Abstract
Crop loss brought on by phytopathogens is a significant issue that affects the entire world. Synthetic pesticides have been used for years to prevent economic losses caused by phytopathogens, raising severe worries about the environment and public health. Therefore, today, there is growing interest in using helpful microorganisms to control plant pathogens. In this way, Aspergillus chevalieri AGH-1 OP593083 and Aspergillus egyptiacus AGH-4 OP593080 isolated from wheat rhizosphere have been applied as plant growth-promoting fungi (PGPF) for combating early blight disease caused by Alternaria solani (A. solani) and inducing the defense system and growth promotional strategies in Vicia faba. Two milligrams per milliliter ethyl acetate crude extracts of A. chevalieri and A. egyptiacus demonstrated effective antifungal efficacy against A. solani with an inhibition zone of 18 and 16 mm respectively compared with 20 mm attained by fluconazole as a positive control. Moreover, the minimum inhibitory concentrations (MIC) were (0.25 and 0.5 mg/ml) for A. chevalieri and A. egyptiacus extracts respectively. Data showed that A. chevalieri was the best isolate producing indole acetic acid (IAA) and siderophores (+ + +) followed by A. egyptiacus (+ +). Also, the best isolates capable of phosphate solubilization in the soil was A. egyptiacus (+ + +), followed by A. chevalieri (+ +). It is interesting that the PGPF isolates used, whether single or mixed, showed a significant improvement in all growth and yield as well as photosynthetic pigments in Vicia faba. Moreover, A. chevalieri and A. egyptiacus played an important role in improving plant immunity by increasing phenol, proline, and antioxidant enzymes (POD and PPO) activities, as well as reducing oxidative stress by reducing MDA and H2O2. We can conclude that A. chevalieri and A. egyptiacus are considered eco-friendly safe strong inducers of Vicia faba plant immunity alternatives to chemical pesticides against A. solani early blight to preserve plant, soil, and human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to plant infections, the quality and quantity of agricultural products around the world continue to progressively diminish each year. In addition to fungi, the release of mycotoxins is thought as one of the most harmful pre- and post-harvest pathogens that can cause the crop to be destroyed entirely or in part [1]. China, Ethiopia, and Egypt are the top three countries that produce Vicia faba [2, 3]. In Egypt, one of the popular legume crops eaten as fresh vegetable is the Vicia faba. The production and profitability of Vicia faba are being affected by several infectious and non-infectious disease factors [4]. Phytopathogenic fungi are the most destructive disease causative agents of Vicia faba; among these, Alternaria is one of the main fungal pathogens attacking Vicia faba, belonging to the anamorphic fungi and the group of Dothideomycetes [5]. The genus Alternaria includes species that are saprophytic, endophytic, and pathogenic [6]. This fungus can flourish on a variety of substrates, including soil, plants, animals, seeds, and agricultural commodities. Alternaria species are responsible for at least 20% of agricultural spoilage, and the worst losses could wipe out up to 80% of the crop [7]. The infection first appears on the smaller leaves and then progresses upwards. Tiny, rounded lesions 2–4 mm with defined ends of brown or black appear on and inside the leaves. The spots increase to wrap the leaf and later fused all together causing dryness and falling down of leaves [8]. Controlling plant diseases by fungicidal agents has a negative impact on human health as well as other living beings in the environment, and in this regard, plant pathogen may become resistant to fungicides and phytotoxic effects on crops. Therefore, it is preferable to discover more safe and efficient strategies for treating plant diseases to minimize these issues without disturbing the environment [9]. Recently, a number of biocontrol agents are being used to manage various plant diseases comprising Alternaria early blight [10]. Previous investigations have demonstrated that using of microorganisms that promote plant development has a considerable antimicrobial effect as well as increasing the systemic immunity of the plant, which is excellent for improving production [11]. PGPF potentially generate chemical substances that have a variety of advantages for the plant. Plant immunity and tolerance to plant stressors can be improved by both biotic and abiotic natural products [12]. Extensive studies have examined Aspergillus’ potential to act as a biological control agent toward fungus-related crop infections [13, 14]. The metabolites of A. chevalieri, a resource strain, have a range of activities, including the suppression of bacterial and fungal plant pathogens’ growth [15, 16]. Aspergillus egyptiacus has been proven to be a rich source of diverse classes of chemical constituents [17, 18]. This research aims to investigate the effectiveness of two Aspergillus isolates as a prospective biocontrol agent under in vitro and in vivo conditions against the early blight of Vicia faba caused by A. solani and, additionally, to assess the effectiveness of PGPF as a substitute and safe strategy in incorporated managing procedures for controlling the early blight disease as well as biostimulant for improving Vicia faba growth.
2 Materials and methods
2.1 Chemicals and reagents
The solvents and chemicals used were of analytic purity sourced from Sigma-Aldrich Chemical Co. (St. Louis, MO, 63,103, USA). Potato dextrose agar (PDA) and potato dextrose broth (PDB) medium was acquired from Difco (UK).
2.2 Source of the pathogen
The pathogenic fungus Alternaria solani (PPLAS 001) was gained from the Plant Pathology laboratory, Faculty of Science, Al-Azhar University, Cairo, Egypt.
2.3 Inoculum preparation
The pathogenic fungus was cultivated for a week at 28 °C on PDA to encourage sporulation. A conventional procedure previously described by Boedo et al. [19] was used to prepare the conidial suspension. The fungal culture was flooded with sterilized water and the conidia were gently removed from the fungal culture using a sterilized needle, and then, the prepared suspension was filtered. The spore density was determined by a hemocytometer and calibrated to 106 spores/ml.
2.4 Isolation and purification of PGPF
Two soil samples were collected from wheat rhizosphere in Giza, Egypt, in clean plastic bags. Ten grams of each soil sample was mixed with 90 ml of saline and allowed to stand for 30 min on shaker at 200 rpm. Serial dilutions (10−1 to 10−6) were made from samples and 0.1 ml was added to PDA plates and then incubated at 28 °C for 5–7 days. Next, the purification of isolated fungal colonies was carried out by agar streak method [20]. The purified isolates were sub-cultured on slants of their own isolation medium and stored at 4 °C for further investigation.
