Abstract
The present study aimed to reduce environmental pollution by using a natural and cheap adsorbent for the removal of Reactive Orange 122, an anionic dye harmful to the environment, from wastewater in the textile industry. The zeolite mineral, abundant in Turkey, easily accessible, and cost-effective, was first utilized for this purpose. However, it was concluded that sufficient adsorption was not provided in the treatment of reactive dyes due to the structure of the natural zeolite mineral. Therefore, to increase the adsorption efficiency of zeolite minerals, published techniques were utilized to modify it with chitosan, and chitosan/zeolite composite was prepared. The adsorbents were characterized by using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and Brunauer–Emmett–Teller (BET) analysis. The study also examined the influence of various parameters including pH, contact time, and temperature on the adsorption of RO122 dye. Furthermore, the adsorption equilibrium was analyzed by evaluating kinetic and isotherm models. In comparison to the pseudo first-order model, the pseudo-second-order model was determined to be the best-fitting model with a coefficient of determination of 0.999. The study also examined two isotherm models, namely Langmuir and Freundlich, to adjust the equilibrium data to achieve optimal alignment. The Freundlich model demonstrated superior efficacy as an adsorption isotherm compared to the Langmuir isotherm model, as evidenced by a coefficient of determination (R2) of 0.9941.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, water is at a critical point, because of increasing water pollution due to urbanization, population growth, people’s lack of consciousness, and changes in the global climate. This increase in water pollution is a threat to society and nature [1].
The release of chemicals used in manufactured industrial products back into nature increases water and environmental pollution. More specifically dyes used in the textile, paper, pharmaceutical, and tannery sectors significantly pollute water resources [2, 3]. Approximately 10–25% of the dyes used in the textile industry are lost. When dyes are mixed with water, they change the color of the water, and naphthalene, benzene, and other aromatic compounds found in dyes that have a carcinogenic effect appear, causing harm to humans and environmental health [4].
Worldwide, approximately 60 thousand tons of dye are discharged into the environment as waste annually, and 80% of this dye is the azo group. The wastewater discharged in the textile industry is approximately 900 million tons per year [5]. Treatment of these dyes, therefore, may reduce harm to the environment and humans. Treatment is possible biologically, physically, or chemically, each of which has different advantages and disadvantages from each other. For example, in traditional biological water treatment, the removal rate is low, while chemical and physical methods can be costly [5,6,7].
Among various water treatment techniques, the adsorption method is relatively cheap and easy. In addition to these advantages, many kinds of substances can be used as adsorbents. In recent years, alongside increasing environmental problems, a number of studies have been conducted on adsorption [8]. Activated carbon is one of the most widely used adsorbents because it has high efficiency and can also be used on many different types of dyes. However, due to the high production cost of activated carbon, its use is limited from an industrial point of view. Thus, instead of activated carbon, it is necessary to find an easily accessible adsorbent that is cheaper and also has high efficiency. Many adsorbents can be used economically, for example, peat, natural clay, fly ash, chitosan, and polymeric resins [9,10,11].
The objective of the current study was to address environmental pollution by utilizing a natural and affordable adsorbent for the removal of Reactive Orange 122, an environmentally harmful anionic dye. Zeolite mineral, abundant in Turkey and readily available at a low cost, was chosen for this purpose. However, it was determined that the natural zeolite mineral’s structure did not provide sufficient adsorption capacity for treating reactive dyes. To enhance the adsorption efficiency of zeolite minerals, it was therefore deemed necessary to modify them with chitosan. As a result, chitosan/zeolite composites were prepared and investigated in the study. The novelty of this study was the method the adjustment of pH of the solution at the final stage of the chitosan/zeolite composite was performed by using acid solution to prevent the use of excess distilled water. Adsorption tests were performed by varying three key parameters: pH, temperature, and adsorbent dosage. These parameters were investigated to evaluate their influence on the adsorption process.
2 Materials and methods
2.1 Chemicals
Natural zeolite was supplied with the support of “Tusorb” company located in Turkey. Zeolite in the form of a 40 and 100-micron powder was used in the study. Chitosan (degree of deacetylation: min 75%; low molecular weight (50.000–190.000 Da)) was supplied by AFG Scientific. The chemical structure of the Reactive Orange 122 (RO122) (C31H20ClN7Na4O16S5) is shown in Fig. 1. The dye solutions were prepared by dissolving 0.1 g RO122 powder in 1 L distilled water for use in the experiment. NaOH and HCl were used for pH adjustment.
