Abstract
The brewery industry generates a huge quantity of brewers’ spent grain (BSG) which can pose waste disposal and pollution problems. Anaerobic digestion of BSG, a recalcitrant lignocellulosic waste, is slow but can be enhanced by bioaugmentation, biostimulation and co-digestion to obtain a higher biogas yield. Hence, the effect of inoculum from brewery wastewater sludge (BWWS), iron (III) chloride (FeCl3) and co-digestion with poultry manure (PM) on the production of biogas from BSG was investigated. Cumulative biogas and biomethane yields of 588.19 NL/kgVS and 400.34 NLCH4/kgVS, respectively, were obtained from a slurry consisting of a blend of 60% BSG and 40% PM plus 15 mg L-1 FeCl3 in BWWS, after 30 day retention time at 37 oC. However, mono-digestion of BSG in only water yielded 402.17 NLbiogas/kgVS and 262.86 NLCH4/kgVS. The synergistic effects of inoculum from BWWS, FeCl3 and poultry manure on anaerobic digestion of BSG resulted in 46% and 52% increases in biogas and methane yields, respectively, compared with BSG mono-digestion. The biogas and biomethane production kinetics were well described by the dual pooled first order, logistic and modified Gompertz models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Brewery wastes are being explored as energy substrates with biogas potential due to rising energy prices and subsidies for renewable energy production [1,2,3,4]. Brewers' spent grain (BSG), brewery wastewater and extra yeast are examples of brewery waste [3, 5]. The yearly global generation of BSG, which accounts for up to 85% of residues in the brewing industry, is about 39 million tons of agro-industrial waste [4]. A significant approach in waste management and production of renewable energy is anaerobic digestion. This process produces biogas, a gaseous biofuel that can be used for e.g., heating, lighting, and generation of electricity, as well as digestate that can be used as organic fertilizer [6]. Anaerobic digestion provides a more effective means of utilizing organic waste than landfill disposal, whereas landfills pose a significant risk of environmental pollution [7, 8]. Hence, the production of biogas from BSG via anaerobic digestion has recently been the topic of research due to the huge quantity of BSG generated annually, its low costs, excellent nutrient content, and rising energy expenses [9,10,11,12].
Biogas production depends on the type of substrates and microbes, as well as the anaerobic digester and operating conditions, so these factors must be carefully considered during the design and operation of anaerobic digestion systems [12, 13]. The main constituents of BSG (a lignocellulosic biomass) are hemicellulose, cellulose, and lignin [2]. BSG has exhibited poor degradability when utilized as a substrate for biogas production due to the complex crystalline cellulose structure that causes a slow degradation process [7]. The poor degradability of crystalline cellulose is due to the formation of p-cresol and phenolic compounds that are toxic to microbes during the digestion of cellulose, thus inhibiting their activities and consequently impeding further degradation of cellulose by microbes and the production of biogas from BSG [13,14,15,16,17].
Various pretreatment procedures such as heat, mechanical, chemical, and enzymatic treatments are targeted at enhancing hydrolysis stages of anaerobic digestion of BSG, however, these incur additional expenses and inhibitory intermediates, for instance, phenolic compounds may be formed [8, 16, 18].
Several approaches can be employed to improve the biodegradation of substrate and enhance the yield of methane during the anaerobic digestion of organic lignocellulosic substrates. One of the methods is biostimulation, which involves supplementing organic wastes with trace metals during biogas production and maintaining process stability [13, 14, 19, 20]. Another method is the anaerobic co-digestion of organic lignocellulosic substrates; this has an advantage over anaerobic mono-digestion as it could address the majority of the flaws associated with substrate properties [8, 21]. Moreover, the bioaugmentation approach can be employed to ensure that there are sufficient anaerobic microbes in the digester for the degradation of the substrate, this is achieved by introducing microbial consortia from another source e.g., a sludge from a previously operated anaerobic biodigester [22].
Trace metals are micronutrients that enhance the growth of microbes that are involved in anaerobic digestion, they are also important co-factors of enzymes essential to anaerobic digestion [23]. Besides, they ensure the stability of the anaerobic process thus enhancing biogas production [24]. Trace metals such as nickel, cobalt and iron as well as salt additives such as FeCl3, NiCl2 and CoCl2 have been reported to stimulate anaerobic digestion of food waste and livestock manure at mesophilic conditions [13], [19]. Bougrier et al. [14] also reported that supplementing BSG with trace elements such as Ni, Mg and Fe at a low concentration in the same mesophilic condition increased methane yield. Notably, the addition of FeCl3 salt to anaerobic digesters at low concentrations has been reported to reduce hydrogen sulphide (H2S) and volatile fatty acids (VFAs) that cause inhibition in biogas production, thereby enhancing the production of biomethane [24,25,26,27]. Zhan et al. [28] observed that FeCl3 acted as an electron acceptor that sped up the biodegradability of complex organic matter and promoted the production of acetic acid from monosaccharides and amino acids.
The co-digestion of BSG with cattle manure, pig dung, sewage sludge and Jerusalem artichoke phytomass, as co-substrates, significantly enhanced degradability and biogas production [22, 29,30,31]. Goberna et al. [22] studied the anaerobic co-digestion of BSG with peach flesh wastes, juice wastes, sewage sludge and swine manure; it was found that the co-digestion of BSG coupled with anaerobic microbial activities using previously digested BSG as a source of inoculum, enhanced the yield of biomethane. Olivera et al. [32] who studied the effect of co-digestion of trub (surplus yeast) and BSG at 1:9 w/w reported an increase in cumulative methane yield compared to BSG mono-digestion. Lebiocka et al. [33] also reported an increment in biogas potential when BSG was added to sewage sludge in an anaerobic co-digestion experiment. Moreover, Mudzanani et al. [31] reported that anaerobic co-digestion of BSG with sewage sludge improved the biodegradability rate of the organic substrate thereby increasing the biogas yield by 104%. Advantages of co-digestion include access of microbes to a wider spectrum of substrates with a more balanced C/N, mitigation of inhibitory effects, supply of essential micronutrients, reduction in biogas production start-up time, a more stable process performance and enhanced biomethane production [8, 34,35,36,37,38,39,40].
The poultry industry generates a huge amount of manure that is also a substrate to produce biogas [41, 42]. There were about 21.3 billon chickens globally in 2014 that produced manure of about 0.85 million ton VS/day or 1.22 million ton TS/day [41]. Poultry manure consists of undigested protein and uric acid, so it is very rich in nitrogen and its C/N is low [43, 44]. The high nitrogen content in poultry manure leads to the generation of excess ammonia during the hydrolysis stage of the anaerobic mono-digestion of the manure [43, 45, 46]. The production of excess ammonia is a major problem affecting the mono-digestion of poultry manure. Excess ammonia is toxic to the methane-producing microbial consortia in the digester, it inhibits the anaerobic digestion and leads to unstable process, thus limiting biogas and biomethane generation from the manure and eventually causing process failure [44,45,46,47].
