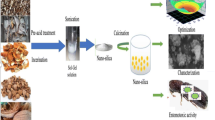
Abstract
This work aims to successfully produce silica nanoparticles (SNPs) from Egyptian white sand using the fungal bioleaching process as a cost-effective and eco-friendly approach. The impact of fungus cultivation techniques (submerged culture SMC and solid-state culture SSC) on the characteristics of the produced SNPs has been investigated. In addition, the most promising fungal isolates for each culture method were selected and identified by morphological and molecular methods. The biosynthesized SNPs were fully characterized by DLS, FTIR, XRD, SEM, EDX, and HRTEM studies. DLS results showed that Aspergillus niger solid-state culture had developed SNPs with a mean particle size distribution of about 3.6 nm, whereas Penicillium crustosum submerged culture developed SNPs with 50.7 nm. SEM images revealed that the prepared SNPs under SMC and SSC have sphere-shaped particles with smooth surfaces and semi-homogeneous characteristics. Moreover, the HRTEM imaging confirmed the spherical shape with an average size of 3.5 and 28.8 nm for the nanosilica synthesized during solid-state and submerged culture, respectively. Based on the results, we recommended using SSC to produce silica nanoparticles from white sand with a small nano-size, high purity, and better economical production. The scientific advances focused on some particular fungi's capacity to manufacture SNPs with high purity, small size, and techniques that were both economical and environmentally beneficial.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Egypt is thought to have one of the best white sands in the world, a vast white gold treasure. It reserves about 20 billion tons of white sand distributed along the Red Sea coast, South Sinai, and Eastern Desert [1]. This sand is the primary supply of silicon, the naturally occurring material that is the foundation for a wide range of modern technology, including the manufacturing of solar cells and electronic chips, which are at the heart of the enormous technological boom that moved the world into the twenty-first century [2, 3]. Therefore, white sand should receive more attention from Egyptian researchers, as it is a strategic treasure that has not been exploited sufficiently. Given the above, we attempt to produce silica nanoparticles (SNPs) from Egyptian white sand using the fungal bioleaching process as a low-cost and environment-friendly method.
Significant efforts have been conducted in the last decade to manufacture SNPs due to their potential usage in various applications, including biosensors, medication delivery, optics, semiconductors, microelectronics, thermal insulators, catalysts, water treatment, and the solar silicon industry [4,5,6,7]. In addition, nano-silica is essential as a filler in plastics, rubbers, and coatings, as well as in food, pesticides, and personal care products due to its unique properties. They are immensely stable and less toxic, with a simple surface structure and a high surface area-to-volume ratio [8, 9].
SNPs can be prepared using various physical, chemical, and biological techniques [10,11,12]. Unfortunately, there are some drawbacks to each approach. The physical processes typically require extreme temperature and pressure and result in low yield, while the chemical methods are more toxic, expensive, eco-hazardous, and have high energy consumption [13]. Therefore, it seems reasonable to develop alternative green biological techniques that, apart from having an essential ecological impact, would be financially competitive with the currently applied approaches. These techniques have great potential as they can employ mild temperature and pressure conditions, being cost-effective and environmentally friendly [14].
Additionally, compared to nanoparticles synthesized chemically, the biological molecules that cover nanoparticles' surfaces make them more biocompatible [15, 16]. Bansal et al. (2005) have developed a biological method for producing nanosilica from white sand using the fungus Fusarium oxysporm. They proposed that the formation of SNPs proceed in a two-step process. The first step involves the extracellular bioleaching of silica from the sand grains in the form of silicic acid by organic acids produced by the fungal biomass, while in the second step, the silicate complex is hydrolyzed by the action of some specific proteins or enzymes in the fungus to silica. Both steps depend on the cultivation conditions and the culture medium, which may have different compositions and sugar/protein ratios [17].
The two primary types of culture that promote the production of enzymes by fungi are submerged and solid-state cultures. Fungi growing on a solid substrate with slight moisture undergo solid-state cultivation, whereas fungi grown in liquid media undergo submerged cultivation [18,19,20]. Examining the ideal culture conditions is beneficial for enhancing production kinetics and fungal metabolites. Where the required variations in fungal morphology, growth kinetics, physiology, and chemical diversities, i.e., generation of secondary metabolites, can be induced by changing culture conditions [21]. In this regard, the novelty of this study aimed to isolate different fungal isolates from Egyptian white sand that could synthesize SNPs, in addition to demonstrating the best type of culturing for the production and characterization of synthesized biogenic nano-silica.
