Abstract
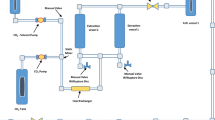
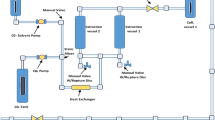
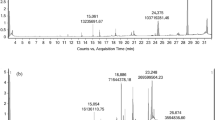
Cupressus sempervirens (C. sempervirens) is a traditional plant that has been used to manage some chronic diseases which make burden to global health concern. Many traditional methods have been applied to derive various effective molecules from C. sempervirens. In the present investigation various pressure levels (20, 30, and 45 Mpa) have been applied to derive various bioactive products from C. sempervirens leaves. Total phenolic compounds and flavonoids were examined in different cases where 20 Mpa produced maximal concentration of these products compared to other pressure levels. HPLC has been used to qualitatively screen polyphenolic compounds in various extracts where the extract using 20 Mpa showed maximal products levels. Antimicrobial assay has been done versus Staphylococcus aureus (ATCC 23235), Enterococcus faecalis (ATCC29212), Escherichia coli (ATCC25922), Klebsiella pneumonia (ATCC13883), Candida albicans (ATCC10231), and Mucor racemosus (ATCC42647) upon using various extracts where C. sempervirens extracted using 20 Mpa had the maximal inhibition zone versus Klebsiella pneumonia (ATCC13883) of 25 ± 0.3 mm and showed higher anti-biofilm impact versus K. pneumonia (ATCC13883) upon using 75% of MBC. Applying 20-Mpa extraction pressure has a maximal antioxidant impact with IC50 = 3.05 ± 0.1 µg/ml. There was a minimal difference in lipase and amylase inhibitions upon using the tree pressure levels. Furthermore, there was a slight change in the antidiabetic action of C. sempervirens upon changing pressure. Collectively, elevation of pressure in supercritical fluid extraction of C. sempervirens decreases the impact of some antimicrobial action and has a minimal impact on other tested applications.







Similar content being viewed by others
Data availability
All raw results available with all authors upon request.
Abbreviations
- SFE :
-
Supercritical fluid extraction
- MPa :
-
Megapascal as a unit of pressure
- HPLC :
-
High performance liquid chromatography
- MIC :
-
Minimal inhibitory concentration
- MBC :
-
Minimum bactericidal concentration or level
- DMSO :
-
Dimethyl sulphoxide
- CFU :
-
Colony forming unit
- TSY :
-
Trypticase soy yeast media
- DPPH :
-
1, 1-Diphenyl-2-picryl hydrazyl
- PNPB :
-
P-nitrophenyl butyrate
- DNSA :
-
3,5-Dinitrosalicylic acid
References
Alsalamah SA, Alghonaim MI, Jusstaniah M, Abdelghany TM (2023) Anti-yeasts, antioxidant and healing properties of henna pre-treated by moist heat and molecular docking of its major constituents, chlorogenic and ellagic acids, with Candida albicans and Geotrichum candidum proteins. Life 13(9):1839. https://doi.org/10.3390/life13091839
Al-Rajhi AMH, Qanash H, Almashjary MN, Hazzazi MS, Felemban HR, Abdelghany TM (2023) Anti-Helicobacter pylori, antioxidant, antidiabetic, and anti-Alzheimer’s activities of laurel leaf extract treated by moist heat and molecular docking of its flavonoid constituent, naringenin, against acetylcholinesterase and butyrylcholinesterase. Life 13:1512
Qanash H, Bazaid AS, Aldarhami A, Alharbi B, Almashjary MN, Hazzazi MS, Felemban HR, Abdelghany TM (2023) Phytochemical characterization and efficacy of Artemisia judaica extract loaded chitosan nanoparticles as inhibitors of cancer proliferation and microbial growth. Polymers 15:391
Qanash H, Alotaibi K, Aldarhami A, Bazaid AS, Ganash M, Saeedi NH, Ghany TA (2023) Effectiveness of oil-based nanoemulsions with molecular docking of its antimicrobial potential. BioResources 18:1554–1576
Al-Rajhi AMH, Qanash H, Bazaid AS, Binsaleh NK, Abdelghany TM (2023) Pharmacological evaluation of Acacia nilotica flower extract against Helicobacter pylori and human hepatocellular carcinoma in vitro and in silico. J Funct Biomater 14:237
Nehdi IA (2013) Cupressus sempervirens var horizentalis seed oil chemical composition, physicochemical characteristics, and utilizations. Ind Crops Prod. 41:381–385
Hasaballah A, Shehata A, Fouda M, Hassan M, Gad M (2018) The biological activity of Cupressus sempervirens extracts against Musca domestica. Asian J Biol 5:1–12