The potential solutions to the problem of inhibition caused by excess ammonia during the anaerobic mono-digestion of poultry manure include co-digestion with a carbon-rich or low nitrogen content substrate (high C/N substrate), ammonia removal, supplementation with trace elements, lowering of the organic loading rate e.g. via water dilution and adaptation of microbes to ammonia stress [48,49,50,51]. Hence, one way to effectively utilize poultry manure for biogas production is through co-digestion with a substrate such as BSG, a carbon rich lignocellulosic biomass that has a high C/N that would result into a nutrient balance suitable for anaerobic digestion microbes [52, 53]. Moreover, the co-digestion of BSG with poultry manure would also introduce additional consortia of microorganisms to the digester thus aiding the initial start-up stage of the substrate degradation [6].
The introduction of inoculum into the digester (bioaugmentation) is employed to ensure that the inoculum: substrate ratio is sufficient for the existence of microbe consortia at the start-up stage of the anaerobic processes necessary to shorten the retention and total reaction times, resulting in a high yield of biogas and biomethane [6]. Brewery wastewater sludge, obtained from the microbial treatment of brewery wastewater, is a good source of consortia of microbes that are readily available as inoculum for the bioaugmentation of the anaerobic digestion of BSG [54,55,56]. The utilization of brewery wastewater sludge in the anaerobic digestion of BSG will provide a means of optimal waste management and resource integration in the brewing industry.
Moreover, the synergistic effects of inoculum from brewery wastewater sludge, poultry manure application and iron (iii) chloride supplementation on the production of biogas and biomethane from the anaerobic digestion of BSG have not been previously reported. It is expected that the combined effects of biostimulation (use of trace metal element or salt additives), co-digestion and bioaugmentation (incorporation of inoculum from another source) would improve the degradation of BSG and enhance the production of methane from this substrate [22].
Hence, this study aimed to investigate the effect of inoculum (obtained from brewery wastewater sludge), co-digestion of poultry manure (an abundant agro-waste substrate) with BSG and the addition of FeCl3 on the anaerobic digestion of BSG and the yields of biogas and biomethane. In addition, the kinetics of biogas and biomethane production from the mono-digestion and co-digestion of BSG were considered. Notably, the novelty of this study is the elucidation of the combined effects of bioaugmentation, co-digestion and biostimulation on the anaerobic digestion of BSG employing inoculum from brewery wastewater sludge, poultry manure and FeCl3 additive, respectively.
2 Materials and methods
2.1 Collection of feedstock
The main substrate (Brewers’ spent grain) and the inoculum (Brewery wastewater treatment sludge) were obtained from the International Brewery Ilesha, Osun state, Nigeria. The co-substrate (poultry manure) was sourced from a poultry farm at the Agricultural Settlement, Ogbomoso, Nigeria. The trace metal that was used to enhance the anaerobic digestion of Brewers’ spent grain was from ferric chloride hexahydrate (FeCl3.6H2O, granulated, 99% purity) with an average density of 1.80 g/cm3.
2.2 Preparation of feedstock
The Brewers’ spent grain (BSG) feedstock was prepared according to Lebiocka et al. [33], the substrate was sun-dried for two days to avoid rapid degradation of the sample and was then mechanically crushed using mortar and pestle, and afterwards screened to achieve a particle size of 2 mm to ensure homogeneity. Unwanted materials (debris with feathers) were removed from the poultry manure. The BSG and poultry manure (PM) samples were stored at 4 °C until usage.
2.3 Analytical methods
The physical and chemical characteristics of the inoculum, substrate and co-substrate were determined according to AOAC (2016) standard [57]. The properties determined were total solids, volatile solids, pH, nitrogen, organic carbon, crude protein, crude lipid, cellulose, hemicellulose, phosphorus, potassium, and ash content. Total solid (TS) was measured as the amount of solid residue left after the sample had been oven dried for 24 hours at 105 °C. Organic total solid (OTS) was measured as the residue obtained from the total solid analysis subjected to temperature of 550 °C for 2 hours. The total nitrogen content of the sample was determined by the Kjeldahl procedure. Cellulose, hemicellulose and lignin contents of the sample were determined by the acid detergent fiber, neutral detergent fiber and acid detergent lignin methods.
The analysis of microbial communities in each of the feedstock (BSG, PM and brewery wastewater sludge) was carried out using the bacteria and fungi identification procedure reported by Olufemi et al. [58]. The identification of microorganism present in the feedstock was carried out before the anaerobic digestion process to determine the characteristics of microorganisms available in each feedstock.
The composition (i.e. the methane constituent) of the biogas produced was determined by a gas analyzer (Geotech, grade-300).
2.4 Batch anaerobic digestion experimental set-up and procedure
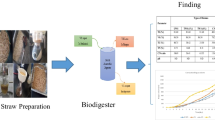
The set-up consists of digestion bottles (bioreactors) of 2.5 L capacity. A thermostatic cabinet, consisting of an installed heater and a thermocouple, was used to keep the reactors at a fixed mesophilic temperature of 37 °C. A 10 mm diameter gas tubing was fitted to each of the digestion bottles through a tightly fitting cork and then connected to a gas collector placed outside the thermostatic cabinet. The gas collector was a cylinder (1000 ml) inverted in a liquid filled graduated 2000 ml cylinder. It has a gas outlet tubing, controlled by a valve, through which the biogas can flow out for analysis.
The fermentation slurries were prepared using the substrate compositions in Table 1 and then poured into seven different digesters. The seven batch digesters were kept at 1.5 L working volume, mesophilic conditions of 37±0.5 °C and substrate-to-inoculum ratio of 1:2. The retention time was 30 days. The organic total solids (OTS) in each of the seven samples (D1 – D7) was set at 53.32 g as shown in Table 1. Batch anaerobic digestion experiments were carried out in laboratory-scale digestion bottles and repeated twice as described by Adebayo et al. [59]. The biogas produced was collected and its volume was measured daily by liquid displacement in the graduated 2000 ml cylinder, using a methyl orange-coloured acidified brine solution. The methane content of the biogas was also determined. Figure 1 shows the schematics of the experimental set-up for the batch anaerobic digestion of BSG.
2.5 Determination of standard gas yields and statistical analysis
The volume (ml) of biogas produced, ambient pressure (mbar) and ambient temperature (°C) were measured and recorded every 24 hours. The biogas yield was then determined at the standard conditions of 273.15 K and 1.01325 bar [59]. The results of the duplicate experimental sets were analyzed using mean and standard error; error bars were used to indicate the duplicate values. A one way analysis of variance was performed on the cumulative biogas and methane yields obtained from all the experimental samples, using the Statistical Package for Social Science software, to determine the existence of a statistically significant difference in the means of the measurements. Sample D1 (BSG + water) was the control experiment, so Dunnett’s method was employed to compare the means of the cumulative biogas and methane yields from samples D2 – D7 with those of the control sample D1 to determine if there were statistically significant differences between the means of the cumulative biogas and methane yields obtained from each of these samples and those of the control experiment. Besides, a two-sample t-test was separately employed to check for a significant difference between the cumulative biogas and methane yields obtained from samples D3 and D4, D5 and D6, as well as between those of samples D4 and D7.