2.5 Screening of isolated fungal strains for plant growth promotion performances
2.5.1 Detection of HCN production
The formation of hydrogen cyanide (HCN) by the isolated fungal strains was assessed according to the methods of Frey et al. [21]. Tested fungi were inoculated on PDA supplemented with glycine 4.4 g/l. Whatman paper discs were soaked in picrate carbonate solution and then placed at the top of each Petri dish. Plates were covered with Para film and then incubated for 4 days at 28 °C.
2.5.2 Indole acetic acid (IAA) production
The efficacy of isolates for (IAA) production was determined by the colorimetric technique according to Leveau and Lindow [22]. One hundred milliliters of PDB containing 0.1% tryptophan was inoculated with 500 µl of fungal cultures and then incubated in the dark at 30 °C for 5 days. Next, centrifugation at 10,000 rpm was conducted for 15 min at 4 °C. Subsequently, 2 ml of supernatant was stirred together with 2 drops of orthophosphoric acid and 4 ml of Salkowski reagent. The formation of pinkish-red color indicates the IAA production.
2.5.3 Detection of siderophore production
The purified isolates were tested for their ability to produce siderophore following the method described by Neilands [23]. The qualitative estimation of hydroxamate type of siderophore in the culture fungal filtrate isolates was tested by using FeCl3 test. To 1 ml of culture filtrate, 5 ml of 1 mM ferric chloride solution was added. The formation of dark yellow to brown color indicated the presence of hydroxamate type of siderophores.
2.5.4 Phosphate solubilization
Phosphate solubilization was determined according to Rezzonico et al. [24]. On top of solidified PVK plates, 0.01 ml of fungal filtrate was loaded. Following incubation at 30 °C for 1 week, the presence of clear halo zone surrounded the fungi was regard as a positive result.
2.5.5 Extraction of bioactive compounds from selected PGPF
The extraction of bioactive compounds was carried as described by the method of Supaphon et al. [25]. Briefly, fungal strains were grown in PDB for three weeks in static circumstances at 28 °C. Filtration of fermentation broth was carried out to eradicate fungal mycelia. Next, the obtained filtrates were extracted twofold by ethyl acetate (EtOAc) (1:1) with continual shaking for 10 min and then tried to settle for 5 min to create two distinct layers. After detaching the organic layer from the aqueous one, the retrieved organic phase was evaporated. The attained fungal crude extract was dissolved in 5% DMSO at a 1 g/ml concentration and then preserved at refrigerator until following experiments.
2.5.6 Efficacy of PGPF against Alternaria solani using well-diffusion method
The sterilized PDA plates were inoculated with Alternaria inoculum. Wells (6 mm) were loaded with 100 µl (2 mg/ml) for each selected fungal extract separately. Fluconazole was used as the standard antifungal (+ control). Antifungal activity was determined in triplicate. The resulted inhibition zones were monitored after incubation at 28 °C for 72 h. Additionally, MIC was conducted, in which wells were filled with various concentrations of each fungal extract (0.125, 0.25, 0.50, 1, 2, and 4 mg/ml).
2.6 Identification of the most promising PGPF isolates
2.6.1 Morphological identification
In this concern, identical methods for morphological and species description were worn out. After 7 days of culturing the fungal isolate on PDA, culture characteristics including color of colony, reverse pigments, texture, and appearance were observed and documented. The isolates were also subjected to microscopic examination using a light microscope (Optika, Italy) [26, 27].
2.6.2 Molecular identification of PGPF
DNA extraction, PCR for rRNA, and sequencing
DNA extraction from fungal isolates AGH-1 and AGH-4 was done according to Al-Bedak et al. [28]. The PCR was achieved by applying the unified primers ITS1 and ITS4 and Sol Gent EF-Taq [29][29].
Alignments and phylogenetic analyses
To create a continuous sequence of the two selected PGPF Aspergillus sp. AGH-1 and Aspergillus sp. AGH-4, the DNASTAR software application (version 5.05) was employed. For phylogenetic studies, the entire ITS dataset—which contained one sequence for Aspergillus sp. AGH-1—was used. Herein, 26 sequences of the Aspergillus species that are most comparable were acquired from GenBank for this study, and an outgroup sequencing for Aspergillus restrictus NRRL 154 was employed as well. A consistent sequence of Aspergillus sp. AGH-4 was constructed using the DNASTAR computer tool (version 5.05). For phylogenetic analysis, the entire ITS data was employed which contained single sequence for Aspergillus sp. AGH-4. Throughout this work, 16 sequences of the most related Aspergillus species retrieved from GenBank, and an outgroup sequence for Aspergillus keveii CBS 209.92. Those sequences were carefully adjusted after being manually matched applying MUSCLE [30]. Maximum-likelihood (ML) examination was conducted as follows: heuristic searches with random step-by-step adding up of 1000 replicates [31] and Tree-Bisection-Regrafting (TBR) rearrangements, employing MEGA X (version 10.2.6) [32]. The best optimal model of nucleotide substitution for the ML analyses was established using Akaike information criterion (AIC) as implemented in Model test 3.7 [33]. Analyses of ML and MP phylogeny were done using MEGA X [32].
Faba bean seeds
In the present investigation, seeds of Vicia faba, L. cv. Giza 3 were obtained from Legume Crops Research Department, Field Crop Research Institute, Agricultural Research Center, Giza, Egypt.
