Abstract
The valorization of biogas as a renewable energy source faces a major obstacle regarding its purification. Siloxane is one of the impurities that cause problems such as damages to equipment of combustion engines, turbines, and boilers used for biogas conversion to heat and electricity. In this review, adsorption for siloxane removal is widely discussed, with two specific approaches: adsorbents sensitivity to water and regeneration, two essential points for industrial application. Thus, determining factors in adsorbents capacity, reusability, and water tolerance including textural properties, surface functional groups, and hydrophobicity are deeply analyzed. Studies oriented to the optimization of traditional adsorbents such as activated carbon, silica gel, and aluminosilicates as well as newly emerging adsorbents such as metal organic frameworks, graphene oxides, and waste-derived materials are studied in detail in terms of reusability and water tolerance. Although activated carbon is commercially used, its low selectivity, pore blockage due to siloxane polymerization, and unsuccessful regeneration make it disadvantageous. Silica gel, however, shows better reusability as a result of less adsorbent-adsorbate dissociation energy. In addition, aluminosilicates, despite its low adsorption capacity, proved to be more practical for real biogas due to their high hydrophobicity. Graphene oxide cost and energy efficiency in their synthesis make them more industrially appealing candidates despite their low adsorption capacity. Finally, metal organic frameworks demonstrated high selectivity, high adsorption capacity, and more efficient regeneration and therefore have more advantages and less drawbacks, although the number of published studies is still limited.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Biogas valorization and utilization as a renewable source of energy has increasingly captured the worldwide attention over the last decades towards a lesser dependence on fossil fuels [1,2,3]. However, its use has always had its own challenges due to the presence of undesirable substances such as carbon dioxide, H2S, siloxanes, H2O, NH3, N2, and so forth [4, 5]. Biogas treatment includes its purification and upgrading process. Purification process typically includes firstly drying by dewatering, and then removing hydrogen sulfide, and finally removing other impurities. Upgrading process simply refers to the separation of methane from carbon dioxide to obtain high methane-enriched biogas which is the so-called biomethane [6]. However, biogas upgrading systems faces challenges such as the massive digestate produced that will add more complication in terms of land use and also the consequent greenhouse gas emissions released from the digestate storage, transport, and manipulation. This challenge can be overcome by integrating some strategies into the anaerobic digestion such as the use of biochar, which will lead to a carbon zero-emission to the environment. Also, the biochar produced from digestate can be easily stored, transported and exploited for soil amendment [7, 8]. In addition, other strategies have been investigated to facilitate biogas upgrading in terms of methane content enrichment and simultaneously removing some potential biogas impurities such as hydrogen sulfide [9]. Siloxane is one of the biogas impurities that brings about various obstacles such as corrosion or coating, as well as damages to some equipment such as combustion engines, turbines and boilers used in the process of valorization and conversion to heat and electricity when biogas is used as a substitute for natural gas in grids or fuel cells [3, 10, 11]. Although the concentration of siloxane in biogas does not exceed 0.4–0.5 mg/L [12], more and more attempts have been made for its elimination due to its adverse effects on the equipment, which imposes high costs of maintenance and frequent equipment replacement [12, 13]. There are two main types of siloxanes including poly dimethyl siloxanes (PDM) and methyl siloxanes which are volatile (VMS). VMS is the principal siloxane that ends up in biogas as a result of PDM hydrolysis and the volatilization of a part of VMS available in the digester during the anaerobic digestion process [14,15,16]. VMS falls into two categories: linear and cyclic. Each category of linear and cyclic also has different types, which will be discussed later. Besides, biogas composition in terms of siloxane content varies mainly because of the different digested organic substrates [17]. Thus, the selection of the methods for siloxane elimination is challenging and needs to be carefully carried out according to the typology of siloxanes found in biogas.
