nanocomposite, ZnO-encapsulated biochar nanocomposite using cotton stalks, and ZnO-betaine-modified biochar nanocomposites, have been prepared using different biomass sources [42,43,44]. ZnO-coated biochar nanocomposites bring the advantages of increased surface area, heightened reactive sites, improved dispersion, robust electron transfer between ZnO and biochar, and amplified photocatalytic activity [45, 46]. Recent advancements have led to porous carbon-supported ZnO nanocomposites, expanding raw materials and enhancing photocatalytic performance [47]. While the full scope of ZnO/biochar nanocomposite’s potential remains untapped, its microstructure has shown promise in augmenting photocatalytic degradation through biochar supplementation [48]. Hence, the fusion of ZnO nanoparticles and biochar holds substantial promise in the photocatalytic degradation of organic pollutants, with the ZnO/biochar nanocomposite demonstrating significant potential for photodegradation studies.
There is currently significant interest in the potential applications of biochar produced through the pyrolysis of biomass. These biochars are made from inexpensive, environmentally friendly, renewable natural resources and involve simple techniques for creating functionalized carbon-based materials [49]. The creation of biochar and ZnO nanocomposites offers several advantages, such as increased adsorption capacity, high surface area, numerous active sites, well-established structures, and improved dispersion of ZnO nanoparticles on the biochar surface [50, 51]. The nanocomposite catalyst developed enhances photocatalytic degradation efficiency [52]. Notably, Leichtweis et al. discovered that the interaction between Cu/Fe2O4 and biochar enhanced the photocatalytic degradation activity of the CuFe2O4/biochar nanocomposite. This interaction also improved charge carrier separation, adsorption capacity, and electron transfer mediation [53]. Meena et al. developed a green biochar-supported ZnFe2O4 composite photocatalyst that effectively degrades MB under visible light irradiation [54]. Additionally, pomegranate peels were used to create a ZnO composite supported by biochar, enhancing the photocatalytic activity of ZnO nanoparticles. The biochar-encapsulated ZnO nanocomposite, with its multiple active sites, supports increased adsorption of organic pollutants and generates highly reactive radicals, thus boosting visible light absorption and photocatalytic efficiency [55, 56]. Sun et al. reported that a hybrid nanocomposite photocatalyst based on g-C3N4/ferrite and biochar demonstrated excellent photocatalytic degradation of dyes and enhanced adsorption of organic pollutants, making it suitable for wastewater treatment systems [57].
To date, no known reports have focused on the work presented in this study. In this investigation, a ZnO/BC nanocomposite was synthesized using the hydrothermal method, utilizing banana peels to produce biochar. The biochar was then modified with ZnO to form the nanocomposite. Characterization of the materials was conducted using techniques such as FT-IR, UV-visible spectroscopy, XRD, SEM, and TEM. The design of the ZnO/BC nanocomposite aimed to enhance light absorption, alleviate photogenerated electron-hole recombination, and fortify photocatalytic capabilities. The experimental evaluation of the materials focused on their photocatalytic degradation of methylene blue (MB) under sunlight irradiation, comparing the photocatalytic efficiency of ZnO with that of the ZnO/BC nanocomposite. As a result, the ZnO/BC nanocomposite emerged as a crucial agent in advancing our understanding of the photocatalytic process and the catalytic mechanisms involved.
2 Experimental section
2.1 Chemicals and reagents
Banana peels were collected from a nearby market in Tenkasi, India (circa N8.96°, E77.31°). Analytical-grade Zinc acetate dihydrate (Zn(OAc)2.2H2O) and methylene blue (MB) were procured from Sigma-Aldrich, India. Sodium hydroxide (NaOH), hydrochloric acid (HCl), and ethanol were sourced from Sisco Research Laboratories (SRL) PVT Ltd., India (www.vijayascientific.com). Distilled water was employed for all experimental procedures.
2.2 Preparation of ZnO nanoparticles
A 0.1M solution of Zn(OAc)2.2H2O was prepared by dissolving 2.0 g of the salt in 80 mL of distilled water and stirring for 30 min. Subsequently, a 2.5 M solution of NaOH was obtained by mixing 1.0 g of NaOH with 10 mL of distilled water stirring for 20 min. This NaOH solution was then gradually added to the Zn(OAc)2.2H2O solution while stirring, resulting in a white precipitate. The mixture was stirred for an additional hour to ensure proper formation of the precipitate.
The resulting solution, now containing the white precipitate, was transferred into a 100-mL Teflon-coated autoclave. This autoclave was placed in a muffle furnace and maintained at a temperature of 180 °C for duration of 12 h. After this thermal treatment, the precipitate was recovered by centrifugation. This centrifugation process was repeated five times, alternating between distilled water and ethanol as the washing agents. The autoclave was allowed to cool to room temperature before starting the washing procedure. To obtain powdered ZnO nanoparticles, the white precipitate was subjected to a final drying step. This involved drying the precipitate at a temperature of 400 °C for duration of 2 h.
2.3 Synthesis of ZnO/BC nanocomposite
The collected banana peels underwent a thorough cleansing process, involving two rounds of washing with water to eliminate any impurities. Following this, they were allowed to naturally dry for 4 days under sunlight. Subsequently, the dried peels were finely ground into powder form. This powder served as a foundational material for crafting a nanocomposite featuring biochar-coated zinc oxide, referred to as ZnO/BC.
To create the nanocomposite, a 250-mL beaker was employed, wherein 4.0 g of banana peel powder and 2.0 g of zinc acetate dihydrate were combined. To this mixture, 80 mL of distilled water was introduced. The mixture was homogenized by stirring the solution using a magnetic stirrer for duration of 30 min. The next step involved transferring the solution into a 100-mL Teflon-coated autoclave, which was then positioned within a muffle furnace set at 180°C for a period of 6 h.
Upon completing the autoclave treatment, the resulting precipitate was gathered through centrifugation at a speed of 5000 rpm for 10 min. Subsequently, the precipitate was cleaned using multiple washes with a combination of water and ethanol to ensure the removal of any residual components. The washed precipitate was then meticulously collected and subjected to drying within an oven maintained at 80 °C for a span of 12 h. This meticulous process culminated in the production of a distinctive black powder. The visual representation of the entire procedure is illustrated in the accompanying Scheme 1.
2.4 Characterization methods
The present study employed a range of characterization techniques for the assessment of materials. The ZnONP and ZnO/BC nanocomposite powder samples were suspended in distilled water prior to measuring the optical properties using a UV-visible spectrometer (Double beam, Model: UH-5300, HITACHI). Functional group analysis of the materials was conducted with Fourier transform infrared (FT-IR) spectroscopy using an ID7ATR Thermo Scientific/NicoLET IS5 instrument.
Fluorescence behavior of ZnONPs and the ZnO/BC nanocomposite was examined with a fluorescence spectrometer (Perkin Elmer). Crystalline properties were assessed using an X-ray diffractometer (Philips PW1729), with Cu Kα radiation (λ = 1.5406 Å) and operating at 45 kV and 40 mA.
Surface morphology and elemental composition analysis were performed using scanning electron microscopy (SEM) with a Carl Zeiss model (Ultra Plus). Size determination of ZnONPs and the ZnO/BC nanocomposite was achieved with transmission electron microscopy (TEM) using a JEOL JEM-2100F instrument, operating at an electron accelerating voltage of 200 kV.
2.5 Photocatalytic degradation method
The investigation into the photocatalytic activity of ZnO nanoparticles (ZnONPs) and the ZnO/biochar (ZnO/BC) nanocomposite for MB dye degradation was carried out through a series of photocatalytic studies. To initiate the experimentation, a stock solution of 0.05 g of MB dye, equivalent to 100 parts per million (100 ppm), was dissolved in distilled water within a standard 1L measuring flask. Subsequently, a quantity of 10 mL from this stock solution, constituting 10 ppm of MB dye, was added to a 100-mL standard measuring flask, wherein it was reconstituted with distilled water. This solution, having the desired concentration, was utilized for the ensuing experiments.
