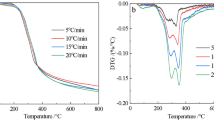
Abstract
Coconut husk is a residue from coconut processing plants that has not been widely utilized. Pyrolysis has the opportunity to convert it into chemicals in the form of bio-crude oil (BCO). Therefore, this study aims to examine the thermal decomposition behavior as well as determine the kinetic and activation thermodynamic parameters of coconut husk pyrolysis in the devolatilization zone using thermogravimetric analysis (TGA). Volatile state Kissinger-Akahira Sunose (KAS), volatile state Flynn–Wall–Ozawa (FWO), volatile state Friedman, and volatile state Coats-Redfern (CR) methods were employed with heating rates of 5, 10, and 20 °C.min−1. The TGA result supports the evidence of the non-isothermal pattern in the mass loss profile. Besides, the average ranges of activation energy, pre-exponential factor, activation enthalpy, activation entropy, and activation Gibbs free energy of coconut husk pyrolysis are 197.56–198.41 kJ.mol−1, 6.08 × 1020–1.41 × 1022 s−1, 192.87–193.73 kJ.mol−1, − 0.24–0.28 kJ.mol−1.K−1, and 152.39–166.12 kJ.mol−1, in a respective term. The pattern of activation energy shows that it initially enhances along with reaction progress and temperature due to low molecular mobility and then the value alleviates as the completion decomposition process to form BCO. On the other side, the results of the activation thermodynamics parameters prove that this process is endothermic, forms a transition state (ordered) before forming the product (disordered), and requires an external supply of heat. As a recommendation, the volatile approach is proven can describe the relationship between activation energy and activation thermodynamic parameters in the transition state.
Graphical Abstract











Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pawar A, Panwar NL, Jain S et al (2023) Thermal degradation of coconut husk waste biomass under non-isothermal condition. Biomass Conv Bioref 13:7613–7622. https://doi.org/10.1007/s13399-021-01657-w
Dinjetbun (2023) Tree crops statistics of Indonesia 2018–2022: Palm and Coconut
Waluyo J, Setianto MM, Safitri NR et al (2023) Characterization of biochar briquettes from coconut shell with the effect of binder: molasses, cow manure and horse manure. Evergreen 10:539–545. https://doi.org/10.5109/6782158
Putri AHI, Steven S, Oktavia FD et al (2024) Pyrolysis of macroalgae residue from the agar industry for silica-rich biochar and other sustainable chemicals: process performances, product applications, and simple business scenario. Biofuels Bioprod Bioref 18:391–409. https://doi.org/10.1002/bbb.2597
Bindar Y, Steven S, Kresno SW et al (2024) Large-scale pyrolysis of oil palm frond using two-box chamber pyrolyzer for cleaner biochar production. Biomass Conv Bioref 14:6421–6434. https://doi.org/10.1007/s13399-022-02842-1
Somerville M, Deev A (2020) The effect of heating rate, particle size and gas flow on the yield of charcoal during the pyrolysis of radiata pine wood. Renew Energy 151:419–425. https://doi.org/10.1016/j.renene.2019.11.036
Zhao B, O’Connor D, Zhang J et al (2018) Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J Clean Prod 174:977–987. https://doi.org/10.1016/j.jclepro.2017.11.013
Anca-Couce A, Obernberger I (2016) Application of a detailed biomass pyrolysis kinetic scheme to hardwood and softwood torrefaction. Fuel 167:158–167. https://doi.org/10.1016/j.fuel.2015.11.062
Steven S, Nugraha PZ, Hernowo P et al (2024) Investigation of high water content in bio-crude oil (BCO) produced from empty oil palm fruit bunches pyrolysis. Biomass Conv Bioref. https://doi.org/10.1007/s13399-024-05297-8
Trirahayu DA, Abidin AZ, Putra RP et al (2022) Process simulation and design considerations for biodiesel production from rubber seed oil. Fuels 3:563–579. https://doi.org/10.3390/fuels3040034
Trirahayu DA, Abidin AZ, Putra RP et al (2023) Process assessment of integrated hydrogen production from by-products of cottonseed oil-based biodiesel as a circular economy approach. Hydrogen 4:272–286. https://doi.org/10.3390/hydrogen4020019
Vyazovkin S (2006) Model-free kinetics. J Therm Anal Calorim 83:45–51. https://doi.org/10.1007/s10973-005-7044-6