To date, there have been various methods and technologies developed for the separation and removal of siloxanes from biogas including absorption, adsorption, cryogenic condensation, biological removal, and membrane separation [13, 18,19,20,21,22]. Absorption of siloxanes can occur either physically or chemically. Physical absorption of biogas components are based on the solubility difference of the components in the solvent without any chemical reaction [23,24,25,26]. Thus, in order to eliminate a specific component from biogas, the appropriate solvent needs to be selected. Siloxanes are hydrophobic and non-polar and therefore cannot be absorbed by polar absorbents like water. Hence, organic solvents with long carbon chains have been traditionally used for the physical absorption of siloxanes [27, 28]. A significant drawback regarding physical absorption is that the siloxanes will strip from the solvent at elevated gas flow rates due to their high volatility. Nonetheless, when siloxanes go through chemical absorption, they can be converted to components with lower volatility, and therefore, the problem of their stripping from the solvent at higher gas flow rates can be minimized. On the other hand, siloxanes demonstrate extreme stability against chemical and biochemical degradation, and only strong acids and bases can be useful for catalyzing the cleavage of Si-O bonds of siloxanes [2, 29]. However, strong acids and bases cannot be practically used because of the consequences in terms of large amounts of precipitated carbonates formed in the presence of CO2 in case of bases and potential corrosion in case of acids [2, 18, 30]. Condensation is another method that at low temperatures and pressures makes the removal of some of the biogas impurities feasible. However, such removal technique neither will lead to a complete removal nor would be economically feasible. Schweigkofler and Niessner’s experiments on the removal of siloxane using condensation at 5 °C concludes with this statement as their results only demonstrated less than 15% siloxane removal from biogas [18, 31]. For this reason, in industrial application, this method is implemented together with other techniques as adsorption. Biological removal of siloxane is another method for siloxane removal, which is based on the fact that VMS (volatile methyl siloxanes) can be degraded by a number of microorganisms such as Arthrobacter, Agrobacterium, Fusarium oxysporum, Methylibium sp., Pseudomonas sp., Phyllobacterium myrsinacearum, Rhodanobacter and Xanthomonadaceae as VMS are an appropriate carbon source for such microorganisms [22, 32,33,34]. Biotrickling filters (BTF) are the reactors typically used for the biological removal of VMS. However, most of the reports demonstrated either low removal efficiencies or long residence times. While siloxane removal methods such as activated carbon adsorption, phosphoric acid absorption, membrane separation, and water scrubbing are widely used at industrial scale for VMS removal, biological methods have been principally examined at laboratory scale [35, 36]. Thus, the performance of such biological systems needs to be enhanced in order to justify their feasibility. Another technique for siloxane removal from biogas is membrane separation system that provides high surface area and occupies low space. Membrane separation system is known to have low energy requirements and high efficiency. In addition, the membrane used for siloxane removal is normally of high selectivity for siloxane, and the resultant methane purity is remarkably high, which means that not only less energy is needed but also less methane is lost in this system [20, 31]. However, while membrane technology has been well developed for CO2 removal, its utilization for siloxane removal is limited. Besides, due to high investment and operational costs, the membrane technology for siloxane removal is still in the research phase. On the other hand, interfering compounds existing in biogas such as H2S and water vapor as well as oil vapors from engines can cause significant damage to the membranes, in addition to the problem regarding their pore blockage and fouling due to the siloxane itself. Consequently, pretreatment of the biogas would be essential as well as the fact that membranes need to be renewed periodically [20, 37].