The photocatalytic degradation reactions were executed by introducing 100 mg of the photocatalysts into a 100 mL solution of 10 ppm MB dye within a glass beaker. To ensure a well-dispersed mixture and attain an adsorption/desorption equilibrium, the amalgam was stirred in the dark for duration of 30 min. Following this, the suspension was subjected to natural sunlight irradiation. At specific intervals of time, 10 mL of the solution was extracted and subjected to filtration using a 0.45-µM syringe filter to eliminate the presence of photocatalysts.
The collected solution underwent assessment for MB dye concentration using a UV–Vis spectrophotometer. Specifically, the absorbance at 664 nm was examined during the degradation process. This procedure allowed for the continuous monitoring and evaluation of the degradation process over different time intervals of 20 min. The percentage of MB degradation efficiency (%) was calculated using the following equation (1) [58, 59]:
where C0 and Ct represent the absorbance of MB solution before and after the photocatalytic degradation activity. The said procedure was also used for the dosages, concentrations of MB, pHs, stability, and scavenger studies. The pHs were adjusted by using 0.1 M HCl or 0.1 M NaOH. Degradation kinetic rate constants of MB with ZnONPs and ZnO/BC nanocomposite were evaluated by using the first-order rate equation as given below [60]:
where C0 is the initial concentration and Ct is the final concentration before and after irradiation time t. k is the degradation rate constant (min−1).
2.6 Scavenger trapping methods
Scavenger trapping methods were used to determine the active species during the photocatalytic degradation of MB under sunlight irradiation. Isopropanol (IPA), phosphoric acid (H3PO4), ethylenediaminetetraacetic acid (EDTA), and sodium persulfate (Na2S2O8) were used as scavengers for quench •OH, •O2−, h+, and e-, respectively. This method was conducted during the photocatalytic degradation of MB by 0.1 M scavengers.
3 Result and discussion
3.1 FT-IR characterizations
FT-IR spectra are used to evaluate the organic functional groups, metal-oxygen vibration bands, and changes in metal-oxygen frequency due to modification. The FT-IR spectra of ZnONPs, BPs, and ZnO/BC nanocomposite are displayed in Fig. 1A (a–c). A broad absorption peak at 3404 cm−1 is associated with the O-H stretching vibration band of hydration on the ZnO surface [61, 62]. A double peak at 2931 cm−1 and 2855 cm−1 is attributed to the =CH2 and C-H bands stretching vibration from the precursors to the acetate group [63]. The peak at 1617 cm−1 and 1318 cm−1 revealed symmetry and asymmetry stretching vibration of C=O bands, a smaller peak at 1016 cm−1 was related to C-O stretching vibration, and a sharp peak at 902 cm−1 was due to C-O bending vibration [64]. A high-intensity peak at 502 cm−1 detected the stretching vibration of the Zn-O band as shown in Fig. 1A (a) [65]. Figure 1A (b) shows that the peaks of BC shifted peak position at 3307 cm−1 due to O-H stretching vibration and other peaks increased intensity compared to ZnONPs. An additional peak appeared at 1722 cm−1 due to C=O stretching vibration in the COOH group of BC as shown in Fig. 1A (b). In Fig. 1A (c), the FT-IR spectrum of the ZnO/BC nanocomposite is depicted, revealing a reduction in peak intensity along with the presence of certain peaks. Notably, shifts in peak positions are observed at 3321 cm−1, 1742 cm−1, and 427 cm−1, accompanied by reduced intensity. This observation indicates the successful coating of BC with ZnO, as evidenced by alterations in peak positions corresponding to O-H, C=O, and Zn-O bonding [66, 67]. The FT-IR spectra indicate that the functional groups in the BC may have contributed to the formation of the ZnO/BC nanocomposite. Table 1 presents the functional groups corresponding to the FT-IR peaks.
A–F A FT-IR spectra (a) ZnONPs, (b) BC, and (c) ZnO/BC nanocomposite; B UV-visible spectra of (a) ZnONPs and (b) ZnO/BC nanocomposite; C Tauc plot of (a) ZnONPs and (b) ZnO/BC nanocomposite, D Fluorescence spectroscopy spectra for (a) ZnONPs and (b) ZnO/BC nanocomposite, E XRD pattern of ZnONPs, and F XRD patterns of (a) BC and (b) ZnO/BC nanocomposite
3.2 UV-visible characterization
UV-visible absorption spectra of ZnONPs and ZnO/BC nanocomposite are shown in Fig. 1B (a, b). Figure 1B (a) shows a sharp absorption peak at 372 nm, which has been described as an intrinsic transition between the valence band (O 2p orbitals) and the conduction band of ZnONPs [80, 81]. Figure 1B (b) shows that the ZnO/BC nanocomposite is shifted to a slightly longer wavelength at 382 nm with lower bandgap energy due to more optically active centers, excitonic-related recombination, and surface traps [82]. Hence, BC is coated on ZnO to improve light harvesting, which is an essential requirement for enhancing photocatalytic activity. Figure 1C (a–c) shows the Tauc plots of ZnONP and ZnO/BC nanocomposite with calculated bandgap energy of 3.04 eV and 2.89 eV, respectively, which are correspond to previous reported results [83]. ZnO/BC nanocomposite has lower bandgap energy than ZnONPs, records a lower electron-holes recombination rate, and is more useful for visible light photocatalysts. ZnO/BC nanocomposite can improve optical properties and enhance the photocatalytic degradation of MB under sunlight irradiation [84].
3.3 Fluorescence spectroscopy characterizations
Defect energy levels, surface vacancies, and charge transfer of photocatalysts were identified by fluorescence spectroscopy [85]. Figure 1D (a, b) shows the fluorescence spectra of ZnONPs and ZnO/BC nanocomposite with excitation at 350 nm and is important for determining the fluorescence properties. Figure 1D (a) shows that the FL spectrum of ZnONPs observed a higher emission peak at 445 nm which is attributed to the oxygen vacancies (Vo) in the valence band transition. The FL spectrum of ZnO/BC nanocomposite shows a low-intensity peak at 385 nm, indicating near-band-edge emission due to the mixing of electrons from the less conduction band with holes of the valence band of ZnONPs as shown in Fig. 1D (b). FL spectra of ZnONPs and ZnO/BC nanocomposite and ZnO/BC nanocomposite reveal lower intensity peak due to BC and quenching fluorescence properties in ZnONPs. Low fluorescence indicates charge transfer and a reduced rate of electron-hole recombination [86, 87]. As a result, the ZnO/BC nanocomposite has excellent photocatalytic activity under sunlight irradiation.