Shen W, He S, Mu M et al (2024) A comprehensive review on the intricate processes involved in algae pyrolysis mechanism and possible migration of undesirable chemical elements. J Anal Appl Pyrolysis 177:106365. https://doi.org/10.1016/j.jaap.2024.106365
Vyazovkin S, Burnham AK, Criado JM et al (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Escalante J, Chen W-H, Tabatabaei M et al (2022) Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: a review of thermogravimetric analysis (TGA) approach. Renew Sustain Energy Rev 169:112914. https://doi.org/10.1016/j.rser.2022.112914
Cai J, Xu D, Dong Z et al (2018) Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sustain Energy Rev 82:2705–2715. https://doi.org/10.1016/j.rser.2017.09.113
Gai C, Dong Y, Zhang T (2013) The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour Technol 127:298–305
Gan DKW, Chin BLF, Loy ACM et al (2018) An in-situ thermogravimetric study of pyrolysis of rice hull with alkali catalyst of CaCO3. IOP ConfSer: Mater Sci Eng 458. https://doi.org/10.1088/1757-899X/458/1/012085
Dhaundiyal A, Singh SB, Hanon MM, Rawat R (2018) Determination of kinetic parameters for the thermal decomposition of Parthenium hysterophorus. Environ Clim Technol 22:5–21. https://doi.org/10.1515/rtuect-2018-0001
Sarkar JK, Wang Q (2020) Characterization of pyrolysis products and kinetic analysis of waste jute stick biomass. Processes 8:837. https://doi.org/10.3390/pr8070837
Anca-Couce A, Tsekos C, Retschitzegger S et al (2020) Biomass pyrolysis TGA assessment with an international round robin. Fuel 276:118002. https://doi.org/10.1016/j.fuel.2020.118002
Tran K-Q, Bui H-H, Chen W-H et al (2024) Pyrolysis kinetics of microalgae residues—a comparative study on DAEM using different distribution functions. In: Environment and Renewable Energy. pp 75–91
Safavi A, Richter C, Unnthorsson R (2022) Mathematical modeling and experiments on pyrolysis of walnut shells using a fixed-bed reactor. ChemEngineering 6:93. https://doi.org/10.3390/chemengineering6060093
Li J, Qiao Y, Zong P et al (2019) Thermogravimetric analysis and isoconversional kinetic study of biomass pyrolysis derived from land, coastal zone, and marine. Energy Fuels 33:3299–3310. https://doi.org/10.1021/acs.energyfuels.9b00331
Vuppaladadiyam AK, Liu H, Zhao M et al (2019) Thermogravimetric and kinetic analysis to discern synergy during the co-pyrolysis of microalgae and swine manure digestate. Biotechnol Biofuels 12:1–18. https://doi.org/10.1186/s13068-019-1488-6
Steven S, Hernowo P, Nadirah N et al (2024) Transformation method in determining kinetic parameters of biomass thermal decomposition from solid-state approach to volatile state approach. Biomass Bioenergy 183:107171. https://doi.org/10.1016/j.biombioe.2024.107171
Cano-Pleite E, Rubio-Rubio M, García-Hernando N, Soria-Verdugo A (2020) Microalgae pyrolysis under isothermal and non-isothermal conditions. Algal Res 51:102031. https://doi.org/10.1016/j.algal.2020.102031
Parthasarathy P, Al-Ansari T, Mackey HR, McKay G (2021) Effect of heating rate on the pyrolysis of camel manure. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01531-9
Hernowo P, Steven S, Restiawaty E, Bindar Y (2022) Nature of mathematical model in lignocellulosic biomass pyrolysis process kinetic using volatile state approach. J Taiwan Inst Chem Eng 139:104520. https://doi.org/10.1016/j.jtice.2022.104520
Amini E, Safdari M-S, Johnson N et al (2021) Pyrolysis kinetics of wildland vegetation using model-fitting methods. J Anal Appl Pyrolysis 157:105167. https://doi.org/10.1016/j.jaap.2021.105167
Said MM, John GR, Mhilu CF (2014) Thermal characteristics and kinetics of rice husk for pyrolysis process. Int J Renew Energy Res 4:275–278. https://doi.org/10.20508/ijrer.84902
Hernowo P, Steven S, Restiawaty E et al (2022) Chemicals component yield prediction and kinetic parameters determination of oil palm shell pyrolysis through volatile state approach and experimental study. J Anal Appl Pyrolysis 161:105399. https://doi.org/10.1016/j.jaap.2021.105399
Liu H, Wang C, Zhang J et al (2020) Pyrolysis kinetics and thermodynamics of typical plastic waste. Energy Fuels 34:2385–2390. https://doi.org/10.1021/acs.energyfuels.9b04152
Kaur R, Gera P, Jha MK, Bhaskar T (2018) Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol 250:422–428. https://doi.org/10.1016/j.biortech.2017.11.077