2.6 Kinetic modelling of biogas production
The nonlinear least square regression modelling was performed using the curve fitting toolbox available in MATLAB (R2015a) to fit nonlinear equations of the dual-pooled first order, exponential rise to maximum, logistic function and modified Gompertz models to the cumulative methane and biogas yield curves [34,35,36,37,38, 35, 38, 40, 60]. The four nonlinear equations searched for the maximum cumulative biogas/methane yield (A), maximum biogas/methane production rate (Rmax) and lag phase (λ) with the main objective of minimizing the sum of squares of the difference between the predicted and experimental values.
The biogas/methane yields predicted from the nonlinear regression analysis were plotted against the biogas/methane yields obtained by experiments to determine the best fit. The coefficient of determination (R2) and root mean square error (RMSE) were obtained by MATLAB (R2015a) software during the fitting to establish the correlation of the model to the experimental data at a confidence interval of 95% for the best fit of the predicted data [60]. Four kinetics models were investigated to estimate the maximum cumulative biogas/methane yields, maximum biogas/methane production rates, lag phase times and kinetic rate constants. The details of these models are presented in Table 2.
3 Results and discussion
3.1 Feedstock characterization results
The physical and chemical properties of the feedstock (BSG, PM and BWWS) are presented in Table 3. In terms of carbon-to-nitrogen ratio, BSG has a higher C/N of 15/1 compared to 9/1 of PM, thus supplying available organic carbon that compensates for the excess nitrogen in PM [61, 62]. The percentage of lignin in BSG (15.05%) is higher than 2.51% in PM; similarly, the crude fibre, crude protein, cellulose and hemicellulose contents of BSG are higher than those of PM. In general, the cellulose, hemicellulose and lignin content of BSG are in agreement with those earlier reported by other investigators in the ranges 16.8-25.4%, 21.8-28.4% and 11.9-27.8%, respectively [63,64,65]. Similarly, the cellulose, hemicellulose and lignin contents of the PM used in this study were close to those reported by Li et al. [66] and Shen et al. [67].
Table 4 indicates that several bacteria were dominantly present in the BSG, PM and BWWS feedstock used for anaerobic digestion. Clostridium perfringens, Shigella flexneri, Bacillus sp and Pseudomonas aeruginosa were dominantly present in Brewery wastewater sludge (BWWS). Bacillus sp, Shigella flexneri and Clostridium perfringens were present in BSG while Escherichia coli, Shigella flexneri, Salmonella enterica and Bacillus sp were dominantly present in poultry manure.
The fungi identified, as shown in Table 4, included Aspergillus niger, A. fumigatus, Fusarium sp and Aspergillus flavies. Fusarium sp was present in BSG and poultry manure substrate while A. fumigatus was only present in BWWS. The highest total count for bacteria was 9.1 x 105 CFU/ml for PM, while the lowest was 1.3 x 105 CFU/ml for BSG. The highest fungal total count was 2.4 x 104 CFU/ml for PM, while the lowest was 1.4 x 103 CFU/ml for BSG.
These findings confirmed that the bioaugmentation and co-digestion of BSG with inoculum from brewery wastewater sludge and poultry manure, respectively, provided a larger consortium of microbes for the anaerobic digestion of BSG compared to the mono-digestion of BSG in only water [6, 56].
3.2 Effect of inoculum on biogas and bio-methane yield of BSG
The daily variations of the biogas and bio-methane yields obtained during 30 days of anaerobic digestion of “BSG + BWWS” and “BSG + Water” are shown in Fig. 2a and 2b, respectively. It was observed that the biogas production peak for “BSG + BWWS” was obtained as 22.0 Nml/gVS after 12 days retention time and the bio-methane yield reached a maximum of 14.8 NmlCH4/gVS after 12 days retention time. However, it was seen that the biogas yield from “BSG + Water” attained a peak of 20.9 Nml/gVS at 19-day retention time while the bio-methane yield reached a maximum of 13.8 NmlCH4/gVS after 19 days retention time. The comparison of the results shows that the BWWS provided more microbial activities to enhance biodegradation of the substrates and thus decreased the lag phase of the anaerobic digestion [6].
Plot of (a) daily biogas yield versus retention time (b) daily methane yield versus retention time (c) cumulative biogas yield versus retention time (d) cumulative methane yield versus retention time, for the anaerobic digestion of “BSG + BWWS” and “BSG + water” showing the effect of inoculum (from BWWS) on anaerobic digestion of BSG
As reported by Goberna et al. [22], the comparable finding showed that bioaugmentation of BSG with inoculum enhanced the biogas production due to the enhanced biodegrading influence provided by the additional microbial consortia. The variation in the biogas and methane yields between the experimental sample D1 (BSG + Water) and D2 (BSG + BWWS) could be a result of the higher anaerobic microbial activity in sample D2 which enhanced the degradation of the cellulose content of BSG, thus speeding up the rate of the digestion process [22].
The cumulative biogas and bio-methane yields in terms of organic dry matter (i.e. volatile solids) for anaerobic fermentation of “BSG + BWWS” and “BSG + Water” are presented in Fig. 2c and 2d, respectively. It was observed that the cumulative biogas and bio-methane yields from the anaerobic digestion of “BSG + BWWS” were 406.08 and 268 NL/kgVS, respectively, after 30 days retention time, whereas the yields from the anaerobic digestion of “BSG + Water” were 402.17 and 262.86 NL/kgVS, respectively, after 30 days retention time. The cumulative biogas and methane yields from sample D2 were 1% and 2% larger than those obtained from sample D1, respectively.
Comparable findings on the effect of inoculum on BSG anaerobic digestion have been reported by Bochmann et al. [7], Olivera et al. [32], Szaja et al. [8] and Gomez et al. [68]. Bochmann et al. [7] reported the high influence of microbial activities of inoculum on methane yield using inoculum sourced from a local wastewater treatment plant and a biogas plant fed with swine manure, maize and grass silage. They observed a high biomethane yield of 386.5 NLCH4/kgVS from BSG at 37 oC after 67 days of retention time. Notably, results obtained in this study are similar to the biomethane yield of 81.1 – 290.1 NLCH4/kgVS from BSG using inoculum obtained from anaerobic sludge blanket reactor at 31 – 59 oC and 8.3 – 19.7 g BSG L-1 reported by Gomez et al. [68]. Also, Szaja et al. [8] reported the bio-methane potential of BSG within the range of 260-390 NLCH4/kgVS. Likewise, Olivera et al. [32] obtained a methane yield of 300 L/kgVS from the digestion of BSG using inoculum sourced from a brewery wastewater treatment plant [32]. On the other hand, Ashraf et al. [54] reported a cumulated methane yield of 10.53 L CH4/kgTVS from the dry anaerobic digestion of BSG using 25% BSG, 45% inoculum and 30% water at 35 oC for 40 days. The mesophilic inoculum utilized in their experiment was obtained from an up-flow anaerobic sludge blanket reactor that treated soft drink wastewater.