2 Material and Methods
2.1 Sampling and characterization of silica sand
A 5 kg sample of naturally occurring white sand was obtained from the Eastern Desert of Egypt. The sample was first cleaned with deionized water to dispose of any clay or other undesirable materials that had adhered to the sand's surface. Next, it was treated with 2M sulfuric acid (98% CAS 7664-93-9 Merk, Molar Mass 98.08 g/mol) and dried in an oven before sieving. The used silica sand has a particle size distribution of 160 to 250 μ. To characterize the prepared sand and determine the chemical composition of the minerals, X-ray fluorescence (XRF) and X-ray diffraction (XRD) were used for analysis.
2.2 Isolation and purification of fungi
About five grams of sand sample was diluted in 9 mL of sterilized distilled water, and 0.5 mL of this suspension was dispersed onto plates containing Sabouraud Dextrose Agar (SDA). Another gram of the sand sample was spread on an SDA plate to isolate fungal isolates that can tolerate silica. The inoculated plates were incubated at 27 °C (±2 °C). As soon as the initial colonies formed, they were transferred to the SDA plate for further purification. Following verification of the colonies' purity, they were sub-cultured on SDA slants, and based on their macroscopic and microscopic examination, colonies were identified [22, 23].
2.3 Silica metal tolerance profiles of isolated fungi
The ability of the fungal isolates to tolerate silica was evaluated using the maximum tolerance concentration (MTC) assay [24]. The examined fungus was inoculated into Sabouraud Dextrose Broth (SDB) with various concentrations of SiO2 (100 to 500 ppm), while the control flasks were free of SiO2. The flasks were incubated at 27 °C (± 2 °C) for at least five days, and the fungal growth was monitored each day. MTC was defined as the highest concentration of silica ions in the medium that permitted fungal growth.
2.4 Organic acid production
Total organic acids generated by the examined fungus were determined by measuring the volumetric titratable acidity using 1% phenolphthalein as an indicator [25]. The fungal broth was centrifuged at 10,000 rpm for five minutes, and the supernatant was separated to measure the organic acids. Citric acid was used as the equivalent factor for the quantification of organic acids as % according to the formula:
The equivalent weight of the acid is 70.
2.5 Submerged culture for SNPs synthesis
The biosynthesis of SNPs from Egyptian white sand is carried out here under submerged culture (SMC) through a two-step method similar to that reported by Bansal et al [17]. Firstly, the fungal biomass was obtained as follows; the examined fungal spores were inoculated into conical flasks containing potato dextrose broth (PDB) at one-third of their capacity. The flasks were then incubated at 27 °C for four days in an orbital shaking incubator to reach the mid-log phase, and the cultivated fungal biomass was harvested and washed many times with distilled water for further use.
Secondly, 100 mL of distilled water containing 10 g of white sand was autoclaved in a 500 mL Erlenmeyer flask, and about 15 g wet weight of the harvested biomass was then added to provide starvation conditions for the fungi. For one day, the fungal biomass and sand reaction was carried out on a shaker incubator (150 rpm) at 27 °C. After filtrating the fungal mycelia from the aqueous phase, the bioleached silica nanoparticles were recovered using a rotary evaporator and dried in an oven at 60 °C for characterization.
2.6 Solid-state culture for SNPs synthesis
In a spectacular one-step method, solid-state culture (SSC) is also employed to produce nanosilica from Egyptian white sand for the first time. A weighted sample of 10 g of white sand was transferred separately into a 500 mL Erlenmeyer flask, and then SDA medium was added to moisten the raw white sand at a level of 100% (w/w). After that, the flask was autoclaved for 15 minutes at 121 °C. Following cooling, 1 mL of the fungal spore suspension (1×107) was added to the flasks containing sand and media, and the contents were gently shaken. The flask was then incubated at 27 °C for four days at 120 rpm.
After incubation, 100 mL of sterile distilled water was added. The weighted sand was allowed to bio-transform into silica nanoparticles by the examined fungi over 24 hours in a shaking incubator at 120 rpm. The sand and fungal mycelia were then separated from the aqueous phase by filtration, and any other remaining substances were removed by centrifuging the filtrate for 10 minutes at 5,000 rpm. Afterward, a rotary evaporator concentrated the supernatant and dried it in an oven at 60 °C.
2.7 Molecular identification of most promising fungi
Molecular methods were used to identify the most promising fungal isolates, which produced large amounts of total organic acids and were tolerant to high SiO2 concentrations [26]. The fungi were cultivated on Czapek's Agar (CZA) medium and incubated for five days at 28 °C. At Assiut University's Molecular Biology Research Unit, DNA was extracted using the pathogens-spin DNA/RNA extraction kit (made by Korea's Intron Biotechnology Company). With the assistance of the SolGent Company, Daejeon, South Korea, polymerase chain reaction (PCR) and sequencing was carried out. Using the universal primers ITS1 (forward) and ITS4 (reverse), which were added to the reaction mixture, the ITS sections of the rRNA gene for Penicillium crustosum were amplified. Primers have the following structure: ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’). With the same primers and the addition of ddNTPs to the reaction mixture, the purified PCR product (amplicons) was sequenced [27].