Yameen MZ, Naqvi SR, AlMohamadi H, Wang S (2023) Process optimization, kinetic, and thermodynamic studies of biodiesel production using KOH-modified bio-carbon catalyst derived from marine macroalgae. Carbon Lett 33:1571–1590. https://doi.org/10.1007/s42823-023-00541-z
Balogun AO, Adeleke AA, Ikubanni PP et al (2021) Kinetics modeling, thermodynamics and thermal performance assessments of pyrolytic decomposition of Moringa oleifera husk and Delonix regia pod. Sci Rep 11:13862. https://doi.org/10.1038/s41598-021-93407-1
Ivanovski M, Petrovic A, Ban I et al (2021) Determination of the kinetics and thermodynamic parameters of lignocellulosic biomass subjected to the torrefaction process. Materials (Basel) 14:7877. https://doi.org/10.3390/ma14247877
Phuakpunk K, Chalermsinsuwan B, Assabumrungrat S (2022) Pyrolysis kinetic parameters investigation of single and tri-component biomass: models fitting via comparative model-free methods. Renew Energy 182:494–507
Hameed S, Sharma A, Pareek V et al (2019) A review on biomass pyrolysis models: kinetic, network and mechanistic models. Biomass Bioenergy 123:104–122. https://doi.org/10.1016/j.biombioe.2019.02.008
Vyazovkin S, Wight CA (1997) Kinetics in solids. Annu Rev Phys Chem 48:125–149. https://doi.org/10.1146/annurev.physchem.48.1.125
Gotor FJ, Criado JM, Malek J, Koga N (2000) Kinetic analysis of solid-state reactions: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A 104:10777–10782. https://doi.org/10.1021/jp0022205
Šesták J, Berggren G (1971) Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta 3:1–12. https://doi.org/10.1016/0040-6031(71)85051-7
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem 110:17315–17328
Font R, Marcilla A, Verdu E, Devesa J (1990) Kinetics of the pyrolysis of almond shells and almond shells impregnated with cobalt dichloride in a fluidized bed reactor and in a pyroprobe 100. Ind Eng Chem Res 29:1846–1855. https://doi.org/10.1021/ie00105a016
Hernowo P, Rasrendra CB, Irawan A et al (2022) Volatile state mathematical models for predicting components in biomass pyrolysis products. J Eng Technol Sci 54:220108. https://doi.org/10.5614/j.eng.technol.sci.2022.54.1.8
Mukherjee A, Okolie JA, Tyagi R et al (2021) Pyrolysis kinetics and activation thermodynamic parameters of exhausted coffee residue and coffee husk using thermogravimetric analysis. Can J Chem Eng 99:1683–1695. https://doi.org/10.1002/cjce.24037
Steven S, Sophiana IC, Murti Z et al (2023) A simple material and energy input–output performance in evaluating silica production from conventional, fume, and biomass thermochemical conversion routes. Waste Biomass Valor. https://doi.org/10.1007/s12649-023-02348-5
Konttinen J, Kallio S, Hupa M, Winter F (2013) NO formation tendency characterization for solid fuels in fluidized beds. Fuel 108:238–246. https://doi.org/10.1016/j.fuel.2013.02.011
Suman S, Gautam S (2017) Pyrolysis of coconut husk biomass: analysis of its biochar properties. Energy Sources, Part A Recover Util Environ Eff 39:761–767. https://doi.org/10.1080/15567036.2016.1263252
Said M, John G, Mhilu C, Manyele S (2014) Analysis of pyrolysis kinetics and energy content of agricultural and forest waste. Open J Renew Energy Sustain Dev 2014:36–44. https://doi.org/10.15764/RESD.2014.01004
Fernandes IJ, Calheiro D, Kieling AG et al (2016) Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 165:351–359. https://doi.org/10.1016/j.fuel.2015.10.086
Osueke CO, Olayanju TMA, Ezugwu CA et al (2018) Comparative calorific evaluation of biomass fuel and fossil fuel. Int J Civ Eng Technol 9:1576–1590
Setiadi, Karubaba JJ (2019) The utilization of biomass waste from coconut fiber to produce rich aromatic bio-oil through catalytic pyrolysis with impregnated nickel and zinc catalysts. In: AIP Conference Proceedings. p 020047
Zhou X, Li W, Mabon R, Broadbelt LJ (2018) A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ Sci 11:1240–1260. https://doi.org/10.1039/C7EE03208K
Ma’ruf A, Pramudono B, Aryanti N (2017) Optimization of lignin extraction from rice husk by alkaline hydrogen peroxide using response surface methodology. Rasayan J Chem 10:407–414. https://doi.org/10.7324/RJC.2017.1021667