However, in this study, statistical analysis revealed that the addition of inoculum from brewery wastewater sludge to the BSG slurry did not significantly (p>0.05) increased the cumulative biogas yield, suggesting that the introduction of additional consortia of bacteria alone into the BSG slurry (i.e. bioaugmentation alone) was not sufficient to significantly enhanced the anaerobic degradation of BSG and production of biogas.
3.3 Effect of co-digestion on biogas and bio-methane yield of BSG
The effect of co-digestion of BSG with PM, in the presence of inoculum from BWWS, on biogas and bio-methane yields was investigated; the daily biogas and bio-methane yields during the 30 days retention time are shown in Fig. 3a and 3b, respectively. The results indicated that the biogas yield from the experimental sample D3 (50%BSG + 50%PM + BWWS) reached a peak of 31.5 Nml/gVS after 7 days of retention time while the highest daily bio-methane yield was 21.7 NmlCH4/gVS after 7 days retention time. In the case of the experimental sample D4 (60%BSG + 40%PM + BWWS), a maximum daily biogas production of 31.0 Nml/gVS was achieved after 8 days with a corresponding maximum daily bio-methane yield of 21.5 NmlCH4/gVS. The two co-digestion experimental sets attained their biogas and bio-methane peaks at shorter retention times than those of experimental samples D1 (BSG + water) and experimental sample D2 (BSG + BWWS), which attained the maximum biogas and bio-methane yields at retention times of 21 and 12 days, respectively. It was observed that an improvement in the biogas and bio-methane yields was achieved when BSG was co-digested with PM. The additional microbial consortia activities present in the co-digestion experimental samples stimulated the formation of methane by methanogens during the anaerobic digestion.
Plot of (a) daily biogas yield versus retention time (b) daily methane yield versus retention time (c) cumulative biogas yield versus retention time (d) cumulative methane yield versus retention time, for the anaerobic digestion of “BSG + BWWS”, “BSG + water”, “50%BSG + 50%PM + BWWS” and “60%BSG + 40%PM + BWWS”, showing the influence of co-digestion of BSG with PM on biogas and bio-methane yields
The cumulative biogas and bio-methane yields for the co-digestion experimental samples are compared with those of the mono-digestion in Fig. 3c and 3d, respectively. The cumulative biogas yields from the anaerobic digestion of samples D3 and D4 were 544.82 and 568.61 NL/kgVS, respectively, while the corresponding bio-methane yields were 371.14 and 389.96 NLCH4/kgVS, respectively. The percentage increases in biogas and methane yields from samples D3 compared to the mono-digestion sample D1 were 35% and 41%, respectively, while those from the second co-digestion sample D4 were 41% and 52%, respectively. The cumulative biogas and methane yields from the anaerobic co-digestion samples D3 and D4 were significantly (p<0.05) higher than the yields from the mono-digestion sample D1. This suggests a higher rate of biodegradation of BSG through the additional influence of the microbial consortia of the PM and inoculum from BWWS. Moreover, the mixture of BSG (C/N of 15) and PM (C/N of 9) in samples D3 and D4 ensured a nutrient balance by maintaining the C/N level of the slurry in the digester at optimum levels. This nutrient balance promoted the growth and activities of anaerobic microbes in these experimental samples. Matheri et al. [62] have reported that optimum C/N ranges from 15-30:1 and that microbes need a C/N of 20 – 30:1 for efficient anaerobic digestion in terms of biogas and bio-methane yields. Olivera et al. [32] who investigated the effect of co-digestion of trub (surplus yeast) and BSG at 1:9 w/w reported an increase in cumulative methane yield compared to BSG mono-digestion. Also, Szaja et al. [8] obtained mean biomethane yields of 270 and 210 NLCH4/kgVS from the co-digestion of BSG and sewage sludge at 35 oC after 18 and 20 days of retention time, respectively. Furthermore, Lebiocka et al. [33] also observed an enhanced biogas yield from the anaerobic co-digestion of BSG with sewage sludge.
It was observed that the biogas and methane yields from the co-digestion sample D4 (60%BSG + 40%PM + BWWS) were significantly (p<0.05) higher than the yields from co-digestion sample D3 (50%BSG + 50%PM + BWWS). This could possibly suggest that a better nutrient balance that was more suitable for the anaerobic microbes was achieved in sample D4 compared to sample D3 [53].
3.4 Effect of FeCl3 additives on biogas and bio-methane yield of BSG
The performance of the anaerobic digestion of “BSG + BWWS” treated with FeCl3 additives was investigated; the daily biogas and bio-methane yields obtained are presented in Fig. 4a and 4b, respectively. It was noticed that adding 10 mg/L FeCl3 to “BSG + BWWS” (sample D5) reduced the lag phase of the biogas and bio-methane production compared to the other experiments “BSG + Water” (sample D1) and “BSG + BWWS” (sample D2) that took more days to achieve the maximum daily biogas and bio-methane yields. Similarly, a positive influence was seen when 15 mg/L of FeCl3 was added to the “BSG + BWWS” slurry where biogas and bio-methane production peak increased up to 29.7 and 19.5 Nml/gVS after 4 days of retention, respectively.
Plot of (a) daily biogas yield versus retention time (b) daily methane yield versus retention time (c) cumulative biogas yield versus retention time (d) cumulative methane yield versus retention time, for the anaerobic digestion of “BSG + Water”, “BSG + BWWS”, “BSG + BWWS+10 mg/L FeCl3” and “BSG + BWWS+15 mg/L FeCl3”, showing the effect of FeCl3 additive on anaerobic digestion of BSG
It was observed that the onset of the production of biogas was improved when the “BSG + BWWS” sample was treated with 10 and 15 mg/L of FeCl3 additives in comparison with the “BSG + BWWS” only sample. Based on the addition of 10 mg/L FeCl3, the biogas yield attained 39 % of the maximum value after four days of retention time and with the addition of 15 mg/L FeCl3 at the same retention time attained 43% of the maximum value. The addition of FeCl3 to the anaerobic digestion slurry enhanced biogas yield by improving the growth of microorganisms at the selected concentration and also stimulated methanogens for the production of methane from the anaerobic digestion of BSG. Abdelsalam et al. [19] have indicated in their study that the addition of 5, 10 and 20 mg/L FeCl3 to cow dung slurry decreased the lag phase and increased the biogas yield after the first day of retention, the biogas yield increased by 1.04, 1.03 and 1.18 times above the control (cow dung slurry only), respectively.
Detailed illustrations of the cumulative biogas and methane production are presented in Fig. 4c and 4d, respectively. It was observed that the addition of 10 and 15 mg/L FeCl3 to the anaerobic digestion of BSG increased the cumulative biogas yield to 483.72 and 501.14 NL/kgVS, respectively, which were 1.19 and 1.23 times the biogas yields from the “BSG + BWWS” slurry, respectively. Likewise, the additions of 10 and 15 mg/L FeCl3 caused the cumulative methane yields to reach 333.54 and 347.66 NLCH4/kgVS, respectively, which were 1.24 and 1.29 times the methane yields from the “BSG + BWWS” slurry, respectively. Moreover, the cumulative biogas and methane yields from samples D5 and D6 containing 10 and 15 mg/L FeCl3 additives were significantly (p<0.05) higher than those from the BSG mono-digestion sample D1. The cumulative biogas and methane yields from sample D5 were 20% and 27% higher than those of sample D1, respectively, while the yields from sample D6 were 25% and 32% higher than those of sample D1, respectively. The introduction of additional micronutrient via FeCl3 additive to the fermentation slurry appreciably stimulated the anaerobic microbes to degrade the substrate and produce biogas. A statistical comparison of samples D5 and D6 revealed that the cumulative biogas and methane yields from samples D6 were significantly (p<0.05) higher than those obtained from sample D5.