Other fungal DNA samples from Aspergillus niger were subjected to PCR and sequencing of the beta-tubulin gene using the primer pairs βtub-F and βtub-R (5'-TGACGGGTGATTGGGATCTC and 5'-CGTCCGCTTCTTCCTTGTTT), respectively. The National Centre of Biotechnology Information's (NCBI) website's Basic Local Alignment Search Tool (BLAST) was used to analyze the acquired sequences. MegAlign (DNA Star) software version 5.05 was used for sequence analysis and the creation of phylogenetic trees. The resulting recognized sequences were deposited with a deposition number in the Culture Collection of the Assiut University Mycological Center.
2.8 Characterization of synthesized SNPs
Various characterization studies have been conducted on the prepared SNPs powder produced by SMC and SSC to determine its structure and particle size. Dynamic light scattering (DLS; Malvern Analytical, Malvern, UK) was used to measure the synthesized SNPs' particle size distribution, hydrodynamic radius, and polydispersity index (PDI). A PerkinElmer infrared spectrophotometer with a resolution of 4 cm-1, 400–4000 cm-1 range, was used to record the FTIR analysis of the biosynthesized silica nanoparticles in the fungal filtrate. The crystalline structure of the prepared nanosilica was examined by X-ray diffraction (XRD) using a Shimadzu XRD-6000 diffractometer, Japan, with CuKα radiation (λ= 0.15408 nm). The diffraction angle was scanned at 2θ range of 5° to 70°. Morphological and stoichiometric analyses of the produced SNPs were gathered using Scanning Electron Microscopy (SEM-EDX; JEOL JSM-5600 LV, Tokyo, Japan). The size and shape of the produced silica were verified using TEM analysis by the drop coating method using a JEOL Transmission Electron Microscope (JEM-1230).
3 Results and discussion
3.1 Mineralogical and chemical composition of silica sand
The results of mineralogical analyses of the raw silica sand using XRD are revealed in Fig. 1. It can be seen that silicon oxide is the main component of the white sand used in this study as compared with the literature review, where the highest peak intensity was recorded at 2θ = 26.58 °.
The XRF results showed that the chemical composition of white sand has a total SiO2 of 94.61 %, along with some undesirable impurities, as shown in (Table 1). These results agreed with that obtained by Wahab et al., who studied the chemical characterization of Wadi Dakhale white sand in Egypt using XRF analysis [1]. The results indicate that the significant element is SiO2, with 95.0 %. Al-Abady et al. showed that silica is the most abundant component, reaching 95.69 % in El-Zaafarana white sand Egypt samples [28].
3.2 Screening of fungi for SiO2 tolerance
By applying the MTC assay at different SiO2 concentrations ranging from 100 to 500 ppm, fungi coded as F2, F4, F6, F8, and F10 exhibited good resistance to silica among all the tested isolated fungi, as seen in Table 2. Thus, these isolates have been subjected to further experiments to select the most potent fungal strain to synthesize SNPs. Various microorganisms decompose and solubilize silicate minerals by secreted metabolites, particularly organic acids [29]. Fungi have a variety of mechanisms for tolerating high levels of metals, including complexation, binding to cell walls, precipitation, production of intracellular/extracellular enzymes, enhanced metal efflux, and sequestration [30].
3.3 Screening of fungi for organic acid production
The use of organic acids, mainly citric and oxalic acid, as a leaching agent in removing metals from industrial minerals has the potential to be interesting [31]. Under certain conditions, several fungi excrete oxalic and citric acid as byproducts of their metabolic processes. Checking the quantity of organic acids produced by the examined fungi is necessary to select the most suitable isolates (see Table 3). According to the previous studies by Ameen et al. (2019), Bist et al. (2020), and Santi et al. (2020), the primary mechanism for silica solubilization is the action of organic acids produced by silicate-solubilizing fungi [32,33,34]. These findings support the hypothesis that citric acid is the cause of the medium's lowered pH, which supplies protons (H+) that enhance silica solubilization.
3.4 Morphological identification of selected fungi
The most promising fungal isolates for the production of organic acids and those that showed the highest resistance to silica were identified by Assiut University, Moubasher Mycological Center (AUMMC). The identification of tested fungi was made morphologically based on macroscopic and microscopic observations and descriptions by Pitt et al. [35] and Domsch et al. [36]. The results are illustrated in Fig. 2.