Toscano G, Duca D, Rossini G et al (2015) Identification of different woody biomass for energy purpose by means of soft independent modeling of class analogy applied to thermogravimetric analysis. Energy 83:351–357. https://doi.org/10.1016/j.energy.2015.02.032
Kasmiarno LD, Panannangan JK, Steven S et al (2024) Exploration of bio-hydrocarbon gases production via pyrolysis of fresh natural rubber: experimental and volatile state kinetic modeling studies. J Anal Appl Pyrolysis 177:106275. https://doi.org/10.1016/j.jaap.2023.106275
Steven S, Restiawaty E, Bindar Y (2022) Simple mass transfer simulation using a single-particle heterogeneous reaction approach in rice husk combustion and rice husk ash extraction. IOP Conf Ser: Earth Environ Sci 963:012050. https://doi.org/10.1088/1755-1315/963/1/012050
Mallick D, Poddar MK, Mahanta P, Moholkar VS (2018) Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour Technol 261:294–305. https://doi.org/10.1016/j.biortech.2018.04.011
Sari RM, Gea S, Wirjosentono B et al (2021) The effectiveness of coconut coir as tar adsorbent in liquid smoke integrated into the pyrolysis reactor. Case Stud Therm Eng 25:100907. https://doi.org/10.1016/j.csite.2021.100907
Di Blasi C (1994) Numerical simulation of cellulose pyrolysis. Biomass Bioenergy 7:87–98. https://doi.org/10.1016/0961-9534(94)00040-Z
Biswas B, Pandey N, Bisht Y et al (2017) Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63. https://doi.org/10.1016/j.biortech.2017.02.046
Soysa R, Choi YS, Kim SJ, Choi SK (2016) Fast pyrolysis characteristics and kinetic study of Ceylon tea waste. Int J Hydrogen Energy 41:16436–16443. https://doi.org/10.1016/j.ijhydene.2016.04.066
Ma Z, Chen D, Gu J et al (2015) Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energy Convers Manag 89:251–259. https://doi.org/10.1016/j.enconman.2014.09.074
Sheng J, Ji D, Yu F et al (2014) Influence of chemical treatment on rice straw pyrolysis by TG-FTIR. IERI Procedia 8:30–34. https://doi.org/10.1016/j.ieri.2014.09.006
Turmanova SC, Genieva SD, Dimitrova AS, Vlaev LT (2008) Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Polym Lett 2:133–146. https://doi.org/10.3144/expresspolymlett.2008.18
Sharma A, Pareek V, Zhang D (2015) Biomass pyrolysis - a review of modelling, process parameters, and catalytic studies. Renew Sustain Energy Rev 50:1081–1096. https://doi.org/10.1016/j.rser.2015.04.193
Di Blasi C (1997) Influences of physical properties on biomass devolatilization characteristics. Fuel 76:957–964. https://doi.org/10.1016/S0016-2361(97)00096-3
Falco G, Guigo N, Vincent L, Sbirrazzuoli N (2018) FA polymerization disruption by protic polar solvents. Polymers (Basel) 10:1–14. https://doi.org/10.3390/polym10050529
Sbirrazzuoli N (2019) Advanced isoconversional kinetic analysis for the elucidation of complex reaction mechanisms: a new method for the identification of rate-limiting steps. Molecules 24:1683. https://doi.org/10.3390/molecules24091683
Vyazovkin S, Sbirrazzuoli N (2006) Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun 27:1515–1532. https://doi.org/10.1002/marc.200600404
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
Zhao S, Lü X, Sun Y, Huang J (2021) Thermodynamic mechanism evaluate the feasibility of oil shale pyrolysis by topochemical heat. Sci Rep 11:5365. https://doi.org/10.1038/s41598-021-84757-x
Acknowledgements
We thank the National Innovation Research Agency (BRIN) in Cibinong for the TGA analysis.
Author information
Authors and Affiliations
Contributions
Pandit Hernowo: conceptualization, writing—original draft, writing—review and editing, and visualization. Soen Steven: writing—review and editing, formal analysis, critical revising, and visualization. Muhammad Maulidin and Alif Gita Arumsari: methodology, investigation, and data curation. Yazid Bindar: conceptualization and formal analysis. Amalia Syauket, Komang Ria Saraswati and Dede Rukmayadi: formal analysis.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernowo, P., Steven, S., Maulidin, M. et al. Thermokinetic study of coconut husk pyrolysis in the devolatilization zone using volatile state approach. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05706-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05706-y