3.4 XRD characterizations
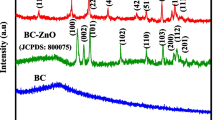
Powder X-ray diffraction serves as an effective method for examining both amorphous and crystalline aspects of prepared materials. As depicted in Figure 1E (a), the XRD pattern of the pure ZnO nanoparticles (ZnONPs) displays a set of distinct diffraction peaks. Notably, these peaks are identified at specific angles: 31.78° (100), 34.26° (002), 36.25° (101), 47.56° (102), 56.75° (110), 62.75° (103), 66.37° (200), 67.95° (112), 69.33° (201), 72.48° (004), and 76.93° (202). These discernible peaks unequivocally indicate the presence of the wurtzite structure characteristic of ZnO. The alignment of these peaks corresponds precisely to the JCPDS card no. 36-1451. Importantly, the observed peaks provide strong evidence of the material’s well-defined crystalline nature [88]. Figure 1F (a, b) shows the X-ray diffraction pattern of BP powder and ZnO/BC nanocomposite. Several peaks were assigned to BP powder, of which two major peaks corresponded to the (002) and (100) plane of disordered graphitic carbon at 2θ of 21.34° and 40.29° in BC as identified by the JCPDS card no. 46–1045 as shown in Fig. 1F (a) [89,90,91]. Figure 1E (b) shows the XRD pattern of the ZnO/BC nanocomposite, which shows peaks indicating the crystallinity of the variants at 28.13° 31.86°, 34.39°, 47.58°, 62.48°, and 66.35° with corresponding to (111), (100), (002), (102), (103), and (200) crystalline planes [92, 93]. Additionally, these peaks were observed as ZnONPs according to the JCPDS card no. 36-1451. Two prominent peaks appear at 2θ = 21.34°, accompanied by a less pronounced peak at 2θ = 40.29°, corresponding to the C (002) and C(100) planes, respectively. This unmistakably signifies the presence of a graphitic structure within the biochar (BC). Furthermore, a sharp peak of significant intensity appears at 28.13° along the (111) plane, indicating that the crystallinity patterns of the BC structure became more defined after forming the ZnO/BC nanocomposite [94, 95]. This result suggests that the ZnO/BC nanocomposite was effectively synthesized using ZnONPs and BC biochar. The average size of the ZnONPs within the ZnO/BC nanocomposite was determined from the maximum crystalline peak of ZnO at 31.86°, which corresponds to the (101) plane. To compute this size, the Debye-Scherrer formula was employed, as outlined by the equation:
where 0.9 is the shape factor, λ is the x-ray wavelength (λ=1.5418 Å), β is the line broadening at half-width maximum intensity (FWHM) in radians, and θ is the Bragg angle. The average size of the ZnO nanoparticles was found to be 18 nm. For the ZnO/BC nanocomposite, the average size was calculated at the 2θ position of the (101) peak of ZnO NPs, which was 30 nm.
3.5 SEM characterizations
Scanning electron microscope was used to find the surface morphology structure and shape of materials. Figure 2A–C shows the SEM images of small ZnONP aggregate with a granular shape at increasing magnifications of (A) 12 kV, 1.15 kx, (B) 12.0 kV, 3.09 kx, and (C) 12.0 kV, 5.12 kx. The EDAX spectrum for elemental determination of Zn and O in ZnONPs was revealed as shown in Fig. 2D. Figure 3A–C shows the SEM image of ZnO/BC nanocomposite at different magnifications of (A) 20.0 kV, 1.50 kx, (B) 20.0 kV, 6.76 kx, and (C) 20.0 kV, 17.7 kx. The nanocomposite at 1.50 kx magnification looks like white particles of ZnO coated on the integrated structure of biochar and irregular size particles are recorded as shown in Fig. 3A. At 6.76 kx magnification it looks like irregular stalks of white particles clustered together irregularly as shown in Fig. 3B. At a higher magnification of 17.7 kx, it clearly shows the irregularly shaped rod and particles as shown in Fig. 3C. EDAX spectrum confirmed the Zn, O, and C elements ZnO/BC nanocomposite as shown in Fig. 3D.
3.6 TEM characterizations
Figure 4A–D shows the TEM images of ZnO (A, B) and ZnO/BC nanocomposite (C, D) at magnifications of (A) 65 kx (200 nm scale bar), (B) 100 kx (100 nm scale bar), and 60 kx (50 nm scale bars insets to both A and B). Distinct shapes of ZnO nanoparticles (ZnONPs) were clearly evident under different magnifications, 65 kx and 100 kx, as illustrated in Fig. 4A and B. At the higher magnification of 60 kx, the hexagonal and square configurations of ZnONPs were captured and are featured in Fig. 4A and B, respectively. These images decisively affirmed the successful synthesis of ZnONPs through the hydrothermal method.
Figure 4C and D presents the images of the ZnO/biochar (ZnO/BC) nanocomposite. In Fig. 4C, observed at 100 kx with a 200-nm scale bar, agglomerated structures integrated with dark particles coated on the biochar substrate are discernible. This amalgamation is attributed to the ZnONPs and BC components. Similarly, Fig. 4D portrays the nanocomposite at 140 kx with a 100 nm scale bar, further highlighting the distinctive features of ZnONPs and BC.
Collectively, these visual representations provide conclusive evidence of the successful formation of ZnONPs and the ZnO/BC nanocomposite using the hydrothermal method. The size of the ZnO/BC nanocomposite, ranging approximately between 20 and 30 nm, was found to be in close agreement with the XRD analysis. This alignment further validates the successful encapsulation of the BP biochar within the ZnO nanoparticles (ZnONPs).
3.7 Photocatalytic degradation studies
The evaluation of catalyst-free photocatalytic degradation involved three scenarios: only sunlight, sunlight with ZnO nanoparticles (Sunlight + ZnONPs), and sunlight with the ZnO/biochar nanocomposite (Sunlight + ZnO/BC), targeting the degradation of methylene blue (MB) using UV-visible spectroscopy. In the standard procedure, the photocatalysts were subjected to a preliminary MB photocatalytic degradation study in the absence of light for 30 minutes to confirm the adsorption/desorption process, which served as the 0-minute reference. Subsequently, UV-visible spectral analysis was used to monitor the progress of MB photocatalytic degradation under sunlight irradiation.
Without a catalyst, the UV-visible spectra of MB showed a persistent peak at 662 nm, indicating incomplete reduction in intensity across the time intervals from 0 to 120 min, as illustrated in Fig. 5A. Introducing ZnONPs as the catalyst exhibited a gradual reduction in the UV-visible spectrum of MB under sunlight irradiation over the same time range, as depicted in Fig. 5B. In contrast, the ZnO/BC nanocomposites proved particularly efficient in degrading MB under sunlight irradiation within the 0 to 120-min span, evident in both the spectral data displayed in Fig. 5C and the inset digital images.
A–F UV-visible absorption spectra of MB with A catalyst free; B ZnONPs and C ZnO/BC nanocomposite with photocatalytic degradation of MB under sunlight irradiation at 0 to 120 min; D plot of the percentage of degradation efficiency vs. time (a) catalyst free, (b) ZnONPs, and (c) ZnO/BC nanocomposite; E plot of ln(Ct/C0) vs. time (a) catalyst free, (b) ZnONPs, and (c) ZnO/BC nanocomposite; and F digital image of successive photocatalytic degration of MB with ZnO/BC nanocomposite
The assessment of photocatalytic efficiency was quantified as depicted in Fig. 5D (a–c), which illustrates the percentage degradation of MB under sunlight irradiation, comparing the scenarios of no catalyst, ZnONPs catalyst, and ZnO/BC nanocomposite catalyst at different time intervals using Equation (2). Significantly, the ZnO/BC nanocomposite demonstrated superior photocatalytic activity, exhibiting a degradation efficiency of 91%, outperforming ZnONPs (72%) and the absence of a catalyst (43%). Notably, ZnO/BC nanocomposite proved to be an exceptional photocatalyst for the degradation of MB under visible light irradiation.
Further insight into the kinetic rate constant and comparative analysis with previous literature related to ZnO nanocomposites are summarized in Tables 2 and 3. Significantly, the ZnO/BC nanocomposite displayed a higher rate constant compared to ZnONPs and the no-catalyst scenario. These findings, supported by digital images in Fig. 5F, reinforce the exceptional photocatalytic performance of the ZnO/BC nanocomposite. Notably, the behavior of the ZnO/BC nanocomposite aligns well with results reported in the existing literature, showcasing its promising potential.
3.8 Effect of photocatalyst dosages
Figure 6 presents a crucial insight into the effect of varying photocatalyst dosages on the removal of dye, particularly highlighting the significance of the 100 mg dose for achieving optimal dye removal. Figure 6A–E effectively portrays the impact of different dosage levels, ranging from 25 to 125 mg of ZnO/biochar (ZnO/BC) nanocomposite, while maintaining an initial concentration of 10 ppm of methylene blue (MB) under sunlight irradiation over a duration of 0 to 120 min.
