Abstract
The presented work aimed to investigate the effect of thermal treatment on sludge from wastewater treatment plants (WWTPs) in the Slovak Republic on the content of pharmaceuticals (PhACs) and illicit drugs. Sludge samples from eight WWTPs (total flows of 6900–62,500 m3/day, number of population equivalents over 40,000 for each WWTP, production of sludge 400–3300 tons DM/year) were treated with thermal processes in the temperature range of 80 to 550 °C. More than 100 compounds were studied in the sludge samples, but in this article, we deeply focused on the fate of the thirteen most concentrated and frequently found PhACs (azithromycin, carbamazepine, cetirizine, citalopram and its metabolite N-desmethylcitalopram, diclofenac, fexofenadine, sertraline and its metabolite norsertraline, telmisartan, trazodone, valsartan, and verapamil). The thermal processes used showed a decrease in PhAC concentrations already at 80 °C when the total concentration of selected PhACs decreased to 81%. In thermally treated sludge samples at 250 °C, only telmisartan at all studied WWTPs and diclofenac at WWTP Banská Bystrica stayed above the limits of quantification (LOQs), while the temperature of 550 °C led to a decrease in all thirteen PhACs below LOQs.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The number of inhabitants on our planet is undeniably growing, and the demands on the quality of living standards are also increasing. This is associated with a significantly increased burden on natural resources and the environment. It is necessary to adapt to trends in an effort to ensure a sustainable state in light of the current global issues brought about by society’s development. Nowadays, the problem of sewage sludge (SS) disposal from wastewater treatment plants (WWTPs) is also related to this case. Although in the past, sludge was used and disposed of on agricultural land without problems; today, this procedure appears to be problematic in several cases. The reason for this is the increasing amount of contaminants in the sludge [1,2,3,4,5]. At present, the regulation of SS land application is only based on the heavy metal content outlined in Council Directive 86/278/EEC [6]. However, this directive is outdated and fails to address the contemporary imperative of safeguarding the agricultural use of sludge. Therefore, finding another suitable alternative for the sludge used or disposed of in this way is necessary.
According to Verlicchi and Zambello [5], SS is produced as a byproduct of the biological and chemical processes that take place in WWTPs. It can consist of a wide variety of organic and inorganic compounds, as well as microorganisms and viruses. The suitability of using sludge for final disposal after dewatering is crucial to understanding its basic physical and chemical properties [7]. The dewatered SS is composed of around 50–70% organic compounds and 30–50% mineral components, including 1–4% inorganic carbon. It also contains 3.4–4.0% nitrogen, 0.5–2.5% phosphorus, and notable quantities of other nutrients, including micronutrients [8]. Heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, adsorbable organohalogens, pesticides, surfactants, hormones, pharmaceuticals (PhACs), personal care products, and many more are just some of the harmful and toxic substances found in SS [9, 10].
Several factors contribute to SS production, such as the effectiveness of primary, secondary, and tertiary treatment; the treatment technology employed (e.g., membrane bioreactor, nutrient removal, phosphorus precipitation, etc.); the method of sludge stabilization (aerobic or anaerobic digestion); and the operating conditions (sludge age and organic load) [3]. Data on the amount of SS produced and disposed of from municipal wastewater (as dry matter, DM) in the EU is available on the Eurostat website [11]. It is estimated that around 10 million tons per year of sewage sludge DM were produced in the EU in the period 2003–2006. This amount increased to 11.5 million tons of DM in 2010, and in 2020, the estimated amount produced is 13.5 million tons of DM [12, 13]. With the improvement of wastewater collection systems and an increasing number of households connected to the sewage system, the production of SS in EU countries is expected to rise. According to Grabic et al. [14], it is expected that around 15 million tons of SS DM will be generated of 5 years. Consequently, appropriate management and disposal of approximately 70 million tons of untreated sludge become imperative.