The morphological identification of selected fungi where, A: F2-Aspergillus niger AUMC 15483, B: F4-Aspergillus flavus AUMC 15485, C: F6-Fusarium sporotrichioides AUMC 15486, D: F8-Penicillium crustosum AUMC 15484, and E: F10- Fusarium konzum AUMC 15487. (a: fungal growth on PDA plates, and b, and c: tested fungi under microscopic)
3.5 SNPs synthesis by submerged culture
The silica bioleaching process from white sand under SMC for 24 h by tested fungi resulted in synthesizing silica nanoparticles of different sizes. The DLS analysis was used to examine the particle size of the biosynthesized silica, and the results are shown in Fig. 3 (a, b, c, d, e, and f). Depending on the DLS histogram, the most promising fungus was Penicillium crustosum, where the produced SNPs have an average size of 50.75 nm and poly-dispersity index (PDI) of 0.892, indicating that they are mono-dispersed particles. According to the International Standards Organizations (ISOs), monodisperse samples tend to have PDI values below 0.05, whereas polydisperse samples tend to have values above 0.7 [37, 38]. By applying the PDI values, it was determined that the synthesized SNPs had modest mono-size dispersion.
FTIR analysis of SNPs synthesized by SMC, where A: F2-Aspergillus niger AUMC 15483, B: F4-Aspergillus flavus AUMC 15485, C: F6-Fusarium sporotrichioides AUMC 15486, D: F8-Penicillium crustosum AUMC 15484, and E: F10- Fusarium konzum AUMC 15487, DLS analysis and PDI of SNPs, where a: F2-Aspergillus niger AUMC 15483, b: F4-Aspergillus flavus AUMC 15485, c: F6-Fusarium sporotrichioides AUMC 15486, d: F8-Penicillium crustosum AUMC 15484, and e: F10- Fusarium konzum AUMC 15487
The FTIR spectra of the produced SNPs are shown in Fig. 3 (A, B, C, D, and E). The broadband of about 3283 cm-1 is caused by the (OH) stretching vibration of the water adsorbed on the surface of the SNPs. The bands at 571 cm-1, 834 cm-1, and 1081 cm-1 are due to the symmetric and asymmetric stretching vibrations of siloxane (Si-O-Si) groups [11, 17].
According to Santi et al. [34], there are three main mechanisms that microorganisms interact with siliceous rocks: i) Microorganisms can solubilize silicate minerals due to their produced organic acids. ii) Microbes use silica by absorbing it into their cells, keeping it in an altered or unchanged state for a while, and then releasing it into the fermentation medium as free silicic acid (H4SiO4). iii) Additionally, microorganisms absorb silica by ingesting it in dissolved form and polymerizing it within the cells; as a result, the mineral becomes a permanent component of the organism and is not expelled before it dies.
3.6 SNPs synthesis by solid-state culture
This study is the first to examine the SNPs bioleaching process from white sand under SSF by fungi. The DLS was used to determine the size of the biosynthesized SNPs. The findings are presented in Fig. 4 (a, b, c, d, e, and f). The most promising fungus under SSF was Aspergillus niger, where the DLS results revealed that the poly-dispersity index (PDI) of synthesized SNPs was 0.178, indicating that the particles were mono-dispersed and had an average size of 3.62 nm. Figure 4 (A, B, C, D, and E) displays the infrared spectra of SNPs measured using FTIR analysis. The broad band at 3280 cm-1 has been explained by the (OH) stretching vibration of the adsorbed water on the surface of the SNPs. The sharp band at 1078 cm-1 is attributed to the asymmetric stretching vibration of siloxane groups. Results obtained by FTIR spectra confirmed the successful preparation of SNPs by the fungal activation power of both culture methods.
FTIR analysis of SNPs synthesized by SSC, where A: F2-Aspergillus niger AUMC 15483, B: F4-Aspergillus flavus AUMC 15485, C: F6-Fusarium sporotrichioides AUMC 15486, D: F8-Penicillium crustosum AUMC 15484, and E: F10- Fusarium konzum AUMC 15487, and DLS analysis and PDI of SNPs, where a: F2-Aspergillus niger AUMC 15483, b: F4-Aspergillus flavus AUMC 15485, c: F6-Fusarium sporotrichioides AUMC 15486, d: F8-Penicillium crustosum AUMC 15484, and e: F10- Fusarium konzum AUMC 15487
3.7 Molecular identification of most promising fungi
By comparing the fungal 18S-rRNA coding gene sequence (493 bp) against sequences recovered from the Gen Bank database using BLAST programs, the F2 isolate was identified as an Aspergillus niger strain that showed 98.9-99.5% similarity and 98-100% coverage with several strains of A. niger registered in the Gen Bank including the type material A. niger NRRL326T with Gen Bank accession no. LC589349, where F8 isolate was identified as Penicillium crustosum AUMC15484, showed 99.8- 100% identity and 100% coverage with several strains of P. crustosum registered in the Gen Bank, including the type material FRR1669T with accession no. NR_077153.