Adsorption is more commonly used for siloxane removal due to advantages such as cost-effectiveness and easy operation, among others [19, 38, 39]. To date, there have been several adsorbents investigated and developed for siloxane removal including activated carbon, silica gel, and aluminosilicate adsorbents as traditional ones [40,41,42,43,44]. However, a great effort of research has been made during the last two decades to find out more efficient adsorbents to optimize the process and to overcome the limitations encountered traditional adsorbents. Particularly, activated carbon and silica gel have been two of the most commonly used adsorbents for siloxane separation from biogas. Activated carbon, thanks to its large surface area and therefore high adsorption capacity, low cost, availability, and stability, has been used commercially [41, 42, 45]. However, some drawbacks such as low selectivity for siloxane due to its affinity for methane adsorption and its probable pore blockage due to the siloxane polymerization phenomenon have been reported, a process that is favored by the biogas water content [40, 43]. Conventionally, this adsorption system is accompanied by a condensation process as a pretreatment to eliminate the water content to enhance its efficiency. Silica gel, as opposed to activated carbon, demonstrated better reusability thanks to the weak adsorbent-adsorbate forces dominating the adsorption mechanism [31, 46]. Researchers also managed to achieve higher adsorption efficiencies and less sensitivity of silica gel to water molecules in biogas by manipulating the effective factors of the adsorbent such as textural and surface properties by means of the modification of the adsorbent synthesis and post treatment process of its preparation [44, 46, 47]. Zeolites and mesoporous aluminosilicates are another category of adsorbents that have been used for siloxane separation from biogas [48, 49]. Although they did not show good performance in terms of adsorption capacity compared to activated carbon, they proved to be competent in terms of sensitivity to biogas water content, due to their high hydrophobicity. Furthermore, the manipulation of their preparation procedure by increasing aluminum dopant and calcination temperature had a positive effect on their pore size and therefore efficiency [46, 49]. The effect of biogas water content on siloxane removal will be also studied in detail in this review. More recently, having unique characteristics such as pore size and shape tunability and high surface area [50,51,52], metal organic frameworks (MOFs) have also been studied as novel adsorbents for siloxane removal and have demonstrated higher adsorption capacity and selectivity compared to conventional adsorbents [53,54,55]. Additionally, their regeneration is reported to be quite successful as opposed to activated carbon and silica gel, a phenomenon that is attributed to the lack of polymerization of siloxane onto the adsorbent surface [56]. In the most recent studies, graphene oxide, an emerging material for environmental applications such as energy storage and separation or water treatment, has gained attention for siloxane removal. Reduced graphene oxide aerogels (rGOA) have been the most used graphene oxides investigated for siloxane adsorption. Although the number of the studies in this regard is few and they are not completely developed, rGOAs have shown high efficiency in terms of reusability in addition to their operation simplicity and the low amount of energy used for their synthesis [57,58,59]. Finally, waste-derived materials and their application in adsorption processes have also gained interest [45, 60, 61]. Taking advantage of waste material and their recycling not only alleviates the environmental burdens of waste management and disposal and environmental consequences, but also eliminates the need for producing raw materials in the framework of circular economy. For instance, activated carbon and carbon nanotubes were successfully synthesized from lignocellulosic biomass waste, with high specific area and pore volume, which can be further tested for the adsorption applications [62, 63] . Regarding siloxane adsorption, a porous silica was synthesized using the residual sand from a WWTP [61], a waste wood-derived biochar was utilized for the adsorption of siloxane [45], a lignocellulosic waste generated in food and wood industry was tested for siloxanes L2 and D4 adsorption [64], and a biochar modified with iron was evaluated for L2 siloxane adsorption [65]. Therefore, adsorbents derived from waste that have been used for siloxane removal will be also reviewed.
This review is intended to focus on the adsorptive removal of siloxane from biogas and discuss the recent advances regarding the adsorption process including the optimization of traditional adsorbents as well as the recent appearance of emerging adsorbents such as graphene oxide, metal organic frameworks (MOFs), and waste-derived materials. Specifically, published studies are critically reviewed in terms of regeneration of the adsorbents used as a factor that determines the adsorbents feasibility at the commercial or industrial scale. In addition to this, a specific focus on the investigation of surface properties of the adsorbents such as porosity, hydrophobicity, and surface functional groups is presented. These parameters are the most important as far as the adsorption capacity and reusability are concerned. The significance of this review is as following: the regeneration of the adsorbents is essential as far as their industrial application is concerned; therefore, in this review, all the adsorbents are scrutinized and compared among them in terms of reusability considering the features of the adsorbents that affect and determine their reusability. These are the bonding forces between the adsorbate and adsorbent that affect the dissociation energy of the adsorbate from the adsorbent surface, as well as issues like polymerization of siloxane in the adsorbents pores that leads to pore blockage and hampers the regeneration process. On the other hand, the adsorbents are compared and evaluated in terms of their sensitivity to water, since moisture is one of the unavoidable components of biogas, and the adsorbents that lose their adsorption capacity in its presence are indeed questionable with real biogas. In this sense, they will not be as practical and efficient as the adsorbents the performance of which is not significantly affected in the presence of water due to their hydrophobicity. Hence, this work will help the trends for the next studies to take into consideration to develop more industrially applicable adsorbents.