Enhancement effect of FeCl3 supplementation on biogas production and methane yield from anaerobic digestion of substrates similar to that seen in this study have been reported. In a study by Yu et al. [25], the addition of FeCl3 to the substrate of waste-activated sludge increased biogas yield by 0.46, 0.83, 1.46 and 1.25 at 0, 36, 72, 108, and 144 hours dosing time, respectively, after 43 days retention. Also, in a separate report by Yu et al. [26], the addition of FeCl3 to the anaerobic digestion of waste-activated sludge resulted in a cumulative methane yield that was 2.93 times the cumulative methane yield from the anaerobic digestion of waste-activated sludge without the FeCl3 additive. Likewise, in another study, Abdelsalam et al. [19] reported the influence of FeCl3 on the anaerobic digestion of cow dung slurry as substrate; it was found that the addition of FeCl3 resulted in a cumulative methane yield that was 1.82 times the yield from the slurry without FeCl3.
3.5 Combined effects of inoculum, co-digestion and FeCl3 on biogas and bio-methane yields of BSG
The daily biogas and bio-methane yields obtained when 15 mg/L FeCl3 was added to the “60%BSG + 40%PM + BWWS” slurry are shown in Fig. 5a and 5b, respectively. The highest daily biogas and bio-methane yields of 35.5 Nml/gVS and 23.9 NmlCH4/gVS, respectively, were obtained after 7 days of retention time. The FeCl3 additive aided the growth of microorganisms resulting in an improved rate of biogas production. Moreover, FeCl3 also stimulated methanogens bacteria in the co-digestion feedstock resulting in a noticeable increase in methane yield as well as improved process stability.
Plot of (a) daily biogas yield versus retention time (b) daily methane yield versus retention time (c) cumulative biogas yield versus retention time (d) cumulative methane yield versus retention time, for the anaerobic digestion of “60%BSG + 40%PM + BWWS” and “60%BSG + 40%PM + BWWS+15 mg/L FeCl3”, showing the effect of FeCl3 additive on the co-digestion of BSG and PM
The cumulative biogas and bio-methane yields gotten from the co-digestion experiments without the addition of FeCl3 are compared with those obtained from the co-digestion experiments involving the addition of FeCl3 as shown in Fig. 5c and 5d. The addition of FeCl3 at 15 mg/L to the co-digestion feedstock significantly (p<0.05) increased the biogas yield; the yield from the feedstock containing FeCl3 was 588.19 NL/kgVS compared to that of 568.61 NL/kgVS from the co-digestion without FeCl3. Similarly, there was a significant (p<0.05) increment in methane yield from 389.96 NLCH4/kgVS to 400.34 NLCH4/kgVS. In a previous study, Zhan et al. [38] observed that the addition of FeCl3 enhanced methane yield from waste-activated sludge by 114.7 – 197.2%, using a FeCl3 concentration of up to 234 mg/L. These results indicate that FeCl3 plays a vital role in promoting the microbial consortia for higher biogas production.
The daily biogas and biomethane yields as well as the cumulative biogas and biomethane yields obtained during the anaerobic digestion of the “BSG+Water” sample were compared with those obtained during the digestion of the “60%BSG+40%PM+BWWS+15 mg/L FeCl3” sample as shown in Fig. 6. This figure shows the synergistic effects of adding inoculum from brewery wastewater sludge and FeCl3 to BSG and its co-digestion with poultry manure on the anaerobic digestion of BSG. It is apparent that bioaugmentation using brewery wastewater sludge, biostimulation using FeCl3 and co-digestion with poultry manure considerably enhanced the anaerobic digestion of the brewers’ spent grain. Maximum daily biogas and biomethane yields of 35.5 Nml/gVS and 23.7 NmlCH4/gVS, respectively were achieved after 7 days by the “60%BSG+40%PM+BWWS+15 mg/L FeCl3” slurry compared to 20.9 Nml/gVS and 13.8 NmlCH4/gVS, respectively, reached by the “BSG+Water” slurry after 19 days.
Plot of (a) daily biogas yield versus retention time (b) daily methane yield versus retention time (c) cumulative biogas yield versus retention time (d) cumulative methane yield versus retention time, for the anaerobic digestion of “BSG + Water” and “60%BSG + 40%PM + BWWS+15 mg/L FeCl3”, showing the combined effect of inoculum, co-digestion and FeCl3 additive on anaerobic digestion of BSG
Similarly, the cumulative biogas and biomethane yields of 588.19 NL/kgVS and 400.34 NLCH4/kgVS, respectively, achieved by the “60%BSG+40%PM+BWWS+15 mg/L FeCl3” sample were significantly (p<0.05) higher than those of 402.17 NL/kgVS and 262.86 NLCH4/kgVS, respectively, gotten from the “BSG+Water” sample. The 46% and 52% increases in biogas and methane yields, respectively, were due to the enhancement of the anaerobic digestion of BSG by the addition of inoculum from brewery wastewater sludge and FeCl3 to BSG as well as its co-digestion with poultry manure. It can be deduced that the nutrients of this experimental sample promoted the growth and activities of microbes involved in the anaerobic digestion.
3.6 Kinetic study
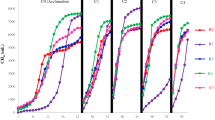
The estimated kinetic model parameters as well as the statistical indicators (R2 and RMSE) obtained when the exponential rise to maximum, modified Gompertz, Logistic and dual pooled first order models were fitted to the experimental cumulative biogas yield data are shown in Table 5 while the parameters and statistical indicators obtained when the experimental biomethane yield data were fitted to the kinetic models are shown in Table 6. To illustrate the fitness of the model, the cumulative biomethane and biogas yields predicted by the four models were also plotted together with the experimental values as depicted in Fig. 7.
Comparison of measured and predicted cumulative yields (a) Exponential rise to maximum model for biogas (b) Exponential rise to maximum model for methane (c) Modified Gompertz model for biogas (d) Modified Gompertz model for methane (e) Logistic model for biogas (f) Logistic model for methane (g) Dual pooled first order model for biogas (h) Dual pooled first order model for methane
The R2 and RMSE values obtained when experimental biogas yields were fitted to the exponential rise to maximum model were 0.9585 – 0.9847 and 21.2166 - 32.1564, respectively, while those obtained when the measured biomethane data were fitted to the model were 0.9547 – 0.9837 and 15.3153 - 22.8621, respectively. The R2 values obtained when the experimental biogas and biomethane yields were fitted to the exponential rise to maximum model (0.9585 – 0.9847 and 0.9547 – 0.9837) were lesser than those gotten when the modified Gompertz (0.9890-0.9973 and 0.9964-0.9991), Logistic (0.9873-0.9957 and 0.9919 – 0.9956) and dual pooled first order (0.9940 – 0.9991 and 0.9940 – 0.9991) models were fitted to the measured data.