Various methods for disposing of SS exist worldwide, including landfilling, agricultural usage, landscaping, thermal treatment, or improper disposal [15,16,17,18,19,20,21]. The predominant method for managing SS remains land application, primarily due to its cost-effectiveness and the presence of valuable nutrients like nitrogen and phosphorus. Additionally, dewatered SS digestate can be used for agricultural purposes. However, safety concerns related to the utilization of SS in this manner have been raised [14]. This activity is controlled in different ways due to the implementation in the Member States of Directive 86/278/CEE, which allows the application of SS (biosolids) on land [22]. Since traditional methods of SS disposal are no longer economically or environmentally viable, thermal processes may become the dominant method of sludge disposal in the future. The organic component of the sludge, as well as any pathogens or pharmaceutical residues, are removed during thermal operations, leaving only the ash component, which is then disposed of in its entirety. According to Goldan et al. [23], the primary objective of thermal processing of SS is to maximize the usage of the energy stored in the sludge while minimizing the negative effects on the environment to comply with increasingly stringent regulations. Thermal treatment options for SS include sludge drying, incineration, pyrolysis, and gasification [21, 24]. Additional drying of SS after dewatering is necessary due to a decrease in the water content of the sludge and an increase in its calorific value [25]. The energy content of SS is similar to that of wood biomass. According to Durdević et al. [24] and Kubonova et al. [26], the calorific value of dry matter in SS varies depending on the type of sludge. Raw sludge has a calorific value of 17–18 MJ/kg, activated sludge has a calorific value of 14–16 MJ/kg, and stabilized sludge has a calorific value of 8–12 MJ/kg. Drying is usually applied before further thermal treatment [16]. Depending on the type of dryer (disc and thin film dryers, drum dryers, belt dryers, or solar drying), the drying of SS can take place in the temperature range of 10–200 °C [25]. Although the melting and boiling points of most PhACs are above 100 °C [27, 28] significant decomposition of PhACs in sludge during this process is not expected. Pyrolysis is a combustion process of SS that occurs at temperatures between 300 and 700 °C in environments with limited or no air [21, 29]. The pyrolysis process produces pyrolysis gas, pyrolysis liquids (bio-oil or pyrolysis oil), and solid biochar [21]. The parameters influencing pyrolysis products are particle size, temperature and heating rate, moisture content, reactor operating principle, gas phase retention time, and carbon, ash, and liquid phase separation. In general, as the temperature increases, solid matter yields decrease, and liquid and gas yields increase [30]. Pyrolysis can be categorized into three types: slow, quick, and flash, based on heating rate and residence time [21]. The slow pyrolysis process, also known as carbonization or torrefaction, is characterized by low heating rates and extended residence times, often ranging from hours to days, and operates at temperatures around 300–700 °C. The primary objective of this method is to optimize the formation of biochar [31]. Torrefaction is typically carried out at temperatures below 400 °C. During the process, there is a reduction in moisture content, accompanied by an increase in the density of the torrefied char, as observed by Gao et al. [32]. The torrefaction process has several advantages when used for sludge treatment including reducing overall volume and effectively eliminating pollutants such as NOx and SOx. Moreover, this method increases the calorific value and C/H ratio of SS, as shown in studies conducted by Atienza-Martínez et al. [33, 34].
Sludge combustion occurs through oxidation at temperatures typically above 1000 °C, where additional oxygen facilitates the combustion of carbon and volatile chemicals [35, 36]. Sludge incineration is considered one of the most effective disposal alternatives [37]. Besides the incineration of sludge, this process is also used to treat other types of waste, such as medical or municipal waste [38]. There are three main aspects to the incineration of SS: the production of heat and energy, the elimination of the effects of hazardous substances present in the sludge, and the reduction of the volume of SS [32]. The primary products of this thermal process are combustible gas, bio-oil, and bio-char [38]. Gasification can be categorized as a variant of pyrolysis, and it is commonly performed at temperatures over 900 °C to attain a substantial production of purified and flammable gases, such as hydrogen (H2), carbon monoxide (CO), and light hydrocarbons [39]. Steam, air–steam mixtures, carbon dioxide, and steam-carbon dioxide mixtures have all been investigated as potential gasifying agents in addition to air [21, 32, 40,41,42]. The considered method shows significant similarities to combustion, except for its reduced ability to tolerate moisture in the reactor, which should not exceed 15% by weight. Additionally, there is a deficiency in stoichiometric oxidants required to achieve complete combustion, as noted by Oladejo et al. [36]. According to Dogru et al. [43], it is a remarkable feature that sludge with a moisture content below 15% undergoes complete moisture loss during the gasification process.
The thermal treatment of SS is used effectively in some European Union (EU) countries, such as the Netherlands, which processes all of its sludge output in this manner. With 97% of its sludge thermally processed, Switzerland (a non-EU country) comes in second, followed by Belgium (89%) and Germany (70%) [25]. The implementation of thermal treatment on sludge is likely to be a necessary measure to fulfill the criteria outlined in the revised Directive 271/1991, particularly in relation to achieving energy neutrality and reducing greenhouse gas emissions.
The presence of emerging contaminants, including PhACs, has raised significant concerns due to their adverse effects on human health as well as aquatic and terrestrial ecosystems [44]. The complexity and heterogeneity of SS make it a difficult matrix to work with. In the literature, there are much fewer articles on the occurrence of PhACs in different types of sludge than in wastewater, possibly because sludge is a more complex and complicated matrix for analysis [2, 14, 45,46,47]. PhACs can occur at high levels in digested sludge, for example, ciprofloxacin (12,858 ng/g DM), ofloxacin (6712 ng/g DM), norfloxacin (6049 ng/g DM), diclofenac (7020 ng/g DM), ibuprofen (4105 ng/g DM), caffeine (2828 ng/g DM), and gemfibrozil (1562 ng/g DM) [3, 47].