2 Biogas sources and its siloxane content
2.1 A short review on the wide variety of biogas sources
Biogas refers to a water-saturated gas that is generated as a result of a biological process by means of microbial organisms on biodegradable organic substrates under anaerobic conditions. It is mainly generated during the anaerobic digestion of biodegradable wastes such as food, manure, municipal, and agricultural wastes in the anaerobic digestion bioreactors and landfills [17, 66]. Developed countries implement advanced large-scale biogas plants to take advantage of biogas. Biogas is regularly applied to generate heat, power, and electricity. In addition, many industrial applications for its use in biogas plants as a substitute to natural gas are being progressed [67]. The composition of biogas is highly influenced by the type of substrate and how the anaerobic process is designed. For instance, the biogas resulting from the biodigesters of agricultural residues will have a composition different from that of a landfill gas recovery system or wastewater treatment plants (WWTPs) [3]. This will obviously affect the biogas upgrading procedure, which is the process to obtain biomethane, removing CO2 and the rest of impurities form biogas.
As mentioned, biogas contains 60–70% methane and 30–40% carbon dioxide and trace amounts of impurities [11, 68]. Many studies have been conducted to eliminate carbon dioxide content of biogas, towards biogas upgrading. This process is typically carried out through technologies such as physical and chemical scrubbing, pressure swing adsorption, separation by membrane, and cryogenic separation [10]. This review will not go further in carbon dioxide removal technologies since the studies and developments on this issue have been largely reviewed [10, 69, 70].
2.2 Siloxane and its effects on biogas valorization
To take advantage of biogas, it must be converted to heat, steam, or electricity or used as a gas fuel substitute in vehicles. For these purposes, biogas needs to be used in different types of equipment such as combustion engines, gas turbine engines, boilers, hot water systems, process heaters for combined heat and power units (CHP), and fuel cells. However, as shown in Table 1, the existing impurities in biogas if not eliminated can cause damage to such equipment, and therefore they hamper the feasibility of biogas valorization process in economical and industrial applications. One of these disruptive components in biogas is siloxanes. Siloxanes are used as additives to cleaning agents, sealants, and pharmaceutical and cosmetic products as well as personal care products such as hairstyle products, lipsticks, creams, and deodorants [16, 71]. Consequently, they appear in wastewater and wastewater treatment plants and landfills [68]. Siloxanes can be categorized into two types: (1) PDM, which has higher molecular weight and low vapor pressure that makes it non-volatile, as opposed to (2) methyl siloxanes, which have lower molecular weight and are known as VMS [14, 15]. For example, owing to their characteristics mentioned above, for siloxanes present in wastewater streams, a part of VMS is volatilized and leaves the liquid stream, and the remaining part is adsorbed onto solid particles and solubilized in liquid medium and, consequently, ends up in the digester. On the other hand, more than 90% of PDM are not volatilized and will be mainly adsorbed on the surface of solid particles and carried out with the sludge to the digester [14, 15]. After the anaerobic digestion process, VMS will be released to the biogas stream due to the hydrolysis of PMD as well as direct volatilization of a part of VMS that has already managed to enter the anaerobic digester through adsorption onto solid particles [16]. Figure 1 graphically depicts the siloxanes fate in a wastewater treatment plant (WWTP) or in a landfill and their occurrence in biogas. Hence, siloxanes existing in biogas are mainly VMS, which are either linear or cyclic. Table 2 shows the main types of VMS existing in biogas [19].