Also, the RMSE (21.2166 - 32.1564 and 15.3153 - 22.8621) obtained when the exponential rise to maximum model was fitted to the measured biogas and biomethane yields were larger than those obtained when the modified Gompertz (8.78818 – 15.2567 and 4.1455 – 6.2817), Logistic (10.3458 – 22.5734 and 6.7474 – 11.7560) and dual pooled first order (6.0801 – 12.1578 and 4.2419 – 8.0591) models were fitted to the measured data. This implies that the exponential rise to maximum model did not fit the measured biogas and bio-methane yields well compared to the other three models. Furthermore, Fig. 7a and 7b confirm that the exponential rise to maximum kinetic model did not well fit the experimental biogas and biomethane yields data compared to the other three models.
The dual pooled first order, Logistic and modified Gompertz models well fitted the experimental cumulative biogas and biomethane yields as shown in Fig. 7c, d, e, f, g and h, so they can be suitably employed to predict the biogas or biomethane yield from the anaerobic digestion of BSG. However, among the three models, the modified Gompertz best described the measured biogas yields from samples D1 and D2, possessing the highest R2 and lowest RMSE values as depicted in Table 5. Similarly, the modified Gompertz model best described the biomethane yields data obtained from samples D1, D2, D3 and D7 as shown by its highest R2 and lowest RMSE values in Table 6. On the other hand, the dual pooled first order model best described the biogas yields data obtained from samples D3-D7 having the highest R2 and lowest RMSE values as depicted in Table 5 while this model best described the biomethane yields data obtained from samples D4, D5 and D7, possessing the highest R2 and lowest RMSE values as shown in Table 6.
The basis for anaerobic digestion kinetics models is the dependence of microbial substrate consumption and growth rates on the concentration of the substrate that limits their development [35]. In previous studies on the kinetics of biomethane production, the production of methane during batch anaerobic digestion has also been described using a few kinetic models including Gompertz, modified Gompertz, dual pooled first order and logistic function [35, 37, 38]. Ashraf et al. [54] reported that the modified Gompertz gave a good fit to the production of methane by the dry anaerobic digestion of BSG. Dennehy et al. [40] found that a dual pooled first order model provided the best fit for the measured biomethane production data, while Aworanti et al. [69] observed that the modified Gompertz model best predicted the cumulative methane yield. Moreover, Zhang et al. [38] and Lim et al. [70] discovered that the modified Gompertz model was the best kinetic model for forecasting methane yield when compared with other kinetic models.
4 Conclusions
The addition of inoculum from brewery wastewater sludge, poultry manure and iron (iii) chloride to brewers’ spent grain in an anaerobic digestion process enhanced the production of biogas and methane yield. The synergistic effects of the addition of brewery wastewater sludge (inoculum) and 15 mg L-1 FeCl3 to a blend of brewers’ spent grain (60%) and poultry manure (40%) in an anaerobic co-digestion process resulted in enhanced cumulative biogas and biomethane yields of 588.19 NL/kgVS and 400.34 NLCH4/kgVS, respectively, after 30-day retention time at 37 °C. Therefore, the anaerobic digestion of brewers’ spent grain with bioaugmentation using inoculum from brewery wastewater sludge, biostimulation by FeCl3 additive and co-digestion with poultry manure can contribute to sustainable waste management and effective bioenergy production in the brewery industry. The dual pooled first order, logistic and modified Gompertz models well described the kinetics of biogas and biomethane production from the anaerobic mono-digestion and co-digestion of brewers’ spent grain.
Data Availability
The data that supports the findings of this study are available within the article.
References
Zupančič GD, Panjičko M, Zelić B (2017) Biogas production from brewer’s yeast using an anaerobic sequencing batch reactor. Food Technol Biotechnol 55(2):187–196. https://doi.org/10.17113/tb.55.02.17.5080
Bachmann SAL, Calvete T, Féris LA (2022) Potential applications of brewery spent grain: Critical an overview. J Environ Chem Eng 10(1):106951. https://doi.org/10.1016/j.jece.2021.106951
Emmanuel JK, Nganyira PD, Shao GN (2022) Evaluating the potential applications of brewers’ spent grain in biogas generation, food and biotechnology industry: A review. Heliyon 8(10):e11140. https://doi.org/10.1016/j.heliyon.2022.e11140
Lisci S, Tronci S, Grosso M, Karring H, Hajrizaj R, Errico M (2022) Brewer’s Spent Grain: its Value as Renewable Biomass and its Possible Applications. Chem Eng Trans 92:259–264. https://doi.org/10.3303/CET2292044
Akunna JC (2015) “Anaerobic treatment of brewery wastes,” in Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste, pp 407–424. https://doi.org/10.1016/B978-1-78242-331-7.00019-8
Aworanti OA et al (2023) Enhancing and upgrading biogas and biomethane production in anaerobic digestion: a comprehensive review. Front Energy Res 11. https://doi.org/10.3389/fenrg.2023.1170133
Bochmann G, Drosg B, Fuchs W (2015) Anaerobic digestion of thermal pretreated brewers’ spent grains. Environ Prog Sustain Energy 34(4):1092–1096. https://doi.org/10.1002/ep.12110
Szaja A, Montusiewicz A, Lebiocka M, Bis M (2020) The effect of brewery spent grain application on biogas yields and kinetics in co-digestion with sewage sludge. PeerJ 8:e10590. https://doi.org/10.7717/peerj.10590
Panjičko M, Zupančič GD, Zelić B (2015) Anaerobic biodegradation of raw and pre-treated brewery spent grain utilizing solid state anaerobic digestion. Acta Chim Slov 62(4):818–827. https://doi.org/10.17344/acsi.2015.1534
Puligundla P, Mok C (2021) Recent advances in biotechnological valorization of brewers’ spent grain. Food Sci Biotechnol 30(3):341–353. https://doi.org/10.1007/s10068-021-00900-4
Lech M, Labus K (2022) The methods of brewers’ spent grain treatment towards the recovery of valuable ingredients contained therein and comprehensive management of its residues. Chem Eng Res Des 183:494–511. https://doi.org/10.1016/j.cherd.2022.05.032
Pabbathi NPP et al (2022) Brewer’s spent grains-based biorefineries: A critical review. Fuel 317:123435. https://doi.org/10.1016/j.fuel.2022.123435
Qiang H, Lang DL, Li YY (2012) High-solid mesophilic methane fermentation of food waste with an emphasis on Iron, Cobalt, and Nickel requirements. Bioresour Technol 103(1):21–27. https://doi.org/10.1016/j.biortech.2011.09.036