Figures 5 and 6 show the phylogenetic relationship between A. niger and P. crustosum with other fungal mitogenomes, respectively.
Phylogenetic tree based on Beta-tubulin gene sequences of the fungal strain isolated in the present study (A. niger AUMC 15483, arrowed) aligned with closely related sequences accessed from the Gen Bank, (A. = Aspergillus). Aspergillus fumigtus is included in the tree as an outgroup strain. A. niger AUMC 15483 showed 98.92% - 99.46% similarity and 98% - 100% coverage with several strains of A. niger registered in the GenBank including the type material A. niger NRRL326T with GenBank accession no. LC589349
Phylogenetic tree based on ITS sequences of rDNA of the fungal strain isolated in the present study (P. crustosum AUMC15484, arrowed) aligned with closely related sequences accessed from the GenBank, (P. = penicillium). Talaromyces (T. funiculosus) is included in the tree as an outgroup strain. P. crustosum AUMC15484 showed 99.83% - 100% identity and 100% coverage with several strains of P. crustosum registered in the GenBank including the type material FRR1669T with accession no. NR_077153
The identified A. niger strain was also preserved with AUMC 15483 in the Culture Collection of Assiut University Mycological Center (AUMC), Assiut, Egypt, where the P. crustosum was held with number AUMC 15484.
3.8 Characterization of synthesized SNPs
Silica nanoparticles synthesized by two different fermentation cultures (SSC and SMC) were characterized by XRD, SEM, EDX, and HRTEM analyses [39,40,41,42]. XRD was used to detect their crystalline structures. Figure 7 shows the XRD pattern of SNPs synthesized from white sand by A. niger and P. crustosum under SSC and SMC. The sharp diffraction peaks for A. niger-SiO2 NPs indicate the presence of a crystalline structure. In contrast, the broad band at 2θ = 22o for P. crustosum-SiO2 NPs shows the disappearance of crystalline phases and confirms the amorphous state of SNPs. These results proved that the fungal culturing methods affect the crystallinity phases of produced nanosilica.
On the other hand, the average crystallite size of the synthesized SNPs was determined by using the Scherrer equation and was found to be 17.0, and 36.4 nm for the synthesized SNPs by A. niger and P. crustosum under SSC and SMC, respectively (Table 4). Additionally, Table 4 displayed FWHM, and d- spacing of different synthesized SiO2 nanoparticles.
SEM images of the prepared SNPs by A. niger under solid-state culture show smooth-surfaced, semi-homogeneous-sized particle spheres, as presented in Fig. 8a. All particles are spherical and have a variable grain nano-size that has been integrated with the fungal culture, as shown in the magnified SNPs (Fig. 8b). For the SNPs synthesized by P. crustosum under SMC (Fig. 8c), the silica spheres were distributed equally with a distinct oval sphere structure over the P. crustosum culture. Furthermore, the scattered composite particles in SEM kept their spherical shape and appeared as bright particles (Fig. 8d). From Fig. 8; SEM images indicated that the synthesized SNPs by A. niger seemed more apparent and smaller than that prepared by P. crustosum and revealed the role of SSC in obtaining more precise and smaller highly distributed SNPs.
The chemical composition and purity check for nanosilica synthesized by SSC and SMC has been analyzed by EDX elemental analysis, as presented in Fig. 9. EDX analysis of the SNPs produced by A. niger under SSC revealed the following pattern of elements (Si and O atoms for SNPs, and C, Mg, Al, P, S, K, Am, Ca, and Te for the remaining fungal media or for impurities leached from sand). Moreover, the elemental quantity determinations (Tables inside Fig. 9) confirmed the semi-purity of the biosynthesized SNPs, as presented in Fig. 9a.
On the other hand, the elemental analysis of the SNPs produced by P. crustosum under SMC revealed the presence of C, O, Na, Al, Si, P, S, Cl, K, and Am peaks. The elemental distribution confirms the biogenic SNPs' semi-purity and successful formation, as represented in Fig. 9b. So we recommend that the synthesized SNPs should undergo calcination for more purification. The atomic ratio of Si and O was about 1: 24 and 1: 19 in SNPs synthesized by A. niger under SSC and P. crustosum under SMC, respectively.