Pot experiment design
The seeds of Vicia faba were soaked for 2 h in the inducers (A. chevalieri and A. egyptiacus) sustention’s 107 CFU, 2 ml/1 g of seeds. In the Research Garden of the Faculty of Science et al.-Azhar University, single seed/pot (30 cm in diameter) comprising of sand and clay (1:3 W/W) weighing a total of 4 kg was sown. Ten replicas of each treatment were delivered along with the pots. The following is how the treatments were set up: T1, healthy control; T2, infected control; T3, healthy plants handled with A. chevalieri; T4, healthy plants handled with A. egyptiacus; T5, healthy plants handled with a mix of A. chevalieri and A. egyptiacus; T6, infected plants handled with A. chevalieri; T7, infected plants handled with A. egyptiacus; T8, infected plants handled with mixture of A. chevalieri and A. egyptiacus. The pathogenic fungus A. solani was used to create an artificial infection 1 week following the seedlings’ germination. After a week of infection, the plants were treated with inducers to evaluate plant resistance and then the disease symptoms were recorded. Morphological measurements were documented at 60 days after seedling (shoot length in centimeter, root length in centimeter, and number of leaves per plant). Biochemical tests and yield characteristics were also denoted after 60 and 75 days of seedling, and the disease was examined.
Disease symptoms and disease index
Symptoms of the disease were evaluated from 30 to 75 days following inoculation. The disease index was determined using a score composed from the following 5 categories: 0 (no symptoms), 1 (slight-yellowing of lower leaves), 2 (moderate plant-yellowing), 3 (yellow halo around browned spots), and 4 (concentric rings of raised and lowered dead tissues) [34] according to the formula:
where n1 to n4 indicate the number of plants in the indicated classes, and nt indicates the total number of tested plants. Percent protection was calculated using the following formula:
where A is the PDI in non-inoculated control plants and B is the PDI in treated plants.
Metabolic indicators for resistance
Chlorophyll and carotenoid pigments were measured following the method of Vernon and Seely [27]. Free proline was established by the method of Bates et al. [35]. Total phenolics were detected using the assay of Supaphon et al. [28]. The activity of peroxidase and polyphenol oxidase was detected according to the methods of Srivastava and Matta [29] respectively. The method described by Nguyen and Lee [30] was used to determine the amount of MDA in fresh faba beans leaves. Hydrogen peroxide (H2O2) content was calculated as depicted by Mukherjee and Choudhuri [36].
2.7 Statistical analyses
The retrieved data were subjected to one-way variance analysis (ANOVA). The statistically significant changes among treatments were shown using the least significant difference (LSD) test at p < 0.05 by CoStat (CoHort, Monterey, CA, USA). The resulted data were presented as means ± standard errors (n = 3).
3 Results
In the current investigation, four fungal strains with distinct colony morphologies were isolated from wheat rhizosphere. Based on morphological features under light microscope, all isolates were recognized as species of Aspergilli.
3.1 Investigation of isolates according to promoting properties
As shown in Table 1, AGH1 was the best isolate capable of producing HCN (+ + +), followed by isolate AGH4 (+ +). The present results confirmed the ability of all isolates to produce IAA, and AGH1 was the best isolate followed by AGH4 (+ +). The results evoked that all isolates showed efficiency of siderophore production except for AGH2. Also, as is evident in the results, the best isolate capable of phosphate solubilization in the soil was AGH4 (+ + +), followed by AGH1 (+ +). Moreover, when the antifungal potential of the four fungal isolates was tested against A. solani, the isolates AGH-1 and AGH-4 were found more effective than the other two strains (AGH-2 and AGH-3).
3.2 Identification of the fungal isolates
The macroscopic identification of Aspergillus sp. AGH-1 illustrated low growth frequency (20 mm) diameter with excellently conidial formation on PDA later 7-day incubation at 28 ± 2 °C. Surface yellow Cleistothecia (teleomorph) with reverse brown pigment deep in center owing to the existence of Cleistothecia surrounded by yellow Hull cells (Fig. 1A, B). The microscopic examination evoked that the ascospores of Aspergillus sp. AGH-1 were with obvious crests like pulley wheels, with two prominent, narrow, longitudinal flanges. Conidial head (anamorph) uniseriate with yellow orange (Fig. 1C, D, and E). Moreover, the macroscopic identification of Aspergillus sp. AGH-4 illustrated fast growth frequency reaching 37 mm in diameter later 7-day incubation at 28 ± 2 °C on PDA. The colonies showing fast growth rate with white olivaceous surface were surrounded with white margin with reverse pale brown pigment (Fig. 2A, B). The microscopic examination evoked that the conidiophores of Aspergillus sp. AGH-4 were long, 9–35 × 1.9–2.5 µm, with globose vesicle 3.0–8.5 µm in diameter, and smooth globose conidia (2.7–04 µm in diameter) (Fig. 2C, D, and E).
3.3 Molecular identification
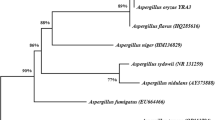
Based on a mega blast search of NCBI GenBank nucleotide database, the closest hits using ITS sequence of Aspergillus sp. AGH-1 are Aspergillus chevalieri strain CBS 522.65 [(GenBank accession number OL772683; identities = 540/540 (100%); Gaps = 0/540 (0%)] and Aspergillus costiformis CBS 101749 [(GenBank accession number OL711815; identities = 540/541 (99.82%); Gaps = 1/541 (0%)]. In this work, Aspergillus sp. AGH-1 was matched to those other Aspergillus species using a genomic analysis that relies on ITS sequencing data. The ITS data collection indicates 28 tracts comprising 576 characters, of which 453 could be correctly aligned (with no gaps or N), 42 noted as variable, and 7 considered informative. The maximum parsimony method was used to create ten trees to deduce the evolutionary record. Figure 3 shows the phylogenetic relationships with the highest log likelihood (− 1030.47), consistency index (1.000000), persistence index (1.000000), and composite index (1.000000) for all sites and parsimony-informative sites. In this investigation, Aspergillus sp. AGH-1 was shown to be grouped with Aspergillus chevalieri strains CBS 522.65 and NRRL 78 (type and ex-type specimens), with a very strong bootstrap support value of 93% ML/88% MP. Aspergillus chevalieri is the name given to the strain of Aspergillus sp. AGH-1 used in the current investigation. ITS sequence of the strain in this study was uploaded to GenBank as OP593083.