Frezza C, De Vita D, Sciubba F, Toniolo C, Tomassini L, Nicoletti M, Franceschin M, Guiso M, Bianco A, Serafini M et al (2022) There is not only Cupressus sempervirens L review on the phytochemistry and bioactivities of the other Cupressus L species. Appl Sci 12:7353 https://doi.org/10.3390/app12147353
Frezza C, Venditti A, Serafini M, Bianco A (2019) Phytochemistry, chemotaxonomy, ethnopharmacology, and nutraceutics of Lamiaceae. Stud Nat Prod Chem 62:125–178
Alghonaim MI, Alsalamah SA, Ali Y, Abdelghany TM (2024) Green mediator for selenium nanoparticles synthesis with antimicrobial activity and plant biostimulant properties under heavy metal stress. BioResources 19(1):898–916. https://doi.org/10.15376/biores.19.1.898-916
Elmongy EI, Negm WA, Elekhnawy E, El-Masry TA, Attallah NGM, Altwaijry N, Batiha GE-S, El-Sherbeni SA (2022) Antidiarrheal and antibacterial activities of Monterey cypress phytochemicals: in vivo and in vitro approach. Molecules 27:346
Ismail A, Mancini E, De Martino L, Hamrouni L, Hanana M, Jamoussi B, Gargouri S, Scognamiglio M, De Feo V (2014) Chemical composition and biological activities of Tunisian Cupressus arizonica Greene essential oils. Chem Biodivers 11:150–160
Abdulkhani A, Sedaghat A, Alizadeh P, Tabil LG (2020) Extraction of bioactive moieties of Cupressus arizonica and Cupressus sempervirens wood knots. Can Biosyst Eng 62:8.1–8.10
Khanyile AT, Andrew JE, Paul V, Sithole BB (2022) A comparative study of supercritical fluid extraction and accelerated solvent extraction of lipophilic compounds from lignocellulosic biomass. Sustain Chem Pharm 26:100608. https://doi.org/10.1016/j.scp.2022.100608
Zorić M, Banožić M, Aladić K, Vladimir-Knežević S, Jokić S (2022) Supercritical CO2 extracts in cosmetic industry: current status and future perspectives. Sustain Chem Pharm 27:100688
Ghasemi E, Raofie F, Najafi NM (2011) Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. Leaves Food Chem 126:1449–1453. https://doi.org/10.1016/j.foodchem.2010.11.135
Kate AE, Singh A, Shahi NC, Pandey JP, Prakash O, Singh TP (2016) Singh Novel eco-friendly techniques for extraction of food based lipophilic compounds from biological materials. Nat Prod Chem Res 4:231–237. https://doi.org/10.4172/2329-6836.1000231
Zhang Q-W, Lin L-G, Ye W-C (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13:1–26. https://doi.org/10.1186/s13020-018-0177-x
Al-Rajhi AMH, Abdelghany TM (2023) Nanoemulsions of some edible oils and their antimicrobial, antioxidant, and anti-hemolytic activities. BioResources 18(1):1465–1481 https://doi.org/10.15376/biores.18.1.1465-1481
Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 9:59
Elizondo-Luévano JH, Gomez-Flores R, Verde-Star MJ, Tamez-Guerra P, Romo-Sáenz CI, Chávez-Montes A, Rodríguez-Garza NE, Quintanilla-Licea R (2022) In vitro cytotoxic activity of methanol extracts of selected medicinal plants traditionally used in Mexico against human hepatocellular carcinoma. Plants (Basel) 11(21):2862. https://doi.org/10.3390/plants11212862
Chai YH, Yusup S, Kadir WNA, Wong CY, Rosli SS, Ruslan MSH, Chin BLF, Yiin CL (2020) Valorization of tropical biomass waste by supercritical fluid extraction technology. Sustainability 13:233. https://doi.org/10.3390/su13010233
Marinova D, FF Ribarova and Atanassova M (2005) Total phenolic and total flavonoids in ulgarian fruits and vegetables. J Univ Chem Technol Metallurgy 40(3):255–260
Sembiring EN, Elya B, Sauriasari R (2018) Phytochemical screening, total flavonoid and total phenolic content, and antioxidant activity of different parts of Caesalpinia bonduc (L.). Roxb Pharmacog J 10(1):123–7
Abdelghany T, Yahya R, Bakri MM, Ganash M, Amin BH, Qanash H (2021) Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int J Pharmacol 17:643–655
Deabes MM, El-Fatah SIA, Salem SH, Naguib KM (2021) Antimicrobial activity of bioactive compounds extract from Saussurea costus against food spoilage microorganisms. Egypt J Chem 64:2833–2843. https://doi.org/10.21608/ejchem.2021.69572.3528