2.2 Preparation of chitosan/zeolite composite
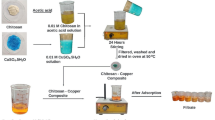
In the present study, pure zeolite and a chitosan/zeolite composite were used as adsorbents for RO122 removal. The chitosan/zeolite composites were prepared according to Lin and Zhan method as follows: 8 g of chitosan was dissolved in 400 mL of a 2 wt.% solution of acetic acid. 40 g of zeolite was dispersed in 400 mL of distilled water for 30 min. Then, the zeolite suspension was mixed with chitosan solution and stirred for 1.5 h at room temperature. After the pH of the mixture was adjusted to 9 by using 2 M sodium hydroxide solution the stirring process was over. Finally, the resulting chitosan/zeolite composite was allowed to precipitate, washed with distilled water until it reached a neutral pH, this is called DZC, and dried at 105 °C [12]. Furthermore, due to the use of excess distilled water in the pH setting of the solution, a slight modification was done, the novelty of this study, and the adjustment of pH of the solution at the final stage of the chitosan/zeolite composite was performed by using 3 M HCl, this is called HZC. After the drying process, the chitosan/zeolite composite was milled and pulverized.
2.3 Characterization of chitosan/zeolite composite
The functional group of the composites was investigated by ATR-FTIR in the region of 400–4000 cm−1 before and after adsorption at 1 cm−1. The surface morphology of the composites was analyzed using scanning electron microscopy (SEM). Brunauer–Emmett–Teller (BET) analysis theory is usually related to surface area, while the Barrett-Joyner-Halenda (BJH) model is usually used for porosity [13]. BET and BJH analyses were performed to measure BET surface area, single point total and BJH cumulative pore volume, and BET pore diameter to study the adsorption properties of zeolite, chitosan, DZC, and HZC materials. For the analysis, the samples were dried in an oven at 70 °C for 24 h and then degassed at 150 °C for 1 day. Moreover, the adsorbent’s point of zero charge (pHpzc) was assessed in 0.01 M KNO3 solutions. The pH value of solutions was adjusted within the 2–12 range using dilute solutions of 0.1 M HCl and NaOH. Subsequently, a specified quantity of the adsorbent was introduced into each 50 mL of the pH-adjusted solutions in glass reactors, and the mixture was stirred for 24 h at ambient temperature. Finally, the recorded pH values of the solutions were graphed against the initial pH to ascertain the pHpzc value [14].
2.4 Adsorption experiments
Adsorption experiments were conducted at various solution pH values, adsorbent amounts, and temperatures in batch mode. One hundred milliliters of 100 ppm RO122 solution were placed in 250 mL Erlenmeyer flask. The initial pH of the solution was adjusted to the desired value by adding either HCl or NaOH solutions. After reaching the desired temperature, required amount of the adsorbent was put into the Erlenmeyer and shaken on an orbital shaker equipped with thermostat at a speed of 200 rpm. After pre-defined time intervals, the aqueous sample was centrifuged, and the residual concentration of RO122 solution in the supernatant was measured by a UV/Vis spectrophotometer at λmax 488 nm.
The adsorption capacity for chitosan/zeolite composites at any time t (qt, mg/g) was calculated using the following equation:
where C0 and Ct are the concentrations of RO122 in aqueous solution at initial time and at any time t, respectively (mg/L); V is the volume of dye solution (L); m is the mass of adsorbent (g). The dye removal efficiency was calculated using the following equation:
Each experiment was repeated three times and the results were given as an average value within the range of ± 5% experiment reproducibility.
2.5 Adsorption kinetic and isotherm models
2.5.1 Adsorption isotherms
The investigation of established interactions between an adsorbent and a given adsorbate greatly relies on the adsorption isotherm, which effectively portrays sorption capacities as a function of the equilibrium adsorbate concentration at a constant temperature [15, 16]
In this study, adsorption data were fitted two-parameter isotherm models of Langmuir and Freundlich isotherms. The underlying assumption of the theoretical Langmuir sorption isotherm is that maximum adsorption takes place when a saturated monolayer of solute molecules exists on the surface of the adsorbent, and the energy of adsorption remains constant. The Langmuir equation can be employed to describe chemisorption. The following equation is used:
where Ce is the concentration of dye solution at equilibrium (mg /L); qe is the amount of adsorbate per unit mass of adsorbent at the equilibrium or adsorption capacity at equilibrium (mg/g); qmax is monolayer of maximal adsorption capacity (mg/g); KL is the adsorption capacity constant (L/mg) [17].