The first of 1000 entirely valid trees constructed by heuristic finding (1000 replications) of Aspergillus chevalieri AGH-1 OP593083 (blue color) in comparison to other Aspergillus ITS sequences found in GenBank. Bootstrap support values for ML/MP equal or above 50% are implied above/below the particular points. The tree is deep-rooted to Aspergillus restrictus NRRL 154 as outgroup (red color)
On the other hand, AGH-4 is Aspergillus egyptiacus CBS 991.72F [(GenBank accession number MN650832; identities = 581/583 (99.66%); Gaps = 2/582 (0%)] and Aspergillus egyptiacus CBS 656.73 [(GenBank accession number OL711762; identities = 581/584 (99.49%); Gaps = 3/584 (0%)]. In this work, Aspergillus sp. AGH-4 was matched to those other Aspergillus species. Employing a genetic analysis depending on ITS sequencing information, the ITS data collection produces 18 sequences comprising 583 characters, of which 526 could be correctly aligned (with no gaps or N), 56 noted as unpredictable, and 38 considered informative. Nine trees were created by the maximum parsimony approach. Here in Fig. 4, it clarifies the phylogenetic relationships for the complete sites and parsimony-informative sites with the highest log likelihood (− 1301.11), consistency index (0.873016), retention index (0.950000), and composite index (0.829365). Aspergillus sp. AGH-4 was clustered with Aspergillus egyptiacus strains CBS 656.73, CBS 991.72, and NRRL 5920 (type specimens), with a very strong bootstrap support value of 99% ML/100% MP. Aspergillus egyptiacus is the name given to the strain of Aspergillus sp. AGH-4. ITS sequence of the strain in this study was uploaded to GenBank as OP593080.
The first of 1000 entirely valid trees constructed by heuristic finding (1000 replications) of Aspergillus egyptiacus AGH-4 OP593080 (blue color) in comparison to other Aspergillus ITS sequences found in GenBank. Bootstrap support values for ML/MP equal or above 50% are implied above/below the particular points. The tree is deep-rooted to Aspergillus keveii CBS 209.92 as outgroup (red color)
3.4 In vitro antifungal efficacy of A chevalieri and A egyptiacus extracts against A. solani
In the present investigation, 2 mg/ml of both A. chevalieri AGH-1 OP593083 and A. egyptiacus AGH-4 OP593080 extracts demonstrated effective antifungal impact against A. solani with an inhibition zone of 18 and 16 mm respectively in comparison with 20 mm attained by fluconazole as a positive control (Fig. 5A). Moreover, the results proclaimed that A. chevalieri and A. egyptiacus AGH-4 extracts exert MIC at 0.25 mg/ml and 0.5 mg/ml giving 6 and 5 mm ZOI respectively (Fig. 5B).
3.5 Disease index (DI) and protection
In this work, as shown in Table 2, A. solani caused severe disease symptoms on Vicia faba plants, which caused DI by 85%. The use of PGPF that stimulate plant growth led to a decrease in DI% and an increase in protection % from the disease. The data exhibited that treatment with a mix of A. chevalieri and A. egyptiacus (T8) and A. chevalieri (T6) has a highly recorded protection by 70.5% and 52.9% and lowering PDI to 25% and 40% and came next A. egyptiacus (T7) that recorded protection by 50.5% and DI by 42.5 compared with untreated infected plants (T2).
3.6 Growth markers
The presented outcomes in Fig. 6 showed that A. solani (T2) affect the shoot and root length as well as the number of leaves (27.5 cm, 3.313 cm, and 9.6 leaf) of Vicia faba in contrast with healthy control (T1) (60.80 cm, 7.79 cm, and 24.80 leaf) respectively. It is interesting that the PGPF isolates used, whether single or mixed, showed a significant improvement in all growth characteristics of healthy Vicia faba, and the results showed that the best treatments were a mix of A. chevalieri and A. egyptiacus (T5), as it recorded an increase in the shoot lengths (92.80 cm), root length (9.28 cm), and number of leaves (37.20), followed by treatment with A. egyptiacus (T4) and came next A. chevalieri (T3). About the effect of PGPF on the challenged plants with A. solani, it was noticed that pretreatment seeds with a mix of A. chevalieri and A. egyptiacus (T8) gave a high significant increase in the shoot lengths (55.50 cm), root length (7.28 cm), and number of leaves (23.32) followed by treatment with A. egyptiacus (T7) and A. chevalieri (T6) respectively compared to infected plants only.
Effect of tested fungi on morphological indicators A shoot length, B root length, and C leaves number/plant; T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data are presented as means ± SE (n = 3). Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
3.7 Yield characteristics
The obtained data in Fig. 7 showed that A. solani (T2) decline the number of pods/plant (13.93) and number of seeds/plant (39.10) as well as the weight of 100 seed (g) (146.93) of Vicia faba plants in parallel with healthy control (T1) (23.43, 53.26, and 186.94) respectively. It is attractive that the inducers used, whether single or mixed, produced a significant increase in all yield tested characteristics, and the results revealed that the greatest inducers were a mix of A. chevalieri and A. egyptiacus (T5), as it showed an increase in the number of pods/plant (25.966) and number of seeds/plant (64.03) as well as the weight of 100 seed (205.83) at level of healthy Vicia faba plant, followed by treatment with A. chevalieri (T3) and came next A. egyptiacus (T4). Regarding to the effect of PGPF on the infected plants, it was observed that pretreatment seeds with a mix of A. chevalieri and A. egyptiacus (T8) passed greatly a significant increase in the number of pods/plant (21.5) and number of seeds/plant (58.3) as well as the weight of 100 seed (167.8), followed by treatment with A. chevalieri (T6) and came next A. egyptiacus (T7), compared to infected plants only.