Yang C-H, Yang C-S, Hwang M-L, Chang C-C, Li R-X, Chuang L-Y (2012) Antimicrobial activity of various parts of Cinnamomum cassia extracted with different extraction methods. J Food Biochem 36:690–698. https://doi.org/10.1111/j.1745-4514.2011.00584.x
French GL (2006) Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J Antimicrob Chemother 58:1107
Alsolami A, Bazaid AS, Alshammari MA et al (2023) Ecofriendly fabrication of natural jojoba nanoemulsion and chitosan/jojoba nanoemulsion with studying the antimicrobial, anti-biofilm, and anti-diabetic activities in vitro. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-05162-0
Kamali E, Jamali A, Izanloo A, Ardebili A (2021) In vitro activities of cellulase and ceftazidime, alone and in combination against Pseudomonas aeruginosa biofilms. BMC Microbiol 21(1):347. https://doi.org/10.1186/s12866-021-02411-y
Calvo MM, Martín-Diana AB, Rico D, López-Caballero ME, Martínez-Álvarez O (2022) Antioxidant, antihypertensive, hypoglycaemic and nootropic activity of a polyphenolic extract from the halophyte ice plant (Mesembryanthemum crystallinum). Foods 11(11):1581. https://doi.org/10.3390/foods11111581
Plaskova A, Mlcek J (2023) New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front Nutr 10:1118761. https://doi.org/10.3389/fnut.2023.1118761
Elbashir SMI, Devkota HP, Wada M, Kishimoto N, Moriuchi M, Shuto T, Misumi S, Kai H, Watanabe T (2018) Free radical scavenging, α-glucosidase inhibitory and lipase inhibitory activities of eighteen Sudanese medicinal plants. BMC Complement Altern Med 18(1):282. https://doi.org/10.1186/s12906-018-2346-y
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6:42. https://doi.org/10.3390/plants6040042
Yahya R, Al-Rajhi AMH, Alzaid SZ, Al Abboud MA, Almuhayawi MS, Al Jaouni SK, Selim S, Ismail KS, Abdelghany TM (2022) Molecular docking and efficacy of Aloe vera gel based on chitosan nanoparticles against Helicobacter pylori and its antioxidant and anti-inflammatory activities. Polymers 14(15):2994. https://doi.org/10.3390/polym14152994
Abdelghany TM, Hassan MM, El-Naggar MA, Abd El-Mongy M (2020) GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol Rep 27:e00496
Tsiaka T, Sinanoglou VJ, Zoumpoulakis P (2017) Extracting bioactive compounds from natural sources using green high-energy approaches: trends and opportunities in lab- and large-scale applications. In: Grumezescu AM, Holban AM (ed) Ingredients extraction by physicochemical methods in food, 4 p 307–365
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha L (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8:1–10. https://doi.org/10.4314/ajtcam.v8i1.60483
Ghany AT (2014) Eco-friendly and safe role of Juniperus procera in controlling of fungal growth and secondary metabolites J Plant Pathol Microbiol 5(3) https://doi.org/10.4172/2157-7471.1000231
Wrona O, Rafińska K, Możeńiska C, Buszewski B (2017) Supercritical fluid extraction of bioactive compounds from plant materials. J AOAC Int 100:1624–1635. https://doi.org/10.5740/jaoacint.17-0232
Nejia H, Séverine C, Jalloul B, Mehrez R, Jean Stéphane VJ (2013) Extraction of essential oil from Cupressus sempervirens: comparison of global yields, chemical composition and antioxidant activity obtained by hydrodistillation and supercritical extraction. Nat Prod Res 27(19):1795–1799. https://doi.org/10.1080/14786419.2012.755680
Orhan IE, Tumen I (2015) Potential of Cupressus sempervirens (Mediterranean Cypress) in health. The Mediterranean Diet 639–47 https://doi.org/10.1016/B978-0-12-407849-9.00057-9
Batiha GES, Teibo JO, Shaheen HM et al (2023) Bioactive compounds, pharmacological actions and pharmacokinetics of Cupressus sempervirens. Naunyn-Schmiedeberg’s Arch Pharmacol 396:389–403. https://doi.org/10.1007/s00210-022-02326-z
Galovičová L, Čmiková N, Schwarzová M, Vukic MD, Vukovic NL, Kowalczewski PŁ, Bakay L, Kluz MI, Puchalski C, Obradovic AD et al (2023) Biological activity of Cupressus sempervirens essential oil. Plants 12(5):1097. https://doi.org/10.3390/plants12051097
Elansary HO, Salem MZM, Ashmawy NA, Yacout MM (2012) Chemical composition, antibacterial and antioxidant activities of leaves essential oils from Syzygium cumini L., Cupressus sempervirens L. and Lantana camara L. from Egypt. J Agric Sci 4:144–152. https://doi.org/10.5539/jas.v4n10p144