The application of the Freundlich isotherm extends to heterogeneous systems and is not limited to monolayer formation. The Freundlich equation predicts that the concentrations of dyes on the adsorbent will increase as long as there is an increase in the dye concentration in the liquid phase. Freundlich equation can be written as follows [16]:
where Kf indicates adsorption capacity (mg/g) and n is parameter related to the intensity of adsorption, which varies with the heterogeneity of adsorbent [18].
2.5.2 Adsorption kinetics
Adsorption kinetics refers to the time dependence of adsorption on solid surfaces. Kinetic models enable us to investigate the adsorption rates and time needed to reach the adsorption equilibrium [19]. The data were modeled using pseudo-first-order kinetic model and pseudo-second-order kinetic model. The pseudo-first-order model describes how the adsorption capacity of solid–liquid systems varies with time can be expressed as:
The linear form of the pseudo-first-order kinetic model by integrating the Eq. 5 is given as follows:
where qt is the amount of dye removed per unit mass of adsorbent at any time t (mg dye/g solid); k1 is pseudo-first-order adsorption rate constant (1/min); t time (min) [20].
The pseudo-second-order kinetic model, also referred to as the Ho model, postulates that the rate of adsorption is directly proportional to the square of the number of remaining free surface sites [21]:
where k2 is the second-order adsorption rate constant (g mg−1 min−1). This model can be linearized as:
3 Result and discussion
3.1 Characterization of DZC and HZC
FT-IR results of chitosan, natural zeolite, DZC, HZC, and DZC after the adsorption (DZC-2), HZC after the adsorption (HZC-2) were shown in Fig. 2. As expected, zeolite has SiO, asymmetric Si4O peak is observed at a wavelength around 1000–1100 cm−1, and symmetric Si4O between wavelengths 700 and 800 cm−1 are shown for zeolite, DZC, HZC, DZC-2, HZC-2. The density of the SiO peak varies for each substance. The SiO peak of the zeolite is the highest; there has not been much change in the SiO density between HZC and HZC-2, but there is a density difference between DZC and DZC-2. In all other substances except chitosan, O–H bending of water peaks is shown wavelength 1627 cm−1. The composite materials, which are DZC, HZC, DZC-2, and HZC-2, have a little peak at around wavelength 2875 cm−1 that represented a C-H bond that comes from the chitosan. O–H stretching peaks are at 3300–3600 cm−1 and Brønsted acid sites are shown at peaks around 3600 cm−1 [22, 23].
It is seen that the peak of the primary amine is at 3345 cm−1, which shows N–H stretching in the FT-IR spectra of chitosan. The peak 3295 cm−1 refers to the O–H stretching bond in the chitosan molecule. It shows the C-H bonds between 2840 and 3000 cm−1. Based on the graph, it appears that chitosan exhibits a distinct peak. At the same time, the chitosan/zeolite composite (DZC, DZC-2, HZC, HZC-2) also have a small peak here. As expected, there is no C-H peak in the zeolite. The presence of a secondary amide molecule is observed at peak 1640–1680 cm−1. The N–H bending is observed around 1590 cm−1. Bending of O–H bonds is observed between 1330 and 1420 cm−1, while the peak observed at 1317 cm−1 indicates the aromatic amine group. C-O stretching of primary alcohols is observed in the range between 1050 and 1085 cm−1 [22, 24, 25].
The scanning electron microscope (SEM) of chitosan, natural zeolite, DZC, HZC, and dye-adsorbed DZC (DZC-2) and HZC (HZC-2) were shown in Fig. 3. While chitosan has a kind of smooth surface, zeolite does not have a smooth surface. After the composition process, DZC and HZC have both smooth and irregular non-smooth surfaces. After the dye removal process, the surface of the DZC and HZC adsorbents has become slightly porous. The reason for the appearance of such a pore structure is due to the attachment of RO122 to the surface of the DZC and HZC. Following dye removal, the adsorbent’s micro surface changed, indicating that there had been structural modifications.
Table 1 shows that the BET surface area of zeolite, which has a porous structure, is the highest as expected, while the surface area of chitosan is quite low, 41.0525 and 0.1219 m2/g, respectively. There was not much change in the BET surface area between the composite materials DZC and HZC, and as expected, it gives a value between the surface area of zeolite and chitosan, 25.1192 and 25.5191 m2/g, respectively. Examining the volume, VSPA and VBJH, datasets reveal nominal disparities in the volumetric capacities of zeolite, DZC, and HZC. In stark contrast, the volumetric profile of chitosan markedly exhibits a significantly small magnitude. DBET pore diameters of DZC and HZC were the largest, followed by zeolite and chitosan, 9.5151, 9.5389, 7.5285, and 6.5885 nm, respectively.