Effect of tested fungi on yield, A seed N/plant, B no of pods/plant, C weight of 100 seed (g); T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data are presented as means ± SE (n = 3). Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
3.8 Photosynthetic pigments
Data represented in Fig. 8 showed that contents of chlorophyll a and b were greatly reduced in A. solani-infected plants (T2). On the other hand, plants handled with a mix of A. chevalieri and A. egyptiacus (healthy T5) showed considerable increases in chl. a and b contents. Also, A. solani-infected plants treated with a mix of A. chevalieri and A. egyptiacus (T8) show major increase in the contents of chl. a and b as well as carotenoid compared with non-treated infected ones. Concerning the effect of PGPF on A. solani-infected plants, it was found that a mix of A. chevalieri and A. egyptiacus (T8) proved substantial enhancement in the contents of chlorophyll a and b and carotenoid, in comparison with A. chevalieri (T6) and A. egyptiacus (T7).
Effect of tested fungi on photosynthetic pigments; A chlorophyll a (mg/g F.Wt), B chlorophyll b (mg/g F.Wt), C carotenoids (mg/g F.Wt); T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data are presented as means ± SE (n = 3). Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
3.9 Free proline and phenol content
Data recorded in Fig. 9 showed that the A. solani-infected plants (T2) naked a rise in the free proline and phenol contents by 176.18% and 175.50%. It is attractive that the PGPF isolates used boosted the resistance of plant by increasing free proline and phenol contents. Plants treated with individual or combination of A. chevalieri and A. egyptiacus showed substantial increases in free proline contents of healthy or A. solani-infected plants. Regarding the effect of PGPF on the infected plants, it was noticed that a mix of A. chevalieri and A. egyptiacus (T8) increased the contents of proline and phenol contents by 96.97% and 49.84% followed by treatment with A. chevalieri by 67.68% and 45.43% and came next A. egyptiacus (T7) by 65.91% and 14.95% respectively.
Effect of tested fungi on A free proline and B phenol content; T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data are presented as means ± SE (n = 3). Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
3.10 H2O2 and MDA
The results illustrated in Fig. 10 indicated that A. solani infection (T2) caused considerable increase in stress indices (H2O2 and MDA) by 57.79% and 1110.29% in contrast to healthy plants. Moreover, treatment with PGPF led to a decrease in the levels of stress indices (H2O2 and MDA) in both healthy and infected plants. Plants treated with individual or combination of A. chevalieri and A. egyptiacus showed substantial decline in stress indices (H2O2 and MDA) of healthy or A. solani-infected plants. The mix of A. chevalieri and A. egyptiacus (T8) treatment was the best treatment, as it recorded a significant decrease in the levels of H2O2 and MDA by 31.48% and 5.70%, then A. egyptiacus (T7) by 26.19% and 40.99%, and then A. chevalieri (T6) by 26.07% and 23.73%, respectively.
Effect of tested fungi on A H2O2 and B MDA; T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data are presented as means ± SE (n = 3). Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
3.11 Antioxidant enzyme activity
As shown in Fig. 11, major activities of POD and PPO were recorded in infected Vicia faba plants. Data recorded in Fig. 11 showed that the A. solani-infected plants (T2) naked a rise in the activities of POD and PPO by 14.95% and 53.63%. Additionally, treatment with PGPF led to boost the activities of POD and PPO. Plants treated with individual or combination of A. chevalieri and A. egyptiacus showed substantial increases in the activities of POD and PPO of healthy or A. solani-infected plants. Regarding the effect of PGPF on the infected plants, it was noticed that the maximum rates of POD and PPO were detected directly to the application of mix of A. chevalieri and A. egyptiacus (T8) by 175.31% and 62.79%, followed by A. chevalieri (T6) by 137.54% and 52.03%, and came next A. egyptiacus (T7) by 115.98% and 40.31%, respectively.
Effect of tested fungi on antioxidant enzymes A POD and B PPO; T1, healthy control; T2, infected control; T3, H + A. chevalieri; T4, H + A. egyptiacus; T5, H + A. chevalieri + A. egyptiacus; T6, I + A. chevalieri; T7, I + A. egyptiacus; and T8, I + A. chevalieri + A. egyptiacus. Data followed by different letters are significantly different following least significant difference (LSD) test at p < 0.05
4 Discussion
The fungi have known to be a substantial resource of active compounds with innovative structures along with different pharmacological actions. Among the major producers of active compounds derived from fungi is Aspergillus. In the current work, Aspergillus chevalieri AGH-1 and Aspergillus egyptiacus AGH-4 demonstrated greet antifungal efficacy against A. solani in vitro. Our findings agreed with prior studies that showed that Aspergillus species could be utilized for plant growth promotion and prevention of fungal infections [37,38,39]. Moreover, A. chevalieri metabolites have a wide range of activities, including the suppression of plant pathogens’ growth [15, 16]. Additionally, Aspergillus egyptiacus is a rich source of multiple different chemical metabolites [17]. On the other hand, Barton and d’Errico [40] revealed that fungi in the genus Aspergillus have positive impacts on plants and are utilized in a variety of forms and treatments to preserve, promote, and enhance plant growth. The application of PGPF can induce both the plant growth and systemic resistance reactions to biotic stresses [41]. Moreover, according to the study of Abdel-Motaal et al. [42], PGPF A. flavus aids in the creation of defenses against Alternaria early blight disease. The trend toward induction of systemic plant resistance has become necessary as an attempt to reduce the use of chemical pesticides and plant diseases [43]. Considering the severe climatic changes that helped spread pathogens and reduce the quality of agricultural crops, which harmed the agricultural economy. The use of growth-stimulating fungi based on the ability against the growth of pathogenic fungi and the production of stimulants and growth stimulants for plants has been recommended by much scientific research recently. In this study, we tended to select new fungal strains A. chevalieri and A. egyptiacus that could inhibit the action of Alternaria solani that causes blight disease. At the same time, the isolated fungi produce HCN, IAA, and siderophores and are effective in phosphate solubilization. HCN is a strong antifungal and has an essential role in the biocontrol of plant pathogens [24, 44, 45]. Indole acetic acid is one of the most