Bougrier C, Dognin D, Laroche C, Gonzalez V, Benali-Raclot D, Cacho Rivero JA (2018) Anaerobic digestion of Brewery Spent Grains: Trace elements addition requirement. Bioresour Technol 247:1193–1196. https://doi.org/10.1016/j.biortech.2017.08.211
Čater M, Fanedl L, Malovrh Š, Marinšek Logar R (2015) Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour Technol 186. https://doi.org/10.1016/j.biortech.2015.03.029
Sežun M, Grilc V, Zupančič GD, Logar RM (2011) Anaerobic digestion of brewery spent grain in a semi-continuous bioreactor: Inhibition by phenolic degradation products. Acta Chim Slov 58(1):158–166
Panjičko M, Zupančič GD, Fanedl L, Logar RM, Tišma M, Zelić B (2017) Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J Clean Prod 166. https://doi.org/10.1016/j.jclepro.2017.07.197
Kainthola J, Shariq M, Kalamdhad AS, Goud VV (2019) Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J Environ Manage 232:188–196. https://doi.org/10.1016/j.jenvman.2018.11.052
Abdelsalam E, Samer M, Abdel-Hadi MA, Hassan HE, Badr Y (2015) Effect of CoCl2, NiCl2 and FeCl3 additives on biogas and methane production. Misr J Agric Eng 32(2):843–862. https://doi.org/10.21608/mjae.2015.98656
González-Suárez A, Pereda-Reyes I, Oliva-Merencio D, Montalvo-Martínez SJ (2020) Speciation of iron, nickel and cobalt in the anaerobic biodegradation of rice straw. Rev Fac Ing Univ Antioq 101:53–63. https://doi.org/10.17533/udea.redin.20200366
Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sustain Energy Rev 36:412–427. https://doi.org/10.1016/j.rser.2014.04.039
Goberna M, Del Mar Camacho M, Lopez-Abadia JA, García C (2013) Co-digestion, biostimulation and bioaugmentation to enhance methanation of brewer’s spent grain. Waste Mgmt Res 31(8):805–810. https://doi.org/10.1177/0734242X13497078
Zhang Y, Feng Y, Yu Q, Xu Z, Quan X (2014) Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Bioresour Technol 159:297–304. https://doi.org/10.1016/j.biortech.2014.02.114
Yu B et al (2015) Variations of organic matters and microbial community in thermophilic anaerobic digestion of waste activated sludge with the addition of ferric salts. Bioresour Technol 179:291–298. https://doi.org/10.1016/j.biortech.2014.12.011
Yu B et al (2015) Dosing time of ferric chloride to disinhibit the excessive volatile fatty acids in sludge thermophilic anaerobic digestion system. Bioresour Technol 189:154–161. https://doi.org/10.1016/j.biortech.2015.03.144
Yu B et al (2015) Methane-rich biogas production from waste-activated sludge with the addition of ferric chloride under a thermophilic anaerobic digestion system. RSC Adv 5(48):38538–38546. https://doi.org/10.1039/c5ra02362a
Yu B, Zhang D, Dai X, Lou Z, Yuan H, Zhu N (2016) The synthetic effect on volatile fatty acid disinhibition and methane production enhancement by dosing FeCl3 in a sludge thermophilic anaerobic digestion system. RSC Adv 6(25):21090–21098. https://doi.org/10.1039/c5ra26245c
Zhan W et al (2021) Mechanistic insights into the roles of ferric chloride on methane production in anaerobic digestion of waste activated sludge. J Clean Prod 296:126527. https://doi.org/10.1016/j.jclepro.2021.126527
Tewelde S, Eyalarasan K, Radhamani R, K. Kaliyaperumal Ph.D. D.Sc (2012) Biogas Production from Co-digestion of Brewery Wastes [BW] and Cattle Dung [CD]. Int J Latest Trends Agric Food Sci 2
Malakhova DV, Egorova MA, Prokudina LI, Netrusov AI, Tsavkelova EA (2015) The biotransformation of brewer’s spent grain into biogas by anaerobic microbial communities. World J Microbiol Biotechnol 31(12):2015–2023. https://doi.org/10.1007/s11274-015-1951-x
Mudzanani K, van Heerden E, Mbhele R, Daramola MO (2021) Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community. Sustainability 13(15):8225. https://doi.org/10.3390/su13158225
Oliveira JV, Alves MM, Costa JC (2018) Biochemical methane potential of brewery by-products. Clean Technol Environ Policy 20(2):435–440. https://doi.org/10.1007/s10098-017-1482-2
Lebiocka M, Montusiewicz A, Szaja A, Rembisz S, Nowakowska E (2019) Thermophilic co-digestion of sewage sludge and brewery spent grain. J Ecol Eng 20(10):118–124. https://doi.org/10.12911/22998993/113139
Latinwo GK, Agarry SE (2015) Modelling the kinetics of biogas production from mesophilic anaerobic co-digestion of cow dung with plantain peels. Int J Renew Energy Dev 4(1):55–63. https://doi.org/10.14710/ijred.4.1.55-63
Xie S et al (2016) Anaerobic co-digestion: A critical review of mathematical modelling for performance optimization. Bioresource Technol 222:498–512. https://doi.org/10.1016/j.biortech.2016.10.015
Aworanti OA, Agarry SE, Ogunleye OO (2017) Biomethanization of Cattle Manure, Pig Manure and Poultry Manure Mixture in Co-digestion with Waste of Pineapple Fruit and Content of Chicken-Gizzard- Part I: Kinetic and Thermodynamic Modelling Studies. Open Biotechnol J 11(1). https://doi.org/10.2174/1874070701711010036
Montecchio D et al (2019) Anaerobic co-digestion of food waste and waste activated sludge: ADM1 modelling and microbial analysis to gain insights into the two substrates’ synergistic effects. Waste Mgmt 97:27–37. https://doi.org/10.1016/j.wasman.2019.07.036
Zhang H, An D, Cao Y, Tian Y, He J (2021) Modeling the Methane Production Kinetics of Anaerobic Co-Digestion of Agricultural Wastes Using Sigmoidal Functions. Energies (Basel) 14(2):258. https://doi.org/10.3390/en14020258
Tabatabaei M et al (2020) A comprehensive review on recent biological innovations to improve biogas production, Part 2: Mainstream and downstream strategies. Renew Energy 146:1392–1407. https://doi.org/10.1016/j.renene.2019.07.047
Dennehy C et al (2016) Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Mgmt 56:173–180. https://doi.org/10.1016/j.wasman.2016.06.032
Chávez-Fuentes JJ, Capobianco A, Barbušová J, Hutňan M (2017) Manure from Our Agricultural Animals: A Quantitative and Qualitative Analysis Focused on Biogas Production. Waste Biomass Valori 8(5). https://doi.org/10.1007/s12649-017-9970-5
Aworanti OA et al (2023) Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables. Energies 16(8). https://doi.org/10.3390/en16083378
Bayrakdar A, Sürmeli RÖ, Çalli B (2017) Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour Technol 244. https://doi.org/10.1016/j.biortech.2017.08.047
Ma J et al (2018) Enhancing performance and stability of anaerobic digestion of chicken manure using thermally modified bentonite. J Clean Prod 183. https://doi.org/10.1016/j.jclepro.2018.02.121