A–G UV-visible absorption spectra of MB with different dosages of A 25 mg, B 50 mg, C 75 mg, D 100 mg, and E 125 mg with ZnO/BC nanocomposite under sunlight irradiation. F Plot of degradation efficiency (%) and G plot of ln(Ct/C0) vs time of (a) 25 mg, (b) 50 mg, (c)75 mg, (d) 100 mg, and (e) 125 mg
Figure 6A–C details the photocatalytic degradation of MB using ZnO/BC nanocomposite dosages of 25 mg, 50 mg, and 75 mg. Under these conditions, the degradation is incomplete due to insufficient catalyst dosages and low photocatalytic activity. In contrast, Fig. 6D and E shows the degradation patterns with 100 mg and 125 mg dosages of ZnO/BC nanocomposite, respectively, where the photocatalytic degradations result in a nearly complete reduction in intensity over 0 to 120 min.
The efficacy of ZnO/BC nanocomposite as a photocatalyst was quantified through degradation efficiency calculations using Equation (1) for various dosages. The resulting degradation percentages for MB were 27% (25 mg), 45% (50 mg), 54% (75 mg), 91% (100 mg), and 96 % (125 mg) as depicted in Fig. 6F (a–e) and summarized in Table 2. These findings corroborate that 100 mg and 125 mg dosages of ZnO/BC nanocomposite yields remarkable MB degradation under sunlight irradiation within the 0 to 120-min timeframe, attributed to the elevated concentration of active photocatalytic sites.
Furthermore, the kinetic degradation rate of MB, utilizing varying catalyst dosages of ZnO/BC nanocomposite under sunlight irradiation, was also assessed. Figure 6G (a–e) shows the ln(Ct/C0) versus time plot, which was used to determine the kinetic rate constant for the photocatalytic degradation of MB using Equation (2), as detailed and summarized in Table 2.
Although the 125 mg dose shown in Fig. 6E achieved slightly higher MB removal, it was slower in the initial phase of degradation. Therefore, the 100 mg dosage provided a better balance between removal rate and degradation efficiency, resulting in the rapid decolorization of the MB dye within 0 to 100 min under visible light irradiation.
3.9 Effect of MB concentration
The impact of varying concentrations of methylene blue (MB)—5 ppm, 15 ppm, 20 ppm, and 25 ppm—on the photocatalytic efficacy when coupled with ZnO/biochar (ZnO/BC) nanocomposite (100 mg) under sunlight irradiation at different time intervals (0 to 120 min) is visualized in Fig. 7A–D.
Figure 7A distinctly depicts the ongoing, continuous decrease in photocatalytic degradation intensity of MB (5 ppm) when treated with ZnO/BC nanocomposite under sunlight irradiation from 0 to 60 min. This contrastingly progressive trend differentiates it from the concentrations of 15 ppm, 20 ppm, and 25 ppm, as illustrated in Fig. 7B–D. The higher concentrations of 20 ppm and 25 ppm, however, exhibited slower degradation rates under sunlight irradiation over the 0 to 120-min duration. This deceleration can be attributed to the abundant presence of MB molecules, which absorb significant sunlight and impede irradiation. As represented in Fig. 7C and D, this light absorption behavior aligns with the observed trend [100].
Quantitatively, the percentages of MB degradation were found to be 98%, 87%, 68%, and 45% for concentrations of 5 ppm, 15 ppm, 20 ppm, and 25 ppm, respectively, as demonstrated in Fig. 7E. Furthermore, the kinetic rate constants, obtained from the analysis illustrated in Fig. 7F, are summarized in Table 2. The results underscore the exceptional degradation efficiency and kinetic rate constant achieved at a concentration of 5 ppm, highlighting its optimal performance.
3.9.1 Effect of pH
The treatment of wastewater involves a broad spectrum of pH values that influence pollutant removal through photocatalytic degradation, mediated by the catalysts’ surface charge Investigation into these pH conditions (pH 3, 5, 8, and 10) involved the ZnO/biochar (ZnO/BC) nanocomposite (100 mg) as a photocatalyst, targeting the degradation of methylene blue (MB) at a concentration of 10 ppm. These studies were conducted under sunlight irradiation, spanning time intervals of 0 to 120 minutes, as elucidated in Fig. S1 (A–D) within the supporting information.
Figire S1 (A, B) captures the photocatalytic degradation spectra of MB at pH 3 and pH 5, revealing that the peak intensity reduction is incomplete. This phenomenon arises from the repulsion between the positive charge of the ZnO/BC nanocomposite and the positive charge of MB [101]. At lower pH values, specifically 3 and 5, the ZnO/BC nanocomposite exhibits a positive surface charge due to the high concentration of H+ ions. This results in reduced MB dye adsorption and lower photocatalytic degradation efficiency, as the electrostatic repulsion between MB and the ZnO/BC nanocomposite diminishes the interaction [102]. In contrast, the photocatalytic process gains momentum at pH 8, as depicted in Fig. S1 (C), where MB undergoes degradation under sunlight irradiation within the 0 to 120-min interval. This shift is attributed to the attractive interplay between the negative charges of the ZnO/BC nanocomposite at higher amount of −OH ions, which can interact with the positive charge of MB for electrostatic attraction [103]. Similarly, Fig. S1 (D) underscores the consistent degradation of MB at pH 10 and the result of electrostatic attraction between the highly negative charge of the ZnO/BC nanocomposite and the positive charge of MB. Moreover, the ZnO/BC nanocomposite surface is predominantly occupied by hydroxyl and oxygen groups at higher pH 8 and pH 10, and surface charges exist as negative values [104]. This nanocomposite showed more affinity toward cationic MB dye, which is necessary for better adsorption in order to achieve increased photocatalytic degradation [105].
The pivotal role of pH 10 is evident, where the photocatalytic degradation of MB with the ZnO/BC nanocomposite takes precedence under sunlight irradiation. Substantiating this, the percentage (%) degradation efficiency of MB is quantified in Fig. 8A (a–d), yielding values of 40%, 65%, 85%, and 94% at pH levels of 3, 5, 8, and 10, respectively. Decay kinetic rate constants were calculated using ln(Ct/C0) versus times and various pHs 3,5,8 and 10 as shown in Fig. 8B (a–d) and reported in Table 2. Interestingly, the best degradation efficiency and kinetic rate constant were demonstrated at pH 10.
A–D A Degradation efficiencies and B plot of kinetic rate constants of MB (a) pH 3, (b) pH 5, (c) pH 8, and (d) pH 10. C Degradation efficiencies and D plot of kinetic rate constants of various scavengers (a) H3PO4, (b) IPA, (c) Na2SO4, and (d) EDTA under sunlight irradiation with ZnO/BC nanocomposite
3.9.2 Effect of scavengers’ studies
Within the framework of this photocatalytic investigation, diverse scavengers were employed to quench reactive species, constituting a vital facet of the experimental procedure. The assessment of photocatalytic activity entailed a range of scavenger probes, each strategically incorporated. In the context of the photocatalytic degradation of methylene blue (MB) at a concentration of 10 ppm, specific scavengers were harnessed to target distinct species. Isopropanol (IPA), hydrogen phosphate (H3PO4), ethylenediaminetetraacetic acid (EDTA), and sodium persulfate (Na2S2O8) were adeptly chosen as effective quenchers for hydroxide radicals (•OH), superoxide radicals (•O2-), holes (h+), and electrons (e-) [106,107,108]. These reactive species manifest during the photocatalytic degradation of MB under sunlight irradiation.