Figure 10 displays HRTEM images of the biosynthesized nanosilica under SSC and SMC. The A. niger-created SNPs have a spherical structure and a diameter that ranges from 2.1 nm to 6.0 nm, with an average size of 3.5 nm. In the case of the bio-fabricated SNPs by P. crustosum under SMC, the faint amorphous SNPs spheres are shown in Fig. 10b with diameter sizes ranging from 20.4 nm to 39.8 nm with an average particle size of 28.8 nm.
Notably, the average particle size for SNPs synthesized by A. niger under SSC (3.5 nm) was smaller than that for SNPs synthesized by P. crustosum under SMC (28.8 nm). Furthermore, HRTEM analysis showed that our mycogenic SNPs under (SSC and SMC) have roughly the same size and spherical shape as those in the recently published article, confirming that the shape and size are appropriate for various biological applications [39,40,41,42]. Particle size measurements in HRTEM were smaller than DLS size measurements. DLS analysis estimates the hydrodynamic radius of SNPs bound by water molecules, resulting in larger particle sizes of the capped nanoparticles, while TEM determines the average particle size of the powder material without the water layer [43]. Based on the obtained results, we concluded that using SSF as a synthetic approach encouraged SNPs production, which seems better production than SMC and produced SNPs with small size and stability. Still, calcination must be taken into consideration for increasing the purity. On top of that, SSC is more economical than SMC because it consumes less media and requires less work to prepare a large biomass than SMC. As a result, we can say that SSC saves energy, effort, money, and time.
4 Conclusion
We conducted a comparative study of the mycogenic production of SNPs with two types of fungal culturing. SMC is the reported method for SNPs bioleaching from sand and SSC, which has not been registered. The SNPs obtained under SSC differed markedly in size and crystallinity phase from those obtained under SMC. The HRTEM imaging confirmed the spherical shape with an average size of 3.5 and 28.8 nm for the SNPs synthesized under SSC and SMC, respectively. The SMC produces amorphous SNPs, whereas SSM produces crystalline SNPs. To our knowledge, each type of mycogenic SNP synthesized has different applications based on their properties, size, and crystallinity phase. Therefore, we recommended using SSC to produce SNPs from white sand with a small nano-size using a cost-effective, eco-friendly, time effort, and energy consumption saver. The advancements in science focused on some particular fungi's capacity to manufacture SNPs with high purity, compact size, and techniques that were both economical and environmentally benign. Future work will be directed toward studying the purification of produced SNPs. In addition, statistical optimization studies would be performed to manage the yield of SNPs. Moreover, the produced SNPs will apply in essential applications. On top of that, the up-scaling of SNPs would be studied.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request
References
Wahab GM, Ibrahim GE-DA, Wahab AAA (2022) Geological and engineering appraisal of hydraulic frac sand in some Egyptian localities as a proppant of oil well drilling. Heliyon 8(8):e10233. https://doi.org/10.1016/j.heliyon.2022.e10233
Vatalis KI, Charalambides G, Benetis NP (2015) Market of high purity quartz innovative applications. Procedia Econom Finance 24:734–742. https://doi.org/10.1016/S2212-5671(15)00688-7
Kuddus A, Islam R, Tabassum S, Md Ismail AB (2020) Synthesis of Si NPs from river sand using the mechanochemical process and its applications in metal oxide heterojunction solar cells. Silicon 12:1723–1733. https://doi.org/10.1007/s12633-019-00267-6
Prabha S, Durgalakshmi D, Rajendran S, Lichtfouse E (2021) Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: a review. Environ Chem Lett 19(2):1667–1691. https://doi.org/10.1007/s10311-020-01123-5
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T (2015) Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med 11(2):313–327. https://doi.org/10.1016/j.nano.2014.09.014
Li M-E, Wang S-X, Han L-X, Yuan W-J, Cheng J-B, Zhang A-N, Zhao H-B, Wang Y-Z (2019) Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J Hazard Mater 375:61–69. https://doi.org/10.1016/j.jhazmat.2019.04.065
Shen Y (2017) Rice husk silica derived nanomaterials for sustainable applications. Renew Sustain Energy Rev 80:453–466. https://doi.org/10.1016/j.rser.2017.05.115