The determined pHpzc value for chitosan/zeolite composite was 6.3, indicating that when the solution pH was below 6.3, the net surface charge of the adsorbent was positive. In such instances, the sorption of anionic dyes was heightened due to the electrostatic attraction between the positively charged surface of composite and the negatively charged surface of reactive dyes. Conversely, when the solution pH exceeded the pHpzc, the surface of composite became negatively charged. As a result, the adsorption capability of chitosan/zeolite composites diminished for anionic solutes [14].
3.2 Effect of contact time
Contact time is usually a factor in adsorption transformation processes. To determine the contact time of the adsorption process, adsorption experiments were performed by using natural zeolite, DZC, and HZC at pH 2 and 25 °C. Figure 4 shows the effect of contact time on the adsorption process of RO122 onto natural zeolite and chitosan/zeolite composites.
As can be seen from Fig. 4, the effect of contact time on the adsorption capacity of natural zeolite, DZC, and HZC for RO122 removal was studied between 5 and 120 min. The dye removal percentage was increased as the contact time increased until equilibrium. The removal percentage increased rapidly in the first 5 min and then gradually up to 60 min. This phenomenon can be attributed to the initially vacant surface sites available at the beginning of the adsorption process. As time progresses, the repulsive forces between the dye molecules adsorbed on the surface of zeolite/chitosan composites and the solution phase intensify. Consequently, it becomes increasingly challenging for the remaining vacant surface sites to be occupied, resulting in a deceleration of the adsorption rate until it eventually reaches a plateau. Therefore, the optimum contact time was found to be 60 min.
The RO122 removal percentage for natural zeolite was 20.8% after 120 min contact time (Fig. 4). That is why it is not usable for reactive dye adsorption. On the other hand, the removal percentages for DZC and HZC were found as 72.2% and 60.4%, respectively, at the end of 120 min. However, there was not much change after 60 min for the chitosan/zeolite composites as seen from Fig. 4. Therefore, it was decided to carry out the adsorption experiments by using DZC and HZC composites with the optimum contact time of 60 min.
3.3 Effect of solution pH
As is known, the pH of the dye solution plays a critical role in the adsorption process because it affects both aqueous charge distribution and the surface binding site of the sorbent. So, the effect of solution pH on the adsorption of RO122 on DZC and HZC was investigated in the pH range 2–6, while initial dye concentration (100 mg/L) and temperature (20 °C) were kept constant. The results are shown in Fig. 5. As can be seen in this figure, the adsorbed RO122 increased with increasing solution pH and reached its maximum at pH = 4 for both zeolite/chitosan composites. The observed phenomenon can likely be attributed to the electrostatic force of attraction between the zeolite/chitosan composites and dye molecules. Reactive dyes, predominantly azo-based chromophores with various reactive groups, interact with the active groups on the surface of the composites. When released into an aqueous solution, reactive dyes form colored anions. Thus, maximum removal of these dyes is achieved under acidic conditions due to the electrostatic force of attraction between the positively charged active groups on the composites’ surface and the negatively charged dye anions in the solution. At lower pH levels, an increased concentration of hydrogen ions is present in the solution, acting as bridging ligands between the dye molecules and the surface of the composites. As pH increases, the zeolite surface is more negatively charged, which results in induced electrostatic repulsion due to changed chemistry of adsorbent active group and a decline was observed in sorption process [26]. The maximum adsorption capacity of the RO122 takes place at around pH 4, which was therefore selected for all further adsorption experiments.
As can be seen from Fig. 5, the removal percentage for DZC at pH 4 is 99.5%, while at pH 6, it was 91.2%. On the other hand, the removal percentage for HZC at pH 4 is 99.5%, while at pH 6, it was 96.7%. The removal percentage of HZC is greater than the removal percentage of DZC. This is probably due to the use of HCl instead of water in the preparation of the zeolite/chitosan composite. There are more H+ ions in the HZC composite, and therefore, it adsorbs better due to the excess of the number of OH− ions, while the medium is more basic.