Interestingly, both EDTA and Na2S2O8 exhibited limited interaction with the holes (h+) and electrons (e-) emanating from the ZnO/BC nanocomposite during the photocatalytic degradation of MB under sunlight irradiation over the 0 to 120-min span. Conversely, holes (h+) and electrons (e-) emerged as the primary agents driving the degradation of MB, accounting for respective percentage degradation efficiencies of 90 % and 66 %, as presented in Fig. 8C (a, b). Intriguingly, the scavengers IPA (55%) and H3PO4 (36%) displayed comparatively lesser involvement in the photocatalytic degradation of MB in conjunction with the ZnO/BC nanocomposite under sunlight irradiation as shown in Fig. 8C (c, d). Notably, hydroxide radicals (•OH) and superoxide radicals (•O2-) were more significantly scavenged, as delineated in Fig. 8C (c, d).
The determination of the kinetic rate constant (k) was derived from the plot of ln(Ct/C0) versus time (t), and slope analysis facilitated the extraction of pertinent information, as illustrated in Fig. 8D (a–d). The derived values, including R2 and k reported in Table 2, unveiled the kinetic characteristics of the scavengers. Specifically, the high degradation rate constants of EDTA and Na2S2O8 were particularly noteworthy, further attesting to their efficacy in degrading MB. The intricate interplay between scavengers and photocatalytic reactions thus adds a layer of understanding to the complex degradation process.
3.9.3 Stability and reusability of studies
The assessment of stability and reusability of the photocatalysts centered on the photocatalytic degradation of methylene blue (MB) at a concentration of 10 ppm, employing the ZnO/biochar (ZnO/BC) nanocomposite (100 mg) under sunlight irradiation spanning 0 to 120 min. Upon completion of the photocatalytic degradation experiment, the ZnO/BC nanocomposite photocatalyst was extracted and separated via centrifugation. It underwent rigorous washing with water and ethanol, followed by drying in a high-temperature oven set at 60°C. Subsequently, the photocatalyst was introduced into a freshly prepared MB solution of the same concentration. This procedure was iteratively executed across 4 consecutive cycles, each encompassing the photocatalytic degradation of MB under sunlight irradiation for durations spanning 0 to 120 min, as depicted in Fig. 9A–D.
A–F A First cycle, B second cycle, C third cycle, and D fourth cycle replication of the UV-visible spectra. E Plot of degradation efficiency vs. number of cycles and F FT-IR spectra of ZnO/BC nanocomposite (a) before and (b) after four cycles for photocatalytic degradation of MB under sunlight irradiation
From the plot portraying (%) removal efficiency against the number of cycles, distinct percentages of degradation efficiency were garnered: 92%, 90%, 75%, and 55%. Remarkably, the photocatalytic degradation efficiencies remained relatively steady at 55% even after the completion of four cycles, underscoring the sustained performance of the ZnO/BC nanocomposite, as showcased in Fig. 9E. A small quantity of catalyst lost during the regeneration process can be used to explain this slight decrease in photocatalytic degradation efficiency [109]. This observation definitively attests to the commendable stability and reusability of the ZnO/BC nanocomposite in the context of photocatalytic MB degradation. Figure 9F (a, b) shows the Fourier-transform infrared (FT-IR) spectra of the ZnO/BC nanocomposite, both before and after the photocatalytic degradation of MB. The results indicate that the spectra exhibit limited alteration following the photocatalytic degradation experiment. This corroborates the persistence of the ZnO/BC nanocomposite compositional integrity and structural attributes throughout the course of the photocatalytic process.
3.9.4 Photocatalytic degradation mechanism of MB with ZnO/BC nanocomposite
Under sunlight, the ZnONPs photocatalyst prompts the excitation of electrons from the valence band (VB) of ZnO to their conduction band (CB), accompanied by the generation of photogenerated holes. These photogenerated electrons are subsequently captured by dissolved oxygen molecules in water, leading to the formation of superoxide radical anions (•O2-). Simultaneously, the photogenerated holes interact with hydroxyl ions, resulting in their absorption onto the photocatalyst’s surface, yielding hydroxyl radicals (•OH). These hydroxide radicals (•OH) and superoxide radical anions (•O2-) are thought to play a role in the degradation of MB and its eventual mineralization [110, 111].
Nevertheless, the facile recombination of these photocatalytically generated electron and hole pairs, due to the significant energy gap (3.04 eV), hampers the overall photocatalytic efficiency of the pristine ZnO catalyst. In contrast, the ZnO/BC nanocomposite (with a narrower energy gap of 2.89 eV) capitalizes on the synergistic effect of ZnO and biochar during the MB degradation process. Upon UV-visible light irradiation, ZnO nanoparticles become activated, inducing the generation of photogenerated electron-hole pairs. Furthermore, the establishment of Zn-O-C bonds, as depicted in the diagram, facilitates the transfer of electrons from the ZnO nanoparticles conduction band to the biochar surface [112]. This notably reduces the recombination rate of the generated e-/h+ pairs within the ZnO/BC nanocomposite. The ZnO/BC nanocomposite FT-IR spectrum following MB degradation showed three prominent peaks with clearly visible decreased intensity and shifted wave number as demonstrated in Fig. 9F (b). The attachment of MB to the BC in the ZnO/BC nanocomposite was responsible for the peak intensity and shifts at 3318 cm−1, 1592 cm−1, and 1012 cm−1, which corresponded to the O-H bond, C=O bond, and C-O bond of BC in the nanocomposite [113, 114].
Moreover, the conduction band electrons (e-) of biochar react with oxygen (O2) to form superoxide radicals (•O2-), while the valence band holes (h+) of ZnO contribute to the generation of hydroxide radicals (•OH) [115]. These radical species efficiently participate in the oxidation of MB, leading to the formation of by-products such as carbon dioxide and water molecules, as elucidated in Scheme 2. The progression of this process is described by the following chemical reactions.
4 Conclusion
This research employs a ZnO-coated biochar nanocomposite, synthesized from banana peels and zinc acetate dihydrate, to enhance the photocatalytic activity of the ZnO/BC nanocomposite. This approach is both cost-effective and environmentally friendly. The ZnO/BC nanocomposite boasts abundant active sites, facilitating a high adsorption capacity for MB and the generation of reactive radicals. Consequently, the photocatalytic degradation efficiency is significantly improved under sunlight irradiation.
The elimination of MB from wastewater using the ZnO/BC nanocomposite was modeled using the pseudo-first-order approach. An in-depth examination of the ZnO/BC nanocomposite’s stability and reusability demonstrated consistent photocatalytic degradation efficiency (70%) for MB even after undergoing four cycles.
Scavenger studies further verified the participation of active radicals, including h+, e-, •O2-, and •OH, in the photocatalytic degradation of MB. Particularly, electron (e-) and hydroxyl (•OH) radicals played prominent roles in the degradation process under sunlight irradiation.
This ZnO/BC nanocomposite holds substantial potential for wide-scale implementation in wastewater treatment and environmental remediation endeavors.