Thermal sludge treatment does not always take place in highly sophisticated and controlled combustion processes. The disposal of SS, mainly from small sources and in rural areas, can take place in simple conditions (domestic combustion) where high temperatures are not reached and the process of decomposition of sludge and organic components may not be perfect. To our knowledge, very few studies have addressed the impact of thermal processes or the effect of temperature on the removal of PhACs, drugs, and their metabolites from SS. Our vision was to perform laboratory tests that aimed at quantifying the concentration of PhACs content in sludge after thermal processes performed at low as well as high temperatures. We desired to prove that thermal processes are safe and that they effectively remove organic constituents from sludge, such as PhACs. Furthermore, the aim was to determine the temperature at which complete decomposition of the studied PhACs occurs. This study is the first in the Slovak Republic to provide a comprehensive description of how thermal processes impact the presence of PhACs in SS. Thus, a further objective of the study is to offer insights into the prospective application of thermal treatment as a method of sludge disposal.
2 Materials and methods
2.1 Characterization of WWTPs
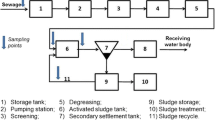
Sewage sludge from eight WWTPs in different parts of Slovakia was selected (see the map in SM) to study the removal of PhACs. Five of them are located in the west (Bratislava—Devínska Nová Ves, Trnava, Trenčín, Piešťany, and Nitra), one in the center (Banská Bystrica), and two of them in the east of Slovakia (Poprad and Košice). All WWTPs utilize anaerobic digestion with biogas production. Basic relevant information on studied WWTPs is given in Table 1.
Each of the WWTPs operates on the mechanical–biological principle of wastewater treatment. Nitrogen removal from wastewater is carried out in nitrification in combination with pre-denitrification, and then phosphorus is removed by increased biological removal or, additionally, with chemical precipitation. Sludge is anaerobically stabilized in digester tanks with biogas production. The generated biogas is energetically recovered in cogeneration units.
2.2 Sampling and analysis
Sludge samples were taken after stabilization and dewatering of the sludge from eight WWTPs. The retention time of the sludge in the WWTP (including anaerobic sludge stabilization processes) ranges between 50 and 100 days; therefore, the sample cannot be defined by time. The collection took place in October 2019. The collected samples were stored at 8 °C until the experiments (approximately 24 h).
For a more efficient thermal process and better handling, the anaerobically stabilized sludge was first crushed into smaller parts, and then, the amount of sludge required for trials was weighed on analytical scales (approximately 10 g). Each sludge was prepared in triplicates. The results are reported as an arithmetic mean.
The thermal processes were done in the laboratory at the Department of Environmental Engineering, Faculty of Chemical and Food Technology, Slovak Technical University in Bratislava. For the experiments, digital drying ovens with a moisture analyzer (MB27 moisture analyzers from the manufacturer OHAUS, 80 °C), a classic laboratory drying oven with natural forced circulation Ecocell (105 and 250 °C) and a laboratory muffle furnace (550 °C) were used. The samples were dried for 2 h. After cooling in the desiccators, the dried sludge samples were weighed for dry matter content (Table 1).
2.3 Calculation of pharmaceutical concentration and removal efficiency in thermal processes
The PhAC concentration was determined in the thermally treated sludge. Target compounds were extracted from sludge samples using the two-step extraction procedure described in detail by Golovko et al. [45]. The filtered sludge extracts were analyzed by liquid chromatography with tandem mass spectrometry (LC–MS/MS) at the Faculty of Fisheries and Protection of Waters, USB, Czech Republic [14]. LC–MS/MS analyses were carried out using a TSQ Quantiva triple-stage quadrupole mass spectrometer from Thermo Fisher Scientific in San Jose, CA, USA. This mass spectrometer was coupled to an Accela 1250 LC pump from Thermo Fisher Scientific as well as an HTS XT-CTC autosampler from CTC Analytics AG, Zwingen, Switzerland. The chromatographic separation was performed using a Hypersil Gold aQ column with dimensions 50 mm × 2.1 mm ID × 5 μm particles (Thermo Fisher Scientific). There is further information available that provides a more in-depth description of MS/MS transitions and analytical methodologies [45, 48, 49].
The concentration of 101 pharmaceuticals, illicit drugs, and their metabolites was determined in sludge samples. Only compounds in sludge samples with values above the limit of quantification (LOQ) were included in the following calculations. Average PhAC concentrations in ng/g units were calculated from triplicates for individual PhAC, which were subsequently used to calculate the content of the given PhAC per gram of sludge dry matter (in units of ng/g dry matter).
The following calculation was used to evaluate the PhACs removal efficiency in sludge during individual thermal processes:
In the equation, C0 expresses the concentration of PhACs in the sludge before the thermal process, and C1,T expresses the concentration of PhACs in the sludge after the thermal treatment at the process temperature T.