3.4 Effect of adsorbent amount
The effect of adsorbent amount on the removal of RO122 was evaluated by varying the amount of the zeolite/chitosan composites, which ranged from 0.1 to 1 g/100 mL. Preliminary results showed that the maximum adsorption capacity was achieved at pH 4, so the influence of adsorbent amount was tested at pH 4 and a temperature of 25 °C for 100 ppm initial RO122 concentration. The effect of adsorbent amount on the adsorption of RO122 on DZC and HZC is shown in Fig. 6.
Figure 6(a) demonstrates that the RO122 removal efficiency of DCZ increased from 50.5 to 99.5% when the amount of adsorbent was increased from 0.1 to 1 g/100 mL. Figure 6(b) demonstrated that the RO122 removal efficiency of HCZ increased from 45.6 to 99.5% when the amount of adsorbent was increased from 0.1 to 1 g/100 mL. The increase in adsorption active sites on the adsorbent surface that occurs with increased adsorbent quantity is what causes the increase in RO122 removal effectiveness. However, the RO122 adsorption capacities for DCZ and HCZ decreased with increasing adsorbent amount. The amount of RO122 adsorbed onto the unit weight of adsorbent decreases with increasing adsorbent dosage because of a split in the flux or concentration gradient between the concentration of RO122 in the solution and the concentration of RO122 on the adsorbent surface.
3.5 Effect of temperature
In this study, the effect of temperature on RO122 adsorption was investigated at temperatures of 25–35-45 °C with pH = 4 using 0.25 g of HZC adsorbent. According to the experiment results, dye removal decreased from 83.2 to 70.8% when the temperature rose from 25 to 45 °C, as shown in Fig. 7. Accordingly, it can be said that the exothermic process controlled the RO122 dye adsorption using HZC adsorbent. The reason for this behavior may be the deactivation of active sites on the composite at higher temperatures. This deactivation leads to a decrease in the sorption capacity of the composite at elevated temperatures. Kausar et al. quoted same results for direct Rose FRN dye removal using chitosan/clay composite [17].
In Fig. 8, the visuals of the RO122 dye removal with respect to the time during the adsorption processes were shown.
3.6 Adsorption isotherm
The collected data in the experiments studied under optimal parameters by adding varying quantities of adsorbent to the dye solution were used to analyze Langmuir and Freundlich isotherms. Isotherm studies were carried out by contacting the RO 122 dye solution at an initial concentration of 100 mg/L and the HZC composite at concentrations of 0.1, 0.25, 0.5, 1 g/100 mL until it reaches equilibrium at 25 °C and pH = 4. The adsorption isotherm models of HZC are shown in Fig. 9. The estimated parameters and statistical values of these models are presented in Table 2. By having a higher correlation coefficient (R2) and a lower standard error than the Langmuir isotherm in the tested range, the Freundlich isotherm model was found to be the most accurate model.
In the Freundlich isotherm model, the constant 1/n represents the sorption intensity of the adsorbent and provides insights into the mechanism of adsorption. In the present study, the 1/n value was obtained as 0.33, indicating that the isotherm is favorable for the adsorption of RO122. This result suggests that the dye is primarily adsorbed onto the heterogeneous surface of the adsorbent, and the adsorption mechanism is likely chemisorption [27].
3.7 Adsorption kinetics
Kinetic modeling studies were performed utilizing the experiments investigated under optimum parameters in order to make a kinetic evaluation of the data gained in the experiments. The mechanism of the adsorption process was studied using pseudo-first order and pseudo-second order kinetic models. The adsorption kinetic models of HZC are shown in Fig. 10. The estimated parameters and statistical data of these models were presented in Table 3. As seen from Table 3, among these models, the pseudo-second-order kinetic model was found to be the most suitable for all experimental data, with high values for the coefficient of determination and low values for the standard error. The pseudo-second-order kinetic model is an approach that considers the sorption capacity of the solid phase. It assumes that the sorption process follows a chemisorption mechanism, which involves the interaction of valence forces. This interaction occurs through the sharing or exchange of electrons between the sorbent (solid phase) and the sorbate (substance being adsorbed) [28].