important and active physiological plant hormones as it controls cell growth, cell division, tissue differentiation, and cell response plant for light and gravity and for the growth and development of plant organs [46]. It has been established that IAA plays an important direct role in cell divisions and stimulation of roots, which contributes to improving plant growth [47]. In this study, the PGPF strains showed a great ability to produce plant growth-promoting substances that support their ability to improve plant vegetative growth, cell division, and root activation. The findings of this work agree with previous studies that support the ability of Aspergillus to produce IAA [48, 49]. The current results are consistent with the studies conducted by Khan et al. and Carvalho et al., who revealed that fungal strains can produce iron carriers to repress iron from the extracellular environment [50, 51]. Siderophore-iron complexes are transported into the cell through receptors in the membrane, which makes these strains able to improve the vital processes of the plant [52]. Organic phosphorus first converted into inorganic phosphorus by the activity of microorganisms [53, 54]. It is earlier recognized that A. egyptiacus and A. chevalieri can induce biochemical resistance in plant [55, 56]. The oppressive effect of the present chosen PGPF strains against A. solani could be associated to the production of HCN, IAA, and siderophores that control the obtainability of iron. The disease index was the earliest influence to control systemic resistance in healed plants by PGPF. In the current work, A. solani caused severe disease symptoms on Vicia faba plants, which caused DI by 85.0% which was in the same line with the observations recorded by Behairy et al. and El-Ammari [57, 58]. The results referred to the ability of the PGPF to produce toxic compounds against the action of the pathogenic fungus. Many studies indicate the ability of the isolates to stimulate plant immunity by producing toxic compounds for pathogens that are stored in the succulent vacuoles of the plant called phytocides [59, 60]. Evidence of pathological infestation showed highly significantly decreased in all morphological parameters. The results recorded a significant damaged on shoot and root length and number of leaves of Vicia faba in contrast with healthy control. These results are consistent with those obtained by Kamei and Singh [61]. The severe deficiency in the morphological parameters because of the fungal infection may be due to the oxidative explosion in the cells and accumulation of reactive oxygen species (ROS), causing growth hormone disorders. In the current study, A. chevalieri and A. egyptiacus were able to create IAA and facilitate phosphate and siderophore which support the results of improved growth and recovery of infected plants [62]. The harmful impacts of A. solani on Vicia faba might be owing to disorders in the mechanisms of growth hormones [63]. Fungal injuries cause failure of the photosynthesis activity [64,65,66]. Results in the current study recorded that contents of chlorophyll a and b were greatly reduced in A. solani-infected plants. The drop in chlorophyll is a consequence of oxidative pressure during disease due to the release of ROS, initiating destruction of chloroplasts; as a result, the plant is unable to absorb light and perform the process of photosynthesis [67,68,69]. The remarkable improvement in photosynthetic pigments because of treatment with A. chevalieri and A. egyptiacus may be due to the reduction of oxidative stress, the building of new cells that retain chloroplasts, and the regularity of vital processes within the cell [70], or could be attributed to enriching the plant and its soil with N2 element [41, 71]. Also, the current data recorded that the A. solani-infected plants naked a rise in the free proline and phenol contents. Our findings were consistent with those obtained by Zohra et al. [72]. Moreover, as the invasion happens as a result of the pathogen’s provocation of the plants, the plant produces new compounds that are not really existed in the healthy plant [73, 74]. These substances stop or restrict the pathogen development [75, 76]. Fungal infection causes oxidative stresses to occur within the cells, which are expressed by an increase in H2O2 and MDA [77]. A. solani led to a significant increase in stress indices (H2O2 and MDA) compared to healthy plants and this was in the same line with the findings obtained by Ikram et al. and Hathout et al. [78, 79]. Reducing levels of H2O2 and MDA due to the use of A. chevalieri and A. egyptiacus provides strong evidence of an improved plant immune response. The current results indicated that the mix of A. chevalieri and A. egyptiacus was the best treatment, as it recorded a significant decrease in the levels of H2O2 and MDA and then A. egyptiacus and A. chevalieri, respectively. These effects related to the study were consistent with the study of Ozden et al. [80] who recorded that the use of PGPF under fungal infection resulted in dipping MDA and H2O2. Several publications reported the induction of plant resistance with bio-inducing agents that increase enzymatic activity to reduce oxidative stress. Increasing the activity of antioxidant enzymes is another way to defend the plant against various stress factors [81].
5 Conclusion
The current study employed a promising practice that was primarily focused on the utilization of PGPF for the invention of systemic resistance of Vicia faba plant against A. solani blight. The isolated PGPF were identified morphologically as well as by molecular characterization as Aspergillus chevalieri AGH-1 and Aspergillus egyptiacus AGH-4 and then deposited in GenBank under accession numbers OP593083 and OP593080. In vitro, A. solani appeared to be susceptible to putative antifungal action from Aspergillus chevalieri AGH-1 and Aspergillus egyptiacus AGH-4. As a result, PGPF are potential agents for use in agriculture and as an efficient biological control against the Alternaria pathogen that causes Vicia faba early blight disease. The recovery of treated plants that were treated with A. chevalieri AGH-1 and A. egyptiacus AGH-4, singly or in combination, is associated with improving the characteristics of growth, yield, and photosynthesis pigments and stimulating plant immunity by increasing the level of phenol, proline, and the activity of antioxidant enzymes, as well as reducing stress factors. We can recommend the application of Aspergillus chevalieri AGH-1 OP593083 and Aspergillus egyptiacus AGH-4 OP593080 as safe and eco-friendly bio-fungicide alternatives to chemical pesticides to preserve plant, soil, and human health.
Data availability
This investigation offers all the data collected or estimated throughout this effort.