Zielonka A, Żymańczyk-Duda E, Brzezińska-Rodak M, Duda M, Grzesiak J, Klimek-Ochab M (2018) Nanosilica synthesis mediated by Aspergillus parasiticus strain. Fungal Biol 122(5):333–344. https://doi.org/10.1016/j.funbio.2018.02.004
Jeelani PG, Mulay P, Venkat R, Ramalingam C (2020) Multifaceted application of silica nanoparticles. A review. Silicon 12:1337–1354. https://doi.org/10.1007/s12633-019-00229-y
Assefi M, Davar F, Hadadzadeh H (2015) Green synthesis of nanosilica by thermal decomposition of pine cones and pine needles. Adv Powder Technol 26(6):1583–1589. https://doi.org/10.1016/j.apt.2015.09.004
Boualem A, Leontie L, Lopera SAG, Hamzaoui S (2022) Synthesis and Characterization of Mesoporous Silica from Algerian River Sand for Solar Grade Silicon: Effect of Alkaline Concentration on the Porosity and Purity of Silica Powder. Silicon 14:5231–5240. https://doi.org/10.1007/s12633-021-01306-x
Eissa D, Hegab RH, Abou-Shady A, Kotp YH (2022) Green synthesis of ZnO, MgO and SiO2 nanoparticles and its effect on irrigation water, soil properties, and Origanum majorana productivity. Sci Rep 12(1):5780. https://doi.org/10.1038/s41598-022-09423-2
Pryshchepa O, Pomastowski P, Buszewski B (2020) Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv Colloid Interface Sci 284:102246. https://doi.org/10.1016/j.cis.2020.102246
Shirsat SD, Mane RS, Dhuldhaj UP (2021) Biogenic synthesis of silicon oxide, titanium oxide and cerium oxide nanomaterials. In: Shende SS et al (eds) Microbial synthesis of nanomaterials. Nova Science Publishers, Inc., New York, pp 137–161
Sharma D, Kanchi S, Bisetty K (2019) Biogenic synthesis of nanoparticles: a review. Arabian journal of chemistry 12(8):3576–3600. https://doi.org/10.1016/j.arabjc.2015.11.002
Azizi M, Sedaghat S, Tahvildari K, Derakhshi P, Ghaemi A (2017) Synthesis of silver nanoparticles using Peganum harmala extract as a green route. Green Chem Lett Rev 10(4):420–427. https://doi.org/10.1080/17518253.2017.1395081
Bansal V, Sanyal A, Rautaray D, Ahmad A, Sastry M (2005) Bioleaching of sand by the fungus Fusarium oxysporum as a means of producing extracellular silica nanoparticles. Adv Mater 17(7):889–892. https://doi.org/10.1002/adma.200401176
Intasit R, Cheirsilp B, Suyotha W, Boonsawang P (2021) Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid state-submerged fermentation and co-cultivation of different filamentous fungi. Biochem Eng J 173:108086. https://doi.org/10.1016/j.bej.2021.108086
Filipe D, Fernandes H, Castro C, Peres H, Oliva-Teles A, Belo I, Salgado JM (2020) Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod Biorefin 14(1):78–91. https://doi.org/10.1002/bbb.2073
Miranda RU, Gómez-Quiroz LE, Mendoza M, Pérez-Sánchez A, Fierro F, Barrios-González J (2014) Reactive oxygen species regulate lovastatin biosynthesis in Aspergillus terreus during submerged and solid-state fermentations. Fungal Biol 118(12):979–989. https://doi.org/10.1016/j.funbio.2014.09.002
Srivastava N, Srivastava M, Ramteke P, Mishra P (2019) Solid-state fermentation strategy for microbial metabolites production: An overview. In: New and future developments in Microbial Biotechnology and Bioengineering, pp 345–354. https://doi.org/10.1016/B978-0-444-63504-4.00023-2
Samson RA, Pitt JI (2000) Integration of modern taxonomic methods for Penicillium and Aspergillus classification, 1st edn. CRC Press, London. https://doi.org/10.1201/9781482284188
Lucking R, Aime MC, Robbertse B, Miller AN, Aoki T, Ariyawansa HA, Cardinali G, Crous PW, Druzhinina IS, Geiser DM (2021) Fungal taxonomy and sequence-based nomenclature. Nat Microbiol 6:540–548. https://doi.org/10.1038/s41564-021-00888-x
El-Saadony MT, Desoky E-S M, Saad AM, Eid RS, Selem E, Elrys AS (2021) Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J Environ Sci 106:1–14. https://doi.org/10.1016/j.jes.2021.01.012
Dezam A, Vasconcellos V, Lacava P, Farinas C (2017) Microbial production of organic acids by endophytic fungi. Biocatal Agric Biotechnol 11:282–287. https://doi.org/10.1016/j.bcab.2017.08.001
Pitt JI, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, Heidelberg Dordrecht London New York. https://doi.org/10.1007/978-0-387-92207-2
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, Inc
Al-Abady A (2022) Characterization and potential upgrading of El-Zaafarana White Sand by Attrition scrubbing. J Univ Shanghai Sci Technol 24(4):79–90
Brindavathy R (2022) Silicate Minerals Induced by Microorganisms. In: Mineral Formation by Microorganisms, pp 125–159. https://doi.org/10.1007/978-3-030-80807-5_5