3 Results and discussions
The concentrations of 101 pharmaceuticals, illicit drugs, and some metabolites (belonging to 20 different therapeutic groups, see Table S1) were quantified in the mentioned sludge samples using the LC–MS/MS method. The therapeutic groups included psychoactive substances and their metabolites (n = 25), antibiotics (n = 16), antihypertensives (n = 15), illicit drugs and their metabolites (n = 16), antihistamines (n = 6), lipid regulators (n = 4), analgesics/NSAIDs (n = 3), and others. The efficacy of thermal processes was evaluated only for the 13 PhACs with the highest concentration that were found in sludge samples from all monitored WWTPs (Table 2). The highest concentrations of PhACs were found in sludge from WWTP Trenčín and WWTP Nitra (the sum of concentrations of 13 selected PhACs were 3900 and 3500 ng/g DM, respectively), and the lowest sum of concentrations was found in sludge sample from WWTP Trnava (1800 ng/g DM). Concentrations of these 13 PhACs in individual WWTPs as well as LOQs are presented in Tables S2.
The selected PhACs were sorted into three categories, namely: psychoactive compounds (n = 6), cardiovascular pharmaceuticals (n = 3), and others (n = 4, this group includes antibiotics, antihistamines, analgesics/NSAIDs). These PhACs are often detected at higher concentration levels in sludge samples not only in Europe but all over the world [3, 5, 50, 51].
Among the PhACs that showed the highest concentrations (based on sludge dry matter) was TEL (Fig. 1), with a minimum concentration value of 1100 ng/g at the WWTP Trnava, and the maximum concentration at the WWTP Trenčín (1800 ng/g). Others included the antihistaminics FEX (minimum concentration − 120 ng/g at the WWTP Trnava and maximum at the WWTP Nitra (500 ng/g)), antidepressant SER (the lowest concentration was 69 ng/g at the WWTP Trnava and the highest concentration at the WWTP Trenčín (430 ng/g)), and CIT (minimum concentration 69 ng/g WWTP Trnava and maximum 300 ng/g WWTP Trenčín) and the NSAID DIC (the lowest concentration was found at the WWTP Banská Bystrica (41 ng/g) and the highest WWTP at the Nitra (200 ng/g)).
3.1 The influence of thermal processes on the concentration of PhACs in sewage sludge
Sludge samples from all monitored WWTPs were subjected to thermal processes in laboratory conditions. Subsequently, PhACs concentrations were evaluated for individual thermal stages (described in Chapter 2.2).
Table 3 contains the total concentrations of 13 selected PhACs in dewatered sludge and sludge after individual thermal processes. The total concentration of all selected PhACs in all sludge samples after thermal treatment at 80 °C decreased. The highest decrease was recorded at the WWTP Nitra (85%, a decrease from a value of 3500 to 510 ng/g DM), while the lowest efficiency was observed in the sludge sample from the WWTP Košice (76%, from a value of 2500 to a value of 590 ng/g DM). This temperature is a real temperature operated in many drying ovens of SS [25]. The original dewatered stabilized sludge contains about 70–80% water, the rest is organic biomass or inorganic matter. PhACs in such a matrix are freely dissolved in the aqueous phase or are physic-chemically bound to solid particles. Such high removal of the monitored PhACs during drying is surprising knowledge not yet observed in literary sources. This is probably due to a change in the structure of the substance under consideration when its state changes from a dissolved form to a solid form, while even a small structural change is evaluated as “decomposition” from a chemical-analytical point of view. Thus, in the process of drying, certain decomposition processes may occur, which will register as a decrease in the concentrations of individual monitored PhACs in the sludge. This phenomenon was also confirmed at 105 °C, when the total concentration decreased to 3000 ng/g DM (86% removal). At this temperature, the highest decrease in the total concentration of selected PhACs was detected at the WWTP DNV (91%) and the lowest decrease in the total concentration of substances (80%) at the WWTP Poprad.
Increasing the temperature to 250 °C resulted in an apparent decrease in PhAC concentrations in all tested sludges. The total concentration at all WWTPs was 35 ng/g DM, which represents almost a 100% decrease from the original dewatered sludge. At a temperature of 550 °C, the concentrations of selected compounds were below the LOQs value; therefore, we assume a complete decomposition of the PhACs at this temperature.
3.1.1 Psychoactive compounds
All substances from this group showed very similar behavior during thermal processes. The highest concentration in DSS was determined for SER—430 ng/g DM and CIT—300 ng/g DM at WWTP Trenčín.
The total concentration of psychoactive substances at all WWTPs was 5300 ng/g DM. After thermal treatment at temperatures of 80 and 105 °C, the decrease was shown to 840 ng/g DM (84%) and 650 ng/g DM (88%), respectively. However, we assume that simple processes, such as dehalogenation, occur during drying at lower temperatures. These assumptions remain hypotheses, as they are not confirmed by the literature.