References
El-Batal AI, El-Sayyad GS, Al-Shammari BM, Abdelaziz AM, Nofel MM, Gobara M et al (2023) Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microb Pathog 180:106131
Beyene AT (2014) Genetic analysis and characterization of faba bean (vicia faba) for resistance to chocolate spot (Botrytis fabae) disease and yield in the Ethiopian highlands (Doctoral dissertation).
FAO F (2017) Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT)
Hashem AH et al (2021) Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in faba bean plants. J Fungi 7(3):195
Eriksson OE, Winka K (1997) Supraordinal taxa of Ascomycota. Myconet 1(1):1–16
DeMers M (2022) Alternaria alternata as endophyte and pathogen. Microbiology 168(3):001153
Nowicki M, Nowakowska M, Niezgoda A, Kozik E (2012) Alternaria black spot of crucifers: symptoms, importance of disease, and perspectives of resistance breeding. Veg Crops Res Bull 76
Gupta S et al (2017) Prevalence, incidence and management of Alternaria blight of rape-seed and mustard (Brassica juncea L) through resistance and integrated approach. Int J Curr Microbiol App Sci 6:2730–2741
Cooper J, Dobson H (2007) The benefits of pesticides to mankind and the environment. Crop Prot 26(9):1337–1348
Abdelaziz AM, Hashem AH, El-Sayyad GS, El-Wakil DA, Selim S, Alkhalifah DH, Attia MS (2023) Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. Trop Plant Pathol:1–23
Hashem AH et al (2023) Potential Impacts of clove essential oil nanoemulsion as bio fungicides against neoscytalidium blight disease of Carum carvi L. Agronomy 13(4):1114
Bartwal A et al (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32:216–232
Kishore G, Pande S, Podile A (2005) Phylloplane bacteria increase seedling emergence, growth and yield of field-grown groundnut (Arachis hypogaea L.). Lett Appl Microbiol 40(4):260–268
Attia MS, Salem MS, Abdelaziz AM (2022) Endophytic fungi Aspergillus spp. reduce fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Convers Biorefin:1–11
Bovio E et al (2019) Marine fungi from the sponge Grantia compressa: biodiversity, chemodiversity, and biotechnological potential. Mar Drugs 17(4):220
Zin WWM et al (2017) Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 141:86–97
SRM I, Mohamed GA, Ahmed HEA (2016) Aegyoxepane: a new oxepane derivative from the fungus Aspergillus aegyptiacus. Lett Org Chem 13(8):560–565
Al-Fakih AA, Almaqtri WQA (2019) Overview on antibacterial metabolites from terrestrial Aspergillus spp. Mycology 10(4):191–209
Boedo C et al (2012) Evaluating aggressiveness and host range of Alternaria dauci in a controlled environment. Plant Pathol 61(1):63–75
Gourama H, Bullerman LB (1995) Antimycotic and antiaflatoxigenic effect of lactic acid bacteria: a review. J Food Prot 58(11):1275–1280
Frey-Klett P et al (2005) Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol 165(1):317–328
Leveau JH, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71(5):2365–2371
Neilands J (1981) Iron absorption and transport in microorganisms. Annu Rev Nutr 1(1):27–46
Rezzonico F et al (2007) Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2, 4-diacetylphloroglucinol really synonymous with higher plant protection? New Phytol 173(4):861–872
Supaphon P et al (2013) Antimicrobial potential of endophytic fungi derived from three seagrass species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE 8(8):e72520
de Gouveia Dal Pino EM, Kowal G (2014) Particle acceleration by magnetic reconnection. In: Magnetic fields in diffuse media. Berlin, Heidelberg, pp 373–398
Nguyen TT, Lee HB (2018) Isolation and characterization of three Zygomycetous fungi in Korea: Backusella circina, Circinella muscae, and Mucor ramosissimus. Mycobiology 46(4):317–327
Al-Bedak OA et al (2020) Impact of fumigation with phosphine on viability of wheat grains stored for six months at two levels of moisture content, in addition to description of four new records of associated fungi and assessment of their potential for enzymatic production. J Basic Appl Mycol (Egypt) 11:77–97
White TJBT (1990) Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: PCR protocols: a guide to methods and applications, pp 315–322
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics (Oxford, England) 14(9):817–818
Kishore PV, Gandhi SK, Singh S (2008) Biological control of Alternaria solani, the causal agent of early blight of tomato. J Biol Control:67–72
Bates L, Waldren RA, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant and soil 39:205–207
Mukherjee S, Choudhuri M (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170
Imran M et al (2021) Multifarious functional traits of free-living rhizospheric fungi, with special reference to Aspergillus spp. isolated from North Indian soil, and their inoculation effect on plant growth. Ann Microbiol 71(1):1–17
Pandya N et al (2018) Plant growth promoting potential of Aspergillus sp. NPF7, isolated from wheat rhizosphere in South Gujarat, India. Environ Sustain 1(3):245–252
Yoo S-J et al (2018) Aspergillus terreus JF27 promotes the growth of tomato plants and induces resistance against Pseudomonas syringae pv. tomato. Mycobiology 46(2):147–153
Barton N, d’Errico F (2012) North African origins of symbolically mediated behaviour and the Aterian. Developments in Quaternary Sciences. Elsevier, pp 23–34
Hossain MM, Sultana F, Islam S (2017) Plant growth-promoting fungi (PGPF): phytostimulation and induced systemic resistance. In: Plant-microbe interactions in agro-ecological perspectives: volume 2: microbial interactions and agro-ecological impacts, pp 135–191
Abdel-Motaal F et al (2020) Early blight suppression and plant growth promotion potential of the endophyte Aspergillus flavus in tomato plant. Ann Agric Sci 65(2):117–123
Alabouvette C, Olivain C, Steinberg C (2006) Biological control of plant diseases: the European situation. Eur J Plant Pathol 114:329–341
Rijavec T, Lapanje A (2016) Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol 7:1785