References
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Public Health Front 8:505570
Lavanya C, Rajesh Dhankar RD, Sunil Chhikara SC, Sarita Sheoran SS (2014) Degradation of toxic dyes: a review. Int J Curr Microbiol App Sci 3:189–199
Mehdizadeh P, Orooji Y, Amiri O, Salavati-Niasari M, Moayedi H (2020) Green synthesis using cherry and orange juice and characterization of TbFeO3 ceramic nanostructures and their application as photocatalysts under UV light for removal of organic dyes in water. Clean Prod 252:119765
Mustafa T, Sarı A, Saleh TA (2018) Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J Environ Manage 206:170–177
Bora LV, Mewada RK (2017) Visible/solar light active photocatalysts for organic effluent treatment: fundamentals, mechanisms and parametric review. Renew Sustain Energy Rev 76:1393–1421
Tiwari JN, Mahesh K, Le NH, Kemp KC, Timilsina R, Tiwari RN, Kim KS (2013) Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 56:173–182
Rashidi HR, Sulaiman NMN, Hashim NA, Hassan CRC, Ramli MR (2015) Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalination and Water Treatment 55(1):86–95
Da Rosa ALD, Carissimi E, Dotto GL, Sander H, Feris LA (2018) Biosorption of rhodamine B dye from dyeing stones effluents using the green microalgae Chlorella pyrenoidosa. J Clean Prod 198:1302–1310
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes forin-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Mollah MYA, Gomes JAG, Das KK, Cocke DL (2010) Electrochemical treatment of Orange II dye solution-use of aluminum sacrificial electrodes and floc characterization. J Hazard Mater 174:851–858
Nguyen CH, Juang RS (2019) Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using platinum-doped titanate nanomaterials. J Taiwan Inst Chem Eng 99:166–179
Kyriakopoulos G, Xiarchos I, Doulia D (2006) Treatment of contaminated water with pesticides via adsorption. Int J Environ Technol Manag 6:515–524
Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368
Sahraei R, Pour ZS, Ghaemy M (2017) Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: removal of heavy metals and dyes from water. J Clean Prod 142:2973–2984
Iqbal T, Khan MA, Mahmood H (2018) Facile synthesis of ZnO nanosheets: structural, antibacterial and photocatalytic studies. Mater Lett 224:59–63
Sirajudeen J, Nagaswarupa HP, Kumar MA, Kumar CR, Gurushantha K, Shekhar TS, Anantharaju KS, Mahesh KV, Sharma SC, Nagabhushana H (2017) Photocatalytic and Photoluminescence studies of ZnO nanomaterials by Banana peel powder. Mater Today Proc 4(11):11827–11836
Peng Y, Ji J, Zhao X, Wan H, Chen D (2013) Preparation of ZnO nanopowder by a novel ultrasound assisted non-hydrolytic sol–gel process and its application in photocatalytic degradation of CI Acid Red 249. Powder Technol 233:325–330
Yang Y, Pignatello JJ, Ma J, Mitch WA (2014) Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ Sci Tech 48(4):2344–2351
Yao Y, Wang L, Sun L, Zhu S, Huang Z, Mao Y, Lu W, Chen W (2013) Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem Eng Sci 101:424–431
Mousa HM, Alenezi JF, Mohamed IM, Yasin AS, Hashem AFM, Abdal-Hay A (2021) Synthesis of TiO2@ ZnO heterojunction for dye photodegradation and wastewater treatment. J Alloys Compd 886:161169
Pellera FM, Giannis A, Kalderis D, Stegmann R, Wang JY, Gidarakos E (2012) Adsorption of Cu (II) ions from aqueous solutions on biochars prepared from agricultural by-products. J Environ Manage 96(1):35–42
Leng L, Yuan X, Zeng G, Shao J, Chen X, Wu Z, Wang H, Peng X (2015) Surface characterization of rice husk bio-char produced by liquefaction and application for cationic dye (Malachite green) adsorption. Fuel 155:77–85
Dias D, Lapa N, Bernardo M, Godinho D, Fonseca I, Miranda M, Pinto F, Lemos F (2017) Properties of chars from the gasification and pyrolysis of rice waste streams towards their valorisation as adsorbent materials. Waste Manag 65:186–194
Costa E, Sotelo JL, Calleja G, Marron C (1981) Adsorption of binary and ternary hydrocarbon gas mixtures on activated carbon: experimental determination and theoretical prediction of the ternary equilibrium data. AIChE J 27(1):5–12
Aydoghmish SM, Hassanzadeh-Tabrizi SA, Saffar-Teluri A (2019) Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Ceram Int 45(12):14934–14942
Iervolino G, Zammit I, Vaiano V, Rizzo L (2020) Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: a critical review. Heterogeneous Photocatalysis: Recent Advances, (pp. 225-264). https://doi.org/10.1007/978-3-030-49492-6_7
Zou Q, Li H, Yang Y, Miao Y, Huo Y (2019) Bi2O3/TiO2 photocatalytic film coated on floated glass balls for efficient removal of organic pollutant. Appl Surf Sci 467:354–360
Ma R, Zhang S, Li L, Gu P, Wen T, Khan A, Li S, Li B, Wang S, Wang X (2019) Enhanced visible-light-induced photoactivity of type-II CeO2/g-C3N4 nanosheet toward organic pollutants degradation. ACS Sustain Chem Eng 7(10):9699–9708
Elango G, Roopan SM (2016) Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J Photochem Photobiol B 155:34–38
Ivetić TB, Finčur NL, Đačanin LR, Abramović BF, Lukić-Petrović SR (2015) Ternary and coupled binary zinc tin oxide nanopowders: synthesis, characterization, and potential application in photocatalytic processes. Mater Res Bull 62:114–121
Jiang D, Xue J, Wu L, Zhou W, Zhang Y, Li X (2017) Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: effects of CuO morphology. Appl Catal B Environ 211:199–204
Abebe B, Murthy HCA, Amare E (2020) Enhancing the photocatalytic efficiency of ZnO: defects, heterojunction, and optimization. Environ Nanotechnol Monit Manag 14:100336
Abdullah FH, Bakar NA, Bakar MA (2020) Low temperature biosynthesis of crystalline zinc oxide nanoparticles from Musa acuminata peel extract for visible-light degradation of methylene blue. Optik 206:164279
Sánchez-Albores R, Cano FJ, Sebastian PJ, Reyes-Vallejo O (2022) Microwave-assisted biosynthesis of ZnO-GO particles using orange peel extract for photocatalytic degradation of methylene blue. J Environ Chem Eng 10(6):108924
Suresh R, Rajendran S, Kumar PS, Vo DVN, Cornejo-Ponce L (2021) Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 274:129734
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sustain Energy Rev 55:467–481
Dawood S, Sen TK, Phan C (2016) Adsorption removal of methylene blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin Water Treat 57(59):28964–28980
Ahmadi S, Ganjidoust H (2021) Using banana peel waste to synthesize BPAC/ZnOnanocomposite for photocatalytic degradation of Acid Blue 25: influential parameters, mineralization, biodegradability studies. J Environ Chem Eng 9(5):106010
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–a critical review. Bioresour Technol 160:191–202
Alessandro AD, De Pergola G (2018) The Mediterranean diet: its definition and evaluation of a priori dietary indexes in primary cardiovascular prevention. Int J Food Sci Nutr 69(6):647–659
Inyang M, Dickenson E (2015) The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: a review. Chemosphere 134:232–240
Nakarmi A, Bourdo SE, Ruhl L, Kanel S, Nadagouda M, Kumar Alla P, Pavel I, Viswanathan T (2020) Benign zinc oxide betaine-modified biochar nanocomposites for phosphate removal from aqueous solutions. J Environ Manag 272:111048
Wang S, Zhou Y, Han S, Wang N, Yin W, Yin X, Gao B, Wang X, Wang J (2018) Carboxymethyl cellulose stabilized ZnO/biochar nanocomposites: enhanced adsorption and inhibited photocatalytic degradation of methylene blue. Chemosphere 197:20–25