The concentration of all these psychoactive drugs was below LOQs at 250 and 550 °C (Table 4). Therefore, we can assume a high efficiency of PhAC removal by thermal processes at higher temperatures. The study conducted by Moško et al. [52] examined the impact of varying pyrolysis temperatures on the elimination of organic contaminants, including PhACs, found in anaerobically stabilized SS. Sludges were tested for the presence of 27 different compounds from different therapeutic groups, but only nine of them were detected in the sludge in the concentration range from 0.1 to 50 ng/g DM. The results reveal that a pyrolysis temperature as low as 400 °C was sufficient to remove the studied pharmaceuticals.
3.1.2 Cardiovascular pharmaceuticals
Of this group of compounds, TEL was especially important. This PhAC is frequently detected in sludge matrices at relatively elevated levels [47, 51, 53]. Notably, among the 13 selected PhACs, TEL exhibited the highest concentration in the Slovak sludge samples, with a maximum concentration of 1800 ng/g DM observed at WWTP TN (Fig. 2). It is evident from the results that already at 80 °C, there is a significant decrease of TEL in all monitored sludges.
TEL was found above its LOQ even after heat treatment at 250 °C. Its concentration ranged from 1.6 (WWTP Poprad) to 9.9 ng/g DM (WWTP Nitra). After thermal treatment at the highest temperature (550 °C), TEL concentrations were below the LOQ.
Table 5 provides an overview of the total concentrations of cardiovascular PhACs, including TEL, VAL, nd VER comparing dewatered sludge to thermally treated sludge at all monitored WWTPs.
As discussed above, TEL was the dominant compound in all sludge samples. Kodešova et al. [54] investigated the effects of soil on the uptake and transfer of PhACs from sewage sludge amended soils to spinach. The results showed that TEL showed high bioaccumulation in plant roots. This fact may be the reason for classifying this substance as potentially hazardous to human health.
3.1.3 Other therapeutic groups of pharmaceuticals
This group includes PhACs such as FEX (antihistaminic), DIC (NSAID), AZI (antibiotics), and CET (antihistaminic). Of these PhACs, FEX showed the highest concentrations in each sludge sample (maximum 500 ng/g DM at WWTP Nitra, minimum 120 ng/g DM at WWTP Trnava). In a study by Ivanová et al. [3], the maximum concentration for FEX was 5600 ng/g DM, which is several times higher than the maximum concentration found in this study (Table 6). Such differences between the values can be explained by the fact that the occurrence and concentrations of PhACs in the effluent on WWTP (subsequently also in the sludge) depend not only on the properties of the PhAC compounds (e.g., log Kow value, volatility, etc.), the properties of the wastewater inflow (i.e., alkalinity and acidity), sorption of pharmaceutical substances to suspended solids and applied treatment processes [55], but also on seasonal changes in consumption of PhAC and treatment processes efficacy during the year.
The concentration of AZI in dewatered sludge was found in the range of 27–160 ng/g DM which is similar to values reported by Mejias et al. [47]. Aydin et al. [50] detected AZI in anaerobically stabilized sludge at a maximum value of 1500 ng/g DM (median 20 ng/g DM). AZI increased in thermally treated sludge at 80 °C at WWTP Poprad by up to 102% compared to the concentration in dewatered sludge (from initial 95 to 190 ng/g DM). At 105 °C, at the same WWTP, a 174% increase in the concentration of AZI (to a concentration of 260 ng/g DM) was observed in the thermally treated sludge sample.
The concentration of DIC in dewatered sludge was found in the range of 41–190 ng/g DM. These values differ from the reported ones. Mejias et al. [47] found diclofenac in anaerobically stabilized sludge in the concentration of 7000 ng/g DM. Contrary, Aydin et al. [50] found a maximum concentration of 19 ng/g DM.
At higher temperatures, these PhACs were below the LOQs. In general, it can be assumed that the strong oxidizing conditions during combustion preclude the presence of residual concentrations of PhAC in the combustion products. However, this assumption has not yet been confirmed in the literature.
4 Conclusion
Thermal processes are a promising way to handle sewage sludge produced in biological wastewater treatment plants. Their advantages include a significant reduction in sludge volume, maximum reduction of organic matter content, and sludge disinfection. Another technological advantage is the possible use of the energy content of sludge in the form of combustible gases, biochar, etc.
This work confirmed the presence of pharmaceuticals, illicit drugs, and some metabolites in anaerobically stabilized sludge from eight Slovak WWTPs. Based on the results, we concluded that thermal processes are an effective method for removing pharmaceutical substances from sludge even at lower process temperatures. Thermal processes at temperatures of 80 °C and 105 °C resulted in a significant decrease in the concentrations of the selected pharmaceuticals. At the minimum operating temperature of 80 °C, the total concentration of pharmaceuticals in all examined sludge samples decreased by nearly 81%. When compared to the total concentration in the samples that were not subjected to thermal treatment, the total concentration of pharmaceuticals reduced by 86% when the temperature increased to 105 °C. Increasing the temperature to 250 °C led to a nearly 100% decrease in concentrations, and at 550 °C, the concentrations were below the limit of quantification, indicating complete decomposition of the pharmaceuticals. Based on the physicochemical properties of pharmaceuticals, we assume that they degrade during thermal processes or that they may volatilize. What exactly happens to pharmaceuticals during thermal processes can be investigated using thermogravimetric analysis (TGA), which may be an ambition for our future study.