Pal K et al (2000) Antifungal characteristics of a fluorescent Pseudomonas strain involved in the biological control of Rhizoctonia solani. Microbiol Res 155(3):233–242
Gomes G, Scortecci K (2021) Auxin and its role in plant development: structure, signalling, regulation and response mechanisms. Plant Biol 23(6):894–904
Shakirova F (2007) Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. Salicylic Acid: Plant Hormone 4:69–89
Hatamzadeh S, Rahnama K, White JF, Oghaz NA, Nasrollahnejad S, Hemmati K (2023) Investigation of some endophytic fungi from five medicinal plants with growth promoting ability on maize (Zea mays L.). J Appl Microbiol 134(1):lxac015
Nafaa M et al (2023) Screening and Identification of the Rhizosphere Fungal Communities Associated with Land Reclamation in Egypt. Agriculture 13(1):215
Khan MA et al (2016) Phylogenetic identification of DNase secreting soil bacteria antagonistic to Fungus aspergillus. Nat Environ Pollut Technol 15(1):19
Carvalho DDC et al (2011) Biocontrol of seed pathogens and growth promotion of common bean seedlings by Trichoderma harzianum. Pesq Agrop Brasileira 46:822–828
Saha M et al (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res 23:3984–3999
Walpola BC, Yoon M-H (2012) Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: a review. Afr J Microbiol Res 6(37):6600–6605
Igiehon NO, Babalola OO (2017) Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Appl Microbiol Biotechnol 101(12):4871–4881
Jangir M et al (2018) Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol Control 123:60–70
Nithyapriya S et al (2021) Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 13(10):5394
Behairy MH et al (2014) Alternaria leaf spot disease control on faba bean in Egypt. J Plant Protect Pathol 5(1):119–130
El-Ammari A (2017) Plant Fungal Diseases of Faba bean in Benghazi [Version 1; awaiting peer review]. ContROL 1:15
Hossain MM, Sultana F (2020) Application and mechanisms of plant growth promoting fungi (PGPF) for phytostimulation. Org Agric:1–31
El-Maraghy S, Tohamy A, Hussein K (2021) Plant protection properties of the plant growth promoting fungi (PGPF): mechanisms and potentiality. Curr Res Environ Appl Mycol 11(1):391–415
Kamei D, Singh A. U. In-vitro studies of different culture media and biocontrol agents on growth and sporulation of Alternaria alternata (Fr.) keissler an incitant of broad bean (Vicia Faba L.) leaf blight.
Karlidag H et al (2007) Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci Hortic 114(1):16–20
Akladious SA, Gomaa EZ, El-Mahdy OM (2019) Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG-4) causing root rot in faba bean (Vicia faba) plants. Eur J Plant Pathol 153:1237–1257
Bassanezi R et al (2001) Accounting for photosynthetic efficiency of bean leaves with rust, angular leaf spot and anthracnose to assess crop damage. Plant Pathol 50(4):443–452
Nogués S, Cotxarrera L, Alegre L, Trillas MI (2002) Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytol:461–470
Singh VK, Singh HB, Upadhyay RS (2017) Role of fusaric acid in the development of ‘Fusarium wilt’symptoms in tomato: physiological, biochemical and proteomic perspectives. Plant Physiol Biochem 118:320–332
Choudhury S, Panda SK (2005) Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut 167(1):73–90
Jahan MS et al (2020) Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol Environ Saf 197:110593
Singh G et al (2021) Bioformulation development via valorizing silica-rich spent mushroom substrate with Trichoderma asperellum for plant nutrient and disease management. J Environ Manage 297:113278
Desoky E-SM et al (2020) Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal Agric Biotechnol 30:101878
Al-Ani RA, Adhab MA (2013) Bean Yellow Mosaic Virus (BYMV) on broadbean: characterization and resistance induced by Rhizobium leguminosarum. J Pure Appl Microbiol 7(1):135–142
Zohra E et al (2021) Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: a comprehensive insight on the mechanistic approach and future perspectives. Green Process Synthesis 10(1):456–475
Doughari J (2015) An overview of plant immunity. J Plant Pathol Microbiol 6(11):10.4172
Liu G et al (2007) Targeted alterations in iron homeostasis underlie plant defense responses. J Cell Sci 120(4):596–605
Ramos MV et al (2019) Laticifers, latex, and their role in plant defense. Trends Plant Sci 24(6):553–567
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plants 10(3):531
Debona D et al (2012) Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 102(12):1121–1129
Ikram M et al (2022) Phytogenic selenium nanoparticles elicited the physiological, biochemical, and antioxidant defense system amelioration of Huanglongbing-Infected ‘Kinnow’Mandarin Plants. Nanomaterials 12(3):356
Hathout TA et al (2010) Biocontrol of Phaseolus vulgaris root rot using arbuscular mycorrhizae. Egypt J Agric Res 88(1):15–29
Ozden M, Demirel U, Kahraman A (2009) Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Scientia Horticulturae 119(2):163–168
Caverzan A, Casassola A, Brammer SP (2016) Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives 17:463–480
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, G.E.D., A.Y.M., A.M.A., and M.S.A.; methodology, G.E.D., A.Y.M., A.M.A., and M.S.A.; software, A.Y.M., A.M.A., and M.S.A.; formal analysis, A.Y.M., A.M.A., and M.S.A.; investigation, A.Y.M., A.M.A., and M.S.A.; resources, G.E.D., A.Y.M., and M.S.A.; data curation, A.Y.M., A.M.A., and M.S.A.; writing original draft preparation, A.Y.M., and M.S.A.; writing review and editing, G.E.D., A.Y.M., A.M.A., M.M.N., and M.S.A.; supervision, A.Y.M. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
There are no experiments on person or animal in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Daigham, G.E., Mahfouz, A.Y., Abdelaziz, A.M. et al. Protective role of plant growth-promoting fungi Aspergillus chevalieri OP593083 and Aspergillus egyptiacus OP593080 as biocontrol approach against Alternaria leaf spot disease of Vicia faba plant. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04510-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04510-4