Iqbal MM, Imran M, Hussain T, Naeem MA, Al-Kahtani AA, Shah GM, Ahmad S, Farooq A, Rizwan M, Majeed A, Khan AR, Ali S (2021) Effective sequestration of Congo red dye with ZnO/cotton stalks biochar nanocomposite: modeling, reusability and stability. J Saudi Chem Soc 25:101176
Akir S, Hamdi A, Addad A, Coffinier Y, Boukherroub R, Omrani AD (2017) Facile synthesis of carbon-ZnO nanocomposite with enhanced visible light photocatalytic performance. Appl Surf Sci 400:461–470
Samadi M, Shivaee HA, Pourjavadi A, Moshfegh AZ (2013) Synergism of oxygen vacancy and carbonaceous species on enhanced photocatalytic activity of electrospun ZnO-carbon nanofibers: charge carrier scavengers mechanism. App Catal A Gen 466:153–160
Gonçalves MG, da Silva Veiga PA, Fornari MR, Peralta-Zamora P, Mangrich AS, Silvestri S (2020) Relationship of the physicochemical properties of novel ZnO/biocharcomposites to their efficiencies in the degradation of sulfamethoxazole and methyl orange. Sci Total Environ 748:141381
Yang C, Wang X, Ji Y, Ma T, Zhang Wang FY, Ci M, Chen D, Jiang A, Wang W (2019) Photocatalytic degradation of methylene blue with ZnO@C nanocomposites: kinetics, mechanism, and the inhibition effect on monoamine oxidase A and B. NanoImpact 15:100174
Rahman QI, Ahmad M, Misra SK, Lohani M (2013) Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater Lett 91:170–174
Matos J (2016) Eco-friendly heterogeneous photocatalysis on biochar-based materials under solar irradiation. Top Catal 59(2):394–402
Colmenares JC, Varma RS, Lisowski P (2016) Sustainable hybrid photocatalysts: titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem 18(21):5736–50
Luo Y, Zheng A, Xue M, Xie Y, Yu S, Yin Z, Xie C, Hong Z, Tan W, Zou W, Dong L (2022) Ball-milled Bi2MoO6/biochar composites for synergistic adsorption and photodegradation of methylene blue: kinetics and mechanisms. Ind Crops Prod 186:115229
Leichtweis J, Silvestri S, Welter N, Vieira Y, Zaragoza-Sánchez PI, Chávez-Mejía AC (2021) Carissimi E. Wastewater containing emerging contaminants treated by residues from the brewing industry based on biochar as a new CuFe2O4/biochar photocatalyst. Process Saf Environ Prot 150:497–509
Meena R, Abdullah M, Vasanthakumar V, Ravichandran D, Murugesan S (2024) Green biochar-supported ZnFe2O4 composite photocatalyst derived from waste banana peel: a sustainable approach for highly efficient visible light-driven degradation of organic pollutants in wastewater. Ionics 28:1–2
Kaur H (2023) Synergistic effect of biochar impregnated with ZnO nano-flowers for effective removal of organic pollutants from wastewater. Appl Surf Sci Adv 12:100339
Kaur H, Roy T (2024) Sustainable removal of organic pollutants using biochar based ZnO composites: experimental and theoretical studies of remediation process. Surf Interfaces 49:104399
Sun J, Lin X, Xie J, Zhang Y, Wang Q, Ying Z (2020) Facile synthesis of novel ternary g-C3N4/ferrite/biochar hybrid photocatalyst for efficient degradation of methylene blue under visible-light irradiation. Colloids Surf A: Physicochem Eng Asp 606:125556
Saravanan R, Gupta VK, Narayanan V, Stephen A (2013) Comparative study on photocatalytic activity of ZnO prepared by different methods. J Mol Liq 181:33–141
Weldegebrieal GK (2020) Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: a review. Inorg Chem Commun 120:108140
Prabakaran E, Pillay K (2021) Self-assembled silver nanoparticles decorated on exfoliated graphitic carbon nitride/carbon sphere nanocomposites as a novel catalyst for catalytic reduction of Cr(VI) to Cr(III) from wastewater and reuse for photocatalytic applications. ACS Omega 6:35221–35243
Bagheri S, Chandrappa KG, Hamid SBA (2013) Facile synthesis of nano-sized ZnO by direct precipitation method. Der Pharma Chemica 5(3):265–270
Ahmad R, Hasan I (2017) Efficient remediation of an aquatic environment contaminated by Cr (VI) and 2, 4-dinitrophenol by XG-g-polyaniline@ZnO nanocomposite. J Chem Eng Data 62(5):1594–1607
Pandey P, Kurchania R, Haque FZ (2015) Controlled hydrothermal synthesis, structural and optical analysis of nanometer-sized ZnO spheres. Optik 126(2):301–303
Yogamalar NR, Bose AC (2011) Tuning the aspect ratio of hydrothermally grown ZnO by choice of precursor. J Solid State Chem 184(1):12–20
Jafarirad S, Mehrabi M, Divband B, Kosari-Nasab M (2016) Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater Sci Eng: C 59:296–302
Zheng QF, Wang YH, Sun YG, Niu HH, Zhou JR, Wang ZM, Zhao J (2014) Study on structural properties of biochar under different materials and carbonized by FTIR. Spectrosc Spectr Anal 34:962–966
Yu F, Tian F, Zou H, Ye Z, Peng C, Huang J, Zheng Y, Zhang Y, Yang Y, Wei X, Gao B (2021) ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511
Lavand AB, Malghe YS (2015) Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram Soc 3(3):305–10
Liu J, Li E, You X, Hu C, Huang Q (2016) Adsorption of methylene blue on an agro-waste oil tea shell with and without fungal treatment. Sci Rep 6(1):38450
Luo J, Wu Y, Jiang M, Zhang A, Chen X, Zeng Y, Wang Y, Zhao Y, Wang G (2023) Novel ZnFe2O4/BC/ZnO photocatalyst for high-efficiency degradation of tetracycline under visible light irradiation. Chemosphere 311:137041
Lu J, Zhang Q, Wang J, Saito F, Uchida M (2006) Synthesis of N-doped ZnO by grinding and subsequent heating ZnO-urea mixture. Powder Technol 162(1):33–7
Han Q, Yang Y, Wang R, Zhang K, Liu N, Hong M (2021) Biochar derived from agricultural wastes as a means of facilitating the degradation of azo dyes by sulfides. Catalysts 11(4):434
Huff MD, Lee JW (2016) Biochar-surface oxygenation with hydrogen peroxide. J Environ Manage 165:17–21
Nahar A, Akbor MA, Shawon MT, Trisha SA, Akher US, Alam MA, Pinky NS, Alam MS, Afrose F, Hossain MS (2024) Biotite and ZnO embedded carbon aerogel composite for enhanced photocatalytic activity to degrade ciprofloxacin under sunlight stimulation. Results Eng. 102524. https://doi.org/10.1016/j.rineng.2024.102524
Kapoor RT, Rafatullah M, Siddiqui MR, Khan MA, Sillanpää M (2022) Removal of reactive black 5 dye by banana peel biochar and evaluation of its phytotoxicity on tomato. Sustainability 14(7):4176
Zhou N, Chen H, Xi J, Yao D, Zhou Z, Tian Y, Lu X (2017) Biochars with excellent Pb (II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour Technol 232:204–210
Foroutan R, Mohammadi R, Ramavandi B, Bastanian M (2018) Removal characteristics of chromium by activated carbon/CoFe2O4 magnetic composite and Phoenix dactylifera stone carbon. Korean J Chem Eng 35:2207–2219
Sun YT, Chen JD, Wei ZH, Chen YK, Shao CL, Zhou JF (2023) Copper ion removal from aqueous media using banana peel biochar/Fe3O4/branched polyethyleneimine. Colloids Surf A: Physicochem Eng Asp 658:130736
Le S, Jiang T, Li Y, Zhao Q, Li Y, Fang W, Gong M (2017) Highly efficient visible-light-driven mesoporous graphitic carbon nitride/ZnO nanocomposite photocatalysts. Appl Catal B: Environ 200:601–10
Tao S, Yang M, Chen H, Zhao S, Chen G (2018) Continuous synthesis of Ag/AgCl/ZnO composites using flow chemistry and photocatalytic application. Ind Eng Chem Res 57(9):3263–3273
Haase M, Weller H, Henglein A (1988) Photochemistry and radiation chemistry of colloidal semiconductors. 23. Electron storage on zinc oxide particles and size quantization. J Phys Chem 92(2):482-487
Smazna D, Rodrigues J, Shree S, Postica V, Neubüse G, Martins AF, Sedrine NB, Jena NK, Siebert L, Schütt F, Lupan O (2018) Buckminsterfullerene hybridized zinc oxide tetrapods: defects and charge transfer induced optical and electrical response. Nanoscale 10(21):10050–10062