References
Dubey M, Mohapatra S, Tyagi VK, Suthar S, Kazmi AA (2021) Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ Pollut 273:116515. https://doi.org/10.1016/j.envpol.2021.116515
Gago-Ferrero P, Borova V, Dasenaki ME, Τhomaidis ΝS (2015) Simultaneous determination of 148 pharmaceuticals and illicit drugs in sewage sludge based on ultrasound-assisted extraction and liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 407:4287–4297. https://doi.org/10.1007/s00216-015-8540-6
Ivanová L, Mackuľak T, Grabic R, Golovko O, Koba O, Vojs Staňová A, Szabová P, Grenčíková A, Bodík I (2018) Pharmaceuticals and illicit drugs – a new threat to the application of sewage sludge in agriculture. Sci Total Environ 634:606–615. https://doi.org/10.1016/j.scitotenv.2018.04.001
Peysson W, Vulliet E (2013) Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography-time-of-flight-mass spectrometry. J Chromatogr A 1290:46–61. https://doi.org/10.1016/j.chroma.2013.03.057
Verlicchi P, Zambello E (2015) Pharmaceuticals and personal care products in untreated and treated sewage sludge: occurrence and environmental risk in the case of application on soil - a critical review. Sci Total Environ 538:750–767. https://doi.org/10.1016/j.scitotenv.2015.08.108
Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture (1986) Official Journal L 181:6–12 ELI. https://data.europa.eu/eli/dir/1986/278/oj
Dey S, Anand U, Bhattacharya S, Kumar V, Dey A (2022) Microbial community composition and functions in activated sludge treatment system. In: Kumar, V., Thakur, I.S. (eds) Omics Insights in Environmental Bioremediation. Springer, Singapore. https://doi.org/10.1007/978-981-19-4320-1_8
Campo G, Cerutti A, Lastella C, Leo A, Panepinto D, Zanetti M, Ruffino B (2021) Production and destination of sewage sludge in the Piemonte region (Italy): the results of a survey for a future sustainable management. Int J Environ Res Public Health 18(7):3556. https://doi.org/10.3390/ijerph18073556
Hudcová H, Vymazal J, Rozkošný M (2019) Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res 14(2):104–120. https://doi.org/10.17221/36/2018-SWR
Kacprzak M, Neczaj E, Fijałkowski K, Grobelak A, Grosser A, Worwag M, Rorat A, Brattebo H, Almås Å, Singh BR (2017) Sewage sludge disposal strategies for sustainable development. Environ Res 156:39–46. https://doi.org/10.1016/j.envres.2017.03.010
Eurostat (2022) Sewage sludge production and disposal from urban wastewater (in dry substance (d.s)). Available at: https://ec.europa.eu/eurostat/web/products-datasets/-/ten00030 (2022), Accessed 14th Sept 2022
Bianchini A, Bonfiglioli L, Pellegrini M, Saccani C (2016) Sewage sludge management in Europe: a critical analysis of data quality. Int J Environ Waste Manag 18:226. https://doi.org/10.1504/IJEWM.2016.080795
Durdević D, Trstenjak M, Hulenić I (2020) Sewage sludge thermal treatment technology selection by utilizing the analytical hierarchy process. Water 12(5):1255. https://doi.org/10.3390/w12051255
Grabic R, Ivanová L, Kodešová R, Grabicová K, Vojs Staňová A, Imreová Z, Drtil M, Bodík I (2022) Desorption of pharmaceuticals and illicit drugs from different stabilized sludge types across pH. Water Res 220:118651. https://doi.org/10.1016/j.watres.2022.118651
Bagheri M, Bauer T, Burgman LE, Wetterlund E (2023) Fifty years of sewage sludge management research: mapping researchers’ motivations and concerns. J Environ Manag 325:116412. https://doi.org/10.1016/j.jenvman.2022.116412
Cieślik BM, Namieśnik J, Konieczka P (2015) Review of sewage sludge management: standards, regulations and analytical methods. J Clean Prod 90:1–15. https://doi.org/10.1016/j.jclepro.2014.11.031
Cieślik B, Konieczka P (2017) A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J Clean Prod 142:1728–1740. https://doi.org/10.1016/j.jclepro.2016.11.116
Ding A, Zhang R, Ngo HH, He X, Ma J, Nan J, Li G (2021) Life cycle assessment of sewage sludge treatment and disposal based on nutrient and energy recovery: a review. Sci Total Environ 769:144451. https://doi.org/10.1016/j.scitotenv.2020.144451
Jiang G, Xu D, Hao B, Liu L, Wang S, Wu Z (2021) Thermochemical methods for the treatment of municipal sludge. J Clean Prod 311:127811. https://doi.org/10.1016/j.jclepro.2021.127811
Qian L, Wang S, Xu D, Guo Y, Tang X, Wang L (2016) Treatment of municipal sewage sludge in supercritical water: a review. Water Res 89:118–131. https://doi.org/10.1016/j.watres.2015.11.047