Oladipo OG, Awotoye OO, Olayinka A, Bezuidenhout CC, Maboeta MS (2018) Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz J Microbiol 49:29–37. https://doi.org/10.1016/j.bjm.2017.06.003
Lazo DE, Dyer LG, Alorro RD (2017) Silicate, phosphate and carbonate mineral dissolution behaviour in the presence of organic acids: A review. Miner Eng 100:115–123. https://doi.org/10.1016/j.mineng.2016.10.013
Ameen F, AlYahya SA, AlNadhari S, Alasmari H, Alhoshani F, Wainwright M (2019) Phosphate solubilizing bacteria and fungi in desert soils: species, limitations and mechanisms. Arch Agron Soil Sci 65(10):1446–1459. https://doi.org/10.1080/03650340.2019.1566713
Bist V, Niranjan A, Ranjan M, Lehri A, Seem K, Srivastava S (2020) Silicon-solubilizing media and its implication for characterization of bacteria to mitigate biotic stress. Front Plant Sci 11:28. https://doi.org/10.3389/fpls.2020.00028
Santi LP (2020) Enhanced solubilization of insoluble silicate from quartz and zeolite minerals by selected Aspergillus and Trichoderma species. Menara Perkebunan 88(2):79–89. https://doi.org/10.22302/iribb.jur.mp.v88i2.381
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press Inc, Ltd
Domsch K, Gams W, Anderson T (2007) Acremonium. Compendium of soil fungi 2:30–38
Yang S-C, Ryu J, Choi K-O, Kang TS, Lee JK, Song CW, Ko S (2014) Dynamic light scattering-based method to determine primary particle size of iron oxide nanoparticles in simulated gastrointestinal fluid. Food Chem 161:185–191. https://doi.org/10.1016/j.foodchem.2014.04.022
Franks K, Kestens V, Braun A, Roebben G, Linsinger TPJ (2019) Non-equivalence of different evaluation algorithms to derive mean particle size from dynamic light scattering data. J Nanopart Res 21(9):195. https://doi.org/10.1007/s11051-019-4630-2
Alhazmi NM (2023) Fungicidal Activity of Silver and Silica Nanoparticles against Aspergillus sydowii Isolated from the Soil in Western Saudi Arabia. Microorganisms 11(1):86. https://doi.org/10.3390/microorganisms11010086
Zaki AG, Hasanien YA, El-Sayyad GS (2022) Novel fabrication of SiO2/Ag nanocomposite by gamma irradiated Fusarium oxysporum to combat Ralstonia solanacearum. AMB Express 12(1):1–18. https://doi.org/10.1186/s13568-022-01372-3
Imoisili PE, Ukoba KO, Jen T-C (2020) Green technology extraction and characterisation of silica nanoparticles from palm kernel shell ash via sol–gel. J Mater Res Technol 9(1):307–313. https://doi.org/10.1016/j.jmrt.2019.10.059
Elbasuney S, El-Sharkawy YH, El-Sayyad GS, Gobara M (2020) Surface modified colloidal silica nanoparticles: Novel aspect for complete identification of explosive materials. Talanta 211:120695. https://doi.org/10.1016/j.talanta.2019.120695
Pabisch S, Feichtenschlager B, Kickelbick G, Peterlik H (2012) Effect of interparticle interactions on size determination of zirconia and silica based systems–A comparison of SAXS, DLS, BET, XRD and TEM. Chemical physics letters 521:91–97. https://doi.org/10.1016/j.cplett.2011.11.049
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
YAH performed the experiments, wrote the manuscript, and participated in manuscript revising and editing. MAM conceived and designed the research, performed the experiments, participated in data analysis and representation, wrote the manuscript, and participated in manuscript revising and editing. ASA conducted experimental methodology and participated in manuscript revising and editing. GSE conceived and designed research, participated in data analysis and representation, provided practical guidance, and participated in the manuscript. EHE suggested the research point, investigated the article, and designed the research. EHB provided the used chemicals, provided practical guidance, and participated in manuscript revising and editing revising and editing. All authors read and approved the article.
Corresponding authors
Ethics declarations
Disclosure of potential conflict of interest
The authors declare no conflict of interest
Funding
None
Research involving Human Participation and/or Animals
Not applicable
Informed consent
Not applicable
Ethical approval
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasanien, Y.A., Mosleh, M.A., Abdel-Razek, A.S. et al. Green synthesis of SiO2 nanoparticles from Egyptian white sand using submerged and solid-state culture of fungi. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04586-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04586-y