4 Conclusions
Composites made of zeolites showed better benefits than raw zeolites for the adsorption of several hazardous substances from water sources. In this study, zeolite/chitosan composite, aiming to increase the adsorption capacity, was used as an adsorbent to remove RO122 textile dye. RO122 removal has been studied under various experimental conditions. The result shows that increased pH would increase the adsorption capacity, while increased adsorbent dose would decrease the adsorption capacity. The experimental data for RO122 is consistent with the pseudo-second order kinetic model. Of the adsorption isotherm models tested, the Freundlich one gave the best fit to the experimental data for RO122. Finally, according to the results, it can be concluded that zeolite/chitosan composite can be employed as a novel, effective, and alternative adsorbent for dye removal and other environmental pollutants.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Strokal M, Kroeze C (2020) Water, society, and pollution in an urbanizing world: recent developments and future challenges. Curr Opin Environ Sustain 46:11–15
Rakanović M, Vukojević A, Savanović MM, Armaković S, Pelemiš S, Živić F, Armaković SJ (2022) Zeolites as adsorbents and photocatalysts for removal of dyes from the aqueous environment. Molecules 27(19):6582
Mahmodi NM, Salehi R, Arami M, Bahrami H (2011) Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 267:64–72
Saini RD (2017) Textile organic dyes: polluting effects and elimination methods from textile wastewater. Int J Chem Eng Res 9(1):121–136
Liu Q (2020) Pollution and treatment of dye waste-water. In IOP Conf Ser: Earth and Environ Sci 514(5):052001
Gilani NS, Tilami SE, Azizi SN (2022) Adsorption properties of low-cost synthesized nanozeolite L for efficient removal of toxic methylene blue dye from aqueous solution. Acta Chim Slov 69(2):458–465
Hidayat E, Yoshino T, Yonemura S, Mitoma Y, Harada H (2022) Synthesis, adsorption isotherm and kinetic study of alkaline-treated zeolite/chitosan/fe3+ composites for nitrate removal from aqueous solution—anion and dye effects. Gels 8(12):782
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8(4):103988
Perrich JR (2018) Activated carbon adsorption for wastewater treatment. CRC Press
Ali NS, Jabbar NM, Alardhi SM, Majdi HS, Albayati TM (2022) Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: Isotherm, kinetics, and thermodynamic studies. Heliyon 8(8):e10276
Wan Ngah WS, Teong LC, Wong CS, Hanafiah MAKM (2012) Preparation and characterization of chitosan–zeolite composites. J Appl Polym Sci 125:2417–2425
Lin J, Zhan Y (2012) Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem Eng J 200:202–213
Chen Z, Qing H, Zhou K, Sun D, Wu R (2020) Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog Mater Sci 108:100618
Dehghani MH, Dehghan A, Najafpoor A (2017) Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutions: kinetics, isotherms, and process optimization. J Ind Eng Chem 51:185–195
Elmorsi TM (2011) Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J Environ Prot 2(6):817–827
Nešić AR, Veličković SJ, Antonović DG (2013) Modification of chitosan by zeolite A and adsorption of Bezactive Orange 16 from aqueous solution. Compos B Eng 53:145–151
Kausar A, Naeem K, Hussain T, Bhatti HN, Jubeen F, Nazir A, Iqbal M (2019) Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: comparison of linear and non-linear regression methods. J Market Res 8(1):1161–1174
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186(1):458–465
Chu HC, Chen KM (2002) Reuse of activated sludge biomass: I. Removal of basic dyes from wastewater by biomass. Process Bio 37:595600
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga SvenskaVetenskapsakademiens. Handlingar 24(4):1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):450–465
Jacox ME (2003) Vibrational and electronic energy levels of polyatomic transient molecules. Supplement B. J Phys Chem Ref Data 32(1):1–441
Bordiga S, Lamberti C, Bonino F, Travert A, Thibault-Starzyk F (2015) Probing zeolites by vibrational spectroscopies. Chem Soc Rev 44(20):7262–7341
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta 396(1–2):153–166
Cardenas G, Miranda SP (2004) FTIR and TGA studies of chitosan composite films. J Chil Chem Soc 49(4):291–295
Saeed M, Nadeem R, Yousaf M (2015) Removal of industrial pollutant (Reactive Orange 122 dye) using environment-friendly sorbent Trapa bispinosa’s peel and fruit. Int J Environ Sci Technol 12:1223–1234
Demirhan E (2020) Response surface methodology approach for adsorptive removal of Reactive Blue 19 onto green pea pod. Water Sci Technol 81(6):1137–1147
El-Bindary MA, El-Deen IM, Shoair AF (2019) Removal of anionic dye from aqueous solution using magnetic sodium alginate beads. J Mater Environ Sci 10(7):604–617
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Gökmen T. Şanlı: investigation, methodology, writing—original draft, writing—review and editing.Elçin Demirhan: investigation, methodology, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Şanlı, G.T., Demirhan, E. Preparation and characterization of chitosan/zeolite composites for Reactive Orange 122 dye removal from aqueous media: isotherm and kinetic studies. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05284-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05284-z