Syed-Hassan SSA, Wang Y, Hu S, Su S, Xiang J (2017) Thermochemical processing of sewage sludge to energy and fuel: fundamentals, challenges and considerations. Renew Sustain Energy Rev 80:888–913. https://doi.org/10.1016/j.rser.2017.05.262
Collivignarelli MC, Abbà A, Frattarola A, Miino MC, Padovani S, Katsoyiannis I, Torretta V (2019) Legislation for the reuse of biosolids on agricultural land in Europe: overview. Sustainability 11(21):6015. https://doi.org/10.3390/su11216015
Goldan E, Nedeff V, Barsan N, Culea M, Tomozei C, Panainte-Lehadus M, Mosnegutu E (2022) Evaluation of the use of sewage sludge biochar as a soil amendment—a review. Sustainability 14(9):5309. https://doi.org/10.3390/su14095309
Durdević D, Blecich P, Jurić Ž (2019) Energy recovery from sewage sludge: the case study of Croatia. Energies 12:1927. https://doi.org/10.3390/en12101927
Schnell M, Horst T, Quicker P (2020) Thermal treatment of sewage sludge in Germany: a review. J Environ Manag 263:110367. https://doi.org/10.1016/j.jenvman.2020.110367
Kubonova L, Janakova I, Malikova P, Drabinova S, Dej M, Smelik R, Skalny P, Heviankova S (2021) Evaluation of waste blends with sewage sludge as a potential material input for pyrolysis. Appl Sci 11(4):1610. https://doi.org/10.3390/app11041610
ChemSpider - chemical structure database. Royal Society of Chemistry. http://www.chemspider.com/ (accessed May 2023).
Drugbank – database for drug and drug target info. University of Alberta and The Metabolomics Innovation Centre. https://go.drugbank.com/ (accessed May 2023).
Pahnila M, Koskela A, Sulasalmi P, Fabritius T (2023) A review of pyrolysis technologies and the effect of process parameters on biocarbon properties. Energies 16(19):6936. https://doi.org/10.3390/en16196936
Al-Rumaihi A, Shahbaz M, Mckay G, Mackey H, Al-Ansari T (2022) A review of pyrolysis technologies and feedstock: a blending approach for plastic and biomass towards optimum biochar yield. Renew Sustain Energy Rev 167:112715. https://doi.org/10.1016/j.rser.2022.112715
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:1126–1140. https://doi.org/10.1016/j.rser.2015.12.185
Gao N, Kamran K, Quan C, Williams PT (2020) Thermochemical conversion of sewage sludge: a critical review. Prog Energy Combust Sci 79:100843. https://doi.org/10.1016/j.pecs.2020.100843
Atienza-Martínez M, Fonts I, Ábrego J, Ceamanos J, Gea G (2013) Sewage sludge torrefaction in a fluidized bed reactor. Chem Eng J 222:534–545. https://doi.org/10.1016/j.cej.2013.02.075
Atienza-Martínez M, Fonts I, Lázaro L, Ceamanos J, Gea G (2015) Fast pyrolysis of torrefied sewage sludge in a fluidized bed reactor. Chem Eng J 259:467–480. https://doi.org/10.1016/j.cej.2014.08.004
Murakami T, Suzuki Y, Nagasawa H, Yamamoto T, Koseki T, Hirose H, Okamoto S (2009) Combustion characteristics of sewage sludge in an incineration plant for energy recovery. Fuel Process Technol 90:778–783. https://doi.org/10.1016/j.fuproc.2009.03.003
Oladejo J, Shi K, Luo X, Yang G, Wu T (2019) A review of sludge-to-energy recovery methods. Energies 12(1):60. https://doi.org/10.3390/en12010060
Ma W, Tang Y, Wu P, Xia Y (2019) Sewage sludge incineration ash for coimmobilization of lead, zinc and copper: mechanisms of metal incorporation and competition. Waste Manag 99:102–111. https://doi.org/10.1016/j.wasman.2019.08.029
Samolada M, Zabaniotou A (2014) Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag 34:411–420. https://doi.org/10.1016/j.wasman.2013.11.003
Udayanga WC, Veksha A, Giannis A, Lisak G, Chang VWC, Lim TT (2018) Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 226:721–744. https://doi.org/10.1016/j.fuel.2018.04.045
Gil-Lalaguna N, Sánchez JL, Murillo MB, Atienza-Martínez M, Gea G (2014) Energetic assessment of air-steam gasification of sewage sludge and of the integration of sewage sludge pyrolysis and air-steam gasification of char. Energy 76:652–662. https://doi.org/10.1016/j.energy.2014.08.061
Choi YK, Mun TY, Cho MH, Kim JS (2016) Gasification of dried sewage sludge in a newly developed three-stage gasifier: effect of each reactor temperature on the producer gas composition and impurity removal. Energy 114:121–128. https://doi.org/10.1016/j.energy.2016.07.166