Niu Q, Qiao W, Qiang H, Hojo T, Li YY (2013) Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour Technol 137. https://doi.org/10.1016/j.biortech.2013.03.080
Bi S et al (2020) Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew Energy 146. https://doi.org/10.1016/j.renene.2019.08.093
Belostotskiy DE, Ziganshina EE, Siniagina M, Boulygina EA, Miluykov VA, Ziganshin AM (2015) Impact of the substrate loading regime and phosphoric acid supplementation on performance of biogas reactors and microbial community dynamics during anaerobic digestion of chicken wastes. Bioresour Technol 193. https://doi.org/10.1016/j.biortech.2015.06.066
Wang F, Pei M, Qiu L, Yao Y, Zhang C, Qiang H (2019) Performance of anaerobic digestion of chicken manure under gradually elevated organic loading rates. Int J Environ Res Public Health 16(12). https://doi.org/10.3390/ijerph16122239
Mahdy A, Bi S, Song Y, Qiao W, Dong R (2020) Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour Technol Rep 9. https://doi.org/10.1016/j.biteb.2019.100359
Shapovalov Y, Zhadan S, Bochmann G, Salyuk A, Nykyforov V (2020) Dry anaerobic digestion of chicken manure: A review. Appl Sci (Switzerland) 10(21). https://doi.org/10.3390/app10217825
Song Y, Qiao W, Zhang J, Dong R (2023) Process Performance and Functional Microbial Community in the Anaerobic Digestion of Chicken Manure: A Review. Energies (Basel) 16(12). https://doi.org/10.3390/en16124675
Bayrakdar A, Molaey R, Sürmeli RÖ, Sahinkaya E, Çalli B (2017) Biogas production from chicken manure: Co-digestion with spent poppy straw. Int Biodeterior Biodegr 119. https://doi.org/10.1016/j.ibiod.2016.10.058
Hassan M, Umar M, Ding W, Mehryar E, Zhao C (2017) Methane enhancement through co-digestion of chicken manure and oxidative cleaved wheat straw: Stability performance and kinetic modeling perspectives. Energy 141. https://doi.org/10.1016/j.energy.2017.11.110
Ashraf A, Ramamurthy R, Rene ER (2021) Wastewater treatment and resource recovery technologies in the brewery industry: Current trends and emerging practices. Sustain Energy Technol Assess 47. https://doi.org/10.1016/j.seta.2021.101432
Simate GS et al (2011) The treatment of brewery wastewater for reuse: State of the art. Desalination 273(2–3). https://doi.org/10.1016/j.desal.2011.02.035
Liu WT, Chan OC, Fang HHP (2002) Characterization of microbial community in granular sludge treating brewery wastewater. Water Res 36(7). https://doi.org/10.1016/S0043-1354(01)00377-3
AOAC (2016) The Association of Official Analytical Chemists International. Off Methods Anal 38(8)
Olufemi FE, Veronica D, Godwin H (2019) Effect of Anaerobic Co-Digestion on Microbial Community and Biogas Production. Biosci Biotechnol Res Asia 16(2):391–401. https://doi.org/10.13005/bbra/2754
Adebayo AO, Jekayinfa SO, Linke B (2015) Anaerobic Digestion Of Selected Animal Wastes For Biogas Production In A Fed-Batch Reactor At Mesophilic Temperature. J Multidiscip Eng Sci Technol 2(7):1875–1880
Tian Y et al (2020) Modelling Biogas Production Kinetics of Various Heavy Metals Exposed Anaerobic Fermentation Process Using Sigmoidal Growth Functions. Waste Biomass Valori 11(9):4837–4848. https://doi.org/10.1007/s12649-019-00810-x
Kigozi R, Aboyade AO, Muzenda E (2014) Sizing of an anaerobic biodigester for the organic fraction of municipal solid waste. Lecture Notes in Engineering and Computer Science, pp 659–663
Matheri AN, Ndiweni SN, Belaid M, Muzenda E, Hubert R (2017) Optimising biogas production from anaerobic co-digestion of chicken manure and organic fraction of municipal solid waste. Renew Sustain Energy Rev 80:756–764. https://doi.org/10.1016/j.rser.2017.05.068
Meneses NGT, Martins S, Teixeira JA, Mussatto SI (2013) Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep Purif Technol 108:152–158. https://doi.org/10.1016/j.seppur.2013.02.015
Bianco A, Budroni M, Zara S, Mannazzu I, Fancello F, Zara G (2020) The role of microorganisms on biotransformation of brewers’ spent grain. Appl Microbiol Biotechnol 104(20):8661–8678. https://doi.org/10.1007/s00253-020-10843-1
Marcus A, Fox G (2021) Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 10(9):2159. https://doi.org/10.3390/foods10092159
Li Y, Zhang R, Chen C, Liu G, He Y, Liu X (2013) Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour Technol 149:406–412. https://doi.org/10.1016/j.biortech.2013.09.091
Shen J, Zhao C, Liu Y, Zhang R, Liu G, Chen C (2019) Biogas production from anaerobic co-digestion of durian shell with chicken, dairy, and pig manures. Energy Convers Manag 198:110535. https://doi.org/10.1016/j.enconman.2018.06.099
Gomes MM, Sakamoto IK, Silva Rabelo CAB, Silva EL, Varesche MBA (2021) Statistical optimization of methane production from brewery spent grain: Interaction effects of temperature and substrate concentration. J Environ Manage 288:112363. https://doi.org/10.1016/j.jenvman.2021.112363
Aworanti OA, Agarry SE, Ogunleye OO (2017) Biomethanization of the Mixture of Cattle Manure, Pig Manure and Poultry Manure in Co-Digestion with Waste Peels of Pineapple Fruit and Content of Chicken-Gizzard - Part II: Optimization of Process Variables. Open Biotechnol J 11(1):54–71. https://doi.org/10.2174/1874070701711010054
Lim YF et al (2022) Evaluation of potential feedstock for biogas production via anaerobic digestion in Malaysia: kinetic studies and economics analysis. Environ Technol 43(16):2492–2509. https://doi.org/10.1080/09593330.2021.1882587
Funding
Open access funding provided by University of Johannesburg. The authors declare that no funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Tunde David Edunjobi, Oluseye Omotoso Agbede and Olufunmilayo Abiola Aworanti. The first draft of the manuscript was written by Tunde David Edunjobi, Oluseye Omotoso Agbede, Olufunmilayo Abiola Aworanti, and Oyetola Ogunkunle. The investigation and supervision were coordinated by Samuel Enahoro Agarry and Ademola Oyejide Adebayo. The resources were made available by Olufunmilayo Abiola Aworanti, Opeyeolu Timothy Laseinde, and Oyetola Ogunkunle. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This study is not applicable to both human and/or animal studies that require ethical approval.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Edunjobi, T.D., Agbede, O.O., Aworanti, O.A. et al. Enhanced anaerobic digestion of brewers’ spent grain: effect of inoculum, poultry manure application and iron (iii) chloride supplementation on biogas production and its kinetics. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04813-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04813-6