Galovičová L, Čmiková N, Schwarzová M, Vukic MD, Vukovic NL, Kowalczewski PŁ, Bakay L, Kluz MI, Puchalski C, Obradovic AD et al (2023) Biological activity of Cupressus sempervirens essential oil. Plants 12:1097. https://doi.org/10.3390/plants12051097
Emami SA, Asili J, Rahimizadeh M, Fazly-Bazzaz BS, Khayyat MH (2006) Chemical and antimicrobial studies of Cupressus sempervirens L. and C. horizentalis mill Essential Oils. Iran J Pharm Sci. 2:103–108 https://doi.org/10.22037/ijps.v2.39558
Pansera MR, Silvestre WP, Sartori VC (2023) Bioactivity of Cupressus sempervirens and Cupressus lusitanica leaf essential oils on Colletotrichum fructicola. J Essent Oil Res 35:51–59
Jose WAN, Renata AC, Magda TDC, Theodora TAC (2017) Antibiofilm activity of natural substances derived from plants. Afr J Microbiol Res 11:1051–1060
Rehman R (2019) Fast and effective identification of the bioactive compounds from Thuja and Cypress via bioactivity guided isolation approach. Ph.D. Thesis, University of Agriculture Faisalabad, Faisalabad, Pakistan
Kırmusaoğlu S (2019) Antimicrobials, antibiotic resistance, antibiofilm strategies and activity methods. Rijeka, Croatia, IntechOpen
Famuyide IM, Fasina FO, Eloff JN, McGaw LJ (2020) The ultrastructural damage caused by Eugenia zeyheri and Syzygium legatii acetone leaf extracts on pathogenic Escherichia coli. BMC Vet Res 16(1):326. https://doi.org/10.1186/s12917-020-02547-5
Blasi F, Ianni F, Mangiapelo L, Pinna N, Cossignani L (2023) In vitro anti-obesity activity by pancreatic lipase inhibition - simple HPLC approach using EVOO as natural substrate. J Sci Food Agric 103(6):2786–2793. https://doi.org/10.1002/jsfa.12417
Neovius M, Johansson K, Rössner S (2008) Head-to-head studies evaluating efficacy of pharmaco-therapy for obesity: a systematic review and meta-analysis. Obes Rev 9(5):420–427. https://doi.org/10.1111/j.1467-789X.2008.00463.x
Jamous RM, Abu-Zaitoun SY, Akkawi RJ, Ali-Shtayeh MS (2018) Antiobesity and antioxidant potentials of selected Palestinian medicinal plants. Evid Based Complement Alternat Med 2018(13):8426752. https://doi.org/10.1155/2018/8426752
Willcox JK, Ash SL, Catignani GL (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44(4):275–295. https://doi.org/10.1080/10408690490468489
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40. https://doi.org/10.1016/j.cbi.2005.12.009
Al-Rajhi AMH, Bakri MM, Qanash H, Alzahrani HY, Halawani H, Algaydi MA, Abdelghany TM (2023) Antimicrobial, antidiabetic, antioxidant, and anticoagulant activities of Cupressus sempervirens in vitro and in silico. Molecules 28:7402. https://doi.org/10.3390/molecules28217402
Kalter-Leibovici O, Atamna A, Lubin F et al (2007) Obesity among Arabs and Jews in Israel: a population-based study. Israel Medical Association Journal 9(7):525–530
Jamous RM, Abu-Zaitoun SY, Akkawi RJ, Ali-Shtayeh MS (2018) Antiobesity and antioxidant potentials of selected Palestinian medicinal plants. Evid Based Complement Alternat Med. 8426752 https://doi.org/10.1155/2018/8426752
Cikoš AM, Jokić S, Šubarić D, Jerković I (2018) Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar Drugs 16(10):348. https://doi.org/10.3390/md16100348
Moges A, Barik CR, Sahoo L, Goud VV (2022) Optimization of polyphenol extraction from Hippophae salicifolia D. Don leaf using supercritical CO2 by response surface methodology. 3 Biotech. 12(11):292 https://doi.org/10.1007/s13205-022-03358-1
Acknowledgements
The author is thankful to the Biology Department, College of Science, at Jazan
University, Jazan, Suadi Arabia.
Author information
Authors and Affiliations
Contributions
Investigation, formal analysis, writing—review and editing, MMA. The author read and agreed to the published version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamed, A. Biological applications of Cupressus sempervirens biomass extracted at various levels of pressure using different critical fluid extraction protocol. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05543-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05543-z