Chiang KY, Lu CH, Liao CK, Ger RHR (2016) Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant. Int J Hydrogen Energy 41(46):21641–21648. https://doi.org/10.1016/j.ijhydene.2016.06.199
Dogru M, Midilli A, Howarth CR (2002) Gasification of sewage sludge using a throated downdraft gasifier and uncertainty analysis. Fuel Process Technol 75:55–82. https://doi.org/10.1016/S0378-3820(01)00234-X
Câmara JS, Montesdeoca-Esponda S, Freitas J, Guedes-Alonso R, Sosa-Ferrera Z, Perestrelo R (2021) Emerging contaminants in seafront zones. Environ Impact Anal Approaches Sep 8(7):95. https://doi.org/10.3390/separations8070095
Golovko O, Koba O, Kodesova R, Fedorova G, Kumar V, Grabic R (2016) Development of fast and robust multiresidual LC-MS/MS method for determination of pharmaceuticals in soils. Environ Sci Pollut Res 23(14):14068–14077. https://doi.org/10.1007/s11356-016-6487-6
Malmborg J, Magnér J (2015) Pharmaceutical residues in sewage sludge: effect of sanitization and anaerobic digestion. J Environ Manage 153:1–10. https://doi.org/10.1016/j.jenvman.2015.01.041
Mejías C, Martín J, Santos JL, Aparicio I, Alonso E (2021) Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: a review on their distribution and environmental risk assessment. Trends Environ Anal Chem 30:e00125. https://doi.org/10.1016/j.teac.2021.e00125
Fedorova G, Randak T, Lindberg RH, Grabic R (2013) Comparison of the quantitative performance of a Q-Exactive high-resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid Commun Mass Spectrom 27(15):1751–1762. https://doi.org/10.1002/rcm.6628
Lindberg RH, Östman M, Olofsson U, Grabic R, Fick J (2014) Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res 58:221–229. https://doi.org/10.1016/j.watres.2014.03.076
Aydın S, Ulvi A, Bedük F, Aydın ME (2022) Pharmaceutical residues in digested sewage sludge: occurrence, seasonal variation and risk assessment for soil. Sci Total Environ 817:152864. https://doi.org/10.1016/j.scitotenv.2021.152864
Sellier A, Khaska S, La Salle CLG (2022) Assessment of the occurrence of 455 pharmaceutical compounds in sludge according to their physical and chemical properties: a review. J Hazard Mater 426:128104. https://doi.org/10.1016/j.jhazmat.2021.128104
Moško J, Pohořelý M, Cajthaml T, Jeremiáš M, Robles-Aguilar AA, Skoblia S, Beňo Z, Innemanová P, Linhartová L, Michalíková K, Meers E (2021) Effect of pyrolysis temperature on removal of organic pollutants present in anaerobically stabilized sewage sludge. Chemosphere 265:129082. https://doi.org/10.1016/j.chemosphere.2020.129082
Castro G, Ramil M, Cela R, Rodríguez I (2021) Identification and determination of emerging pollutants in sewage sludge driven by UPLC-QTOF-MS data mining. Sci Total Environ 778:146256. https://doi.org/10.1016/j.scitotenv.2021.146256
Kodešová R, Klement A, Golovko O, Fér M, Kočárek M, Nikodem A, Grabic R (2019) Soil influences on uptake and transfer of pharmaceuticals from sewage sludge amended soils to spinach. J Environ Manage 250:109407. https://doi.org/10.1016/j.jenvman.2019.109407
Khasawneh OFS, Palaniandy P (2021) Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf Environ Prot 150:532–556. https://doi.org/10.1016/j.psep.2021.04.045
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic This work was supported by the Slovak Research and Development Agency under the contracts No. APVV 0119–17 and APVV-22–0292, by Norway through the Norway Grants project: “Innovative carbon-based sorbents as an effective method of wastewater treatment”, grant number 3213200008, by the Ministry of Agriculture of the Czech Republic—project of National Agency for Agricultural Research (NAZV) No. QK21020080 and the project CENAKVA (LM2018099) granted by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Contributions
Dóra Varjúová: writing, methodology, visualization; Andrea Vojs Staňová: writing, formal analysis, investigation, validation; Kateřina Grabicová: writing, investigation, validation, resources; Ronald Zakhar: methodology, software; Igor Bodík: conceptualization, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varjúová, D., Staňová, A.V., Grabicová, K. et al. Thermal methods of sludge processing—are they suitable for pharmaceuticals and illicit drugs removal from sewage sludge?. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05409-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05409-4