Jing H, Ji L, Li Z, Wang Z, Li R, Ju K (2023) Zn/Fe bimetallic modified Spartina alterniflora-derived biochar heterostructure with superior catalytic performance for the degradation of malachite green. Biochar 5(1):29
He Y, Wang Y, Hu J, Wang K, Zhai Y, Chen Y, Duan Y, Wang Y, Zhang W (2021) Photocatalytic property correlated with microstructural evolution of the biochar/ZnO composites. J Mater Res Technol 11:1308–1321
Bai X, Wang L, Zong R, Lv Y, Sun Y, Zhu Y (2013) Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 29:3097–3105
Son DI, Yang HY, Kim TW, Park WI (2013) Photoresponse mechanisms of ultraviolet photodetectors based on colloidal ZnO quantum dot-graphene nanocomposites. Appl Phys Lett 102:021105
Dhandapani KV, Anbumani D, Gandhi AD, Annamalai P, Muthuvenkatachalam BS, Kavitha P, Ranganathan B (2020) Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal Agric Biotechnol 24:101517
Li X, Sun P, Yang T, Zhao J, Wang Z, Wang W, Liu Y, Lu G, Du Y (2013) Template-free microwave-assisted synthesis of ZnO hollow microspheres and their application in gas sensing. CrystEngComm 15:2949–2955
Han J, Kwon JH, Lee JW, Lee JH, Roh KC (2017) An effective approach to preparing partially graphitic activated carbon derived from structurally separated pitch pine biomass. Carbon 118:431–437
Patel M, Kumar R, Pittman CU Jr, Mohan D (2021) Ciprofloxacin and acetaminophen sorption onto banana peel biochars: environmental and process parameter influences. Environ Res 201:111218
Van Thuan T, Quynh BT, Nguyen TD, Bach LG (2017) Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf Interfaces 6:209–217
Lourenço MA, Zeng J, Jagdale P, Castellino M, Sacco A, Farkhondehfal MA, Pirri CF (2021) Biochar/zinc oxide composites as effective catalysts for electrochemical CO2 reduction. ACS Sustain Chem Eng 9(15):5445–5453
Gonçalves NP, Lourenço MA, Baleuri SR, Bianco S, JagdaleP Calza P (2022) Biochar waste-based ZnO materials as highly efficient photocatalysts for water treatment. J Environ Chem Eng 10(2):107256
Sabaa HM, El-Khatib KM, El-Kady MY, Mahmoud SA (2022) Spinel structure of activated carbon supported MFe2O4 composites as an economic and efficient electrocatalyst for oxygen reduction reaction in neutral media. J Solid State Electrochem 26(12):2749–2763
Azzam AB, Tokhy YA, Dars FM, Younes AA (2023) Heterogeneous porous biochar-supported nano NiFe2O4 for efficient removal of hazardous antibiotic from pharmaceutical wastewater. Environ Sci Pollut Res 30(56):119473–90
Hung MC, Yuan SY, Hung CC, Cheng CL, Ho HC, Ko TH (2014) Effectiveness of ZnO/carbon-based material as a catalyst for photodegradation of acrolein. Carbon 66:93e104
Chen M, Bao C, Hu D, Jin X, Huang Q (2019) Facile and low-cost fabrication of ZnO/biochar nanocomposites from jute fibers for efficient and stable photodegradation of methylene blue dye. J Anal Appl Pyrol 139:319–332
Edison TNJI, Atchudan R, Sethuraman MG, LeeY R (2016) Reductive-degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J Photoch Photob B: Bio 162:604–610
Yu S, Zhou J, Ren Y, Yang Z, Zhong M, Feng X, Su B, Lei Z (2023) Excellent adsorptive-photocatalytic performance of zinc oxide and biomass derived N, O-contained biochar nanocomposites for dyes and antibiotic removal. Chem Eng J 451:138959
Prabakaran E, Pillay K (2019) Synthesis of N-doped ZnO nanoparticles with cabbage morphology as a catalyst for the efficient photocatalytic degradation of methylene blue under UV and visible light. RSC Adv 9:7509–7535
Salem IA, El-Maazawi MS (2000) Kinetics and mechanism of color removal of methylene blue with hydrogen peroxide catalyzed by some supported alumina surfaces. Chemosphere 41(8):1173–80
Wang C, Zhang Y, Yu L, Zhang Z, Sun H (2013) Oxidative degradation of azo dyes using tourmaline. J Hazard Mater 260:851–9
El-Bahy ZM, Ismail AA, Mohamed RM (2009) Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J Hazard Mater 166(1):138–43
Duan W, Wang N, Xiao W, Zhao Y, Zheng Y (2018) Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J Mol Liq 269:874–81
Sharma A, Dutta RK (2018) Se-doped CuO NPs/H2O2/UV as a highly efficient and sustainable photo-Fenton catalytic system for enhanced degradation of 4-bromophenol. J Clean Prod 185:464–75
Lou W, Kane A, Wolbert D, Rtimi S, Assadi AA (2017) Study of a photocatalytic process for removal of antibiotics from wastewater in a falling film photoreactor: scavenger study and process intensification feasibility. Chem Eng Process: Process Intensification 122:213–221
Patel K, Roondhe B, Dabhi SD, Jha PK (2018) A new flatland buddy as toxic gas scavenger: a first principles study. J Hazard Mater 351:337–345
Li J, Li Z, Yang Y, Kong B, Wang C (2018) Laboratory study on the inhibitory effect of free radical scavenger on coal spontaneous combustion. Fuel Process Technol 171:350–360
Rather GA, Nanda A, Pandit MA, Yahya S, Barabadi H, Saravanan M (2021) Biosynthesis of Zinc oxide nanoparticles using Bergenia ciliate aqueous extract and evaluation of their photocatalytic and antioxidant potential. Inorg Chem Commun. 134:109020
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B: Environ 31(2):145–157
Prasad AR, Anagha M, Shamsheera K, Joseph A (2020) BiofabricatedZnO nanoparticles: direct sunlight-drivenselective photodegradation, antibacterial activity, and thermoluminescence-emission characteristics. New J Chem 44:8273–8279
Thi Luyen N, Van Nguyen K, Van Dang N, Quang Huy T, Hoai Linh P, Thanh Trung N, Nguyen VT, Thanh DV (2023) Facile one-step pyrolysis of ZnO/Biochar nanocomposite for highly efficient removal of methylene blue dye from aqueous solution. ACS omega 8(30):26816–26827
Wu Z, Zhong H, Yuan X, Wang H, Wang L, Chen X, Zeng G, Wu Y (2014) Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res 67:330–344
Cai K, Shen W, Ren B, He J, Wu S, Wang WA (2017) A phytic acid modified CoFe2O4 magnetic adsorbent with controllable morphology, excellent selective adsorption for dyes and ultra-strong adsorption ability for metal ions. Chem Eng J 330:936–46
Li B, Wang Y (2013) Facile synthesis and enhanced photocatalytic performance of flowerlike ZnO hierarchical microstructures. J Phys Chem C 114:890–896
Acknowledgements
We thank the University of Pretoria (Postdoctoral fellowship) for the financial support and the University of Johannesburg, Johannesburg, South Africa, for providing research facilities for this work. I am also thankful to Sri Paramakalyani College, Alwarkurichi (Department of Chemistry) providing laboratory facilities.
Funding
Open access funding provided by University of Pretoria. This work further received support from the Austrian Federal Ministry of Education, Science and Research (BMBWF) through Austria’s Agency for Education and Internationalization (OeAD) [Grant Numbers: Africa UNINET P058 and APPEAR Project 341].
Author information
Authors and Affiliations
Contributions
Prabakaran Eswaran planned the project, data interpretation and wrote the first draft. Priya Dharshini Madasamy contributed to this work in the laboratory. Kriveshini Pillay contributed in visualization and study. Hendrik Brink contributed in supervision, writing, review, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eswaran, P., Madasamy, P.D., Pillay, K. et al. Sunlight-driven photocatalytic degradation of methylene blue using ZnO/biochar nanocomposite derived from banana peels. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05999-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05999-z