The irreversible decomposition reaction of siloxanes to silica has been reported to damage the existing facilities since silica deposits and coats devices such as valves, spark plugs, and compressors, which results in shortening the lifetime of the engines and an increase in the maintenance costs [13]. The oxidation reaction of siloxanes to form silicon dioxide (SiO2) is presented in Eq. 1:
Taking into account such obstacles caused by the presence of VMS in biogas, its removal is essential for further utilization of biogas as a renewable source of energy. In the following section, adsorption will be discussed as a promising technology for VMS removal from biogas.
3 Adsorptive removal of siloxane
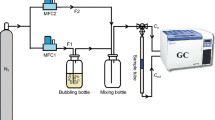
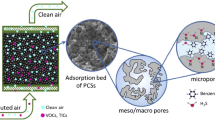
Adsorption can be defined as the change in concentration of a substance at the interface with the neighboring phases and it falls into four categories of liquid-gas, liquid-liquid, solid-liquid, and solid-gas depending on the type of phases in contact [72]. Having excellent properties such as good cost-efficiency, high potential of regenerability, and a specific design and manufacture for a specific need, adsorption process has been extensively used in environmental processes such as water and wastewater treatment [73, 74] and gas separation and purification [75, 76], among the most important ones. Adsorption process occurs through physical attraction of siloxane molecule to an available site on the external and internal solid surface of the adsorbent. What determines the accessibility of siloxane molecules to the internal adsorption surface is the pore size of the adsorbent [77]. That is the reason why, as will be discussed throughout the paper, one of the major attempts in published studies has been the manipulation of the textural properties of the adsorbents such as pore size and pore volume to enhance the adsorption capacity of the tested adsorbents. However, adsorbents also encounter a number of process-related obstacles. For example, a pretreatment step for biogas can be necessary to eliminate the impurities including water, which will disrupt the performance of some adsorbents. Another issue is the heat produced due to the intrinsic exothermic nature of the adsorption process, which will add complication to the adsorption process schemes. Another possible challenge regarding the development of manufacturing of adsorbents is to keep a good balance between reducing the particle size for the enhancement of intra-particle diffusion kinetics and increasing the particle size to limit the pressure drop [78]. Hence, more attempts need to be made to alleviate the challenges and obstructions that inevitably face the adsorbents. Decontamination of biogas from siloxane by means of adsorption has been investigated to date. Since the characteristics and concentration of siloxane in biogas vary depending on the source of the waste stream [19, 31], a universal adsorbent does not exist. Thus, depending on the type of siloxane, the adsorbent needs to be selected and further used. The traditional adsorbents include activated carbon, silica gel, molecular sieves, activated alumina, and alkaline oxides [19, 31, 79]. The most recent adsorbents are reduced graphene oxide and metal organic frameworks (MOFs), which proved to be effective for the removal of specific types of siloxanes. The numerical results of the adsorption studies, such as the adsorption capacity of the adsorbents, the conditions of the adsorption process, and the regeneration results, are presented in Table 3 and Table 4. Additionally, the schematic mechanism of siloxane adsorption from biogas is displayed in Fig. 2, whereas the common regeneration system is shown in Fig. 3.
One of the significant issues that needs to be taken into account as far as adsorption experiments are concerned is the initial concentration of the adsorbate. Typically, it is observed in adsorption studies that the adsorption capacity values increase as the initial concentration of the adsorbate in the solution is higher. However, in order to have reproducible results in terms of adsorption capacity in the real and large scale situation, it is highly recommended that the adsorption experiments are carried out with the initial concentration of the adsorbate in the same range as they are in real situation. Typically, the concentration of siloxane in real biogas is not more than 0.5 mg/L [5]. Hence, the studies that carried out their experimentation using initial siloxane concentrations close to these values as shown in Table 3 present more realistic results. However, in case of the studies that used extremely high initial siloxane concentrations and achieved high adsorption capacities, the reported results are hard to be generalized to the real situation. Summarizing, in the following sections, each types of adsorbents including activated carbon, silica gel, aluminosilicates, rGOA, MOFs, and waste-derived adsorbents will be elaborately discussed and compared in terms of adsorption efficiency, reusability, and sensitivity to water, which are determined and affected by the adsorbents properties such as surface textural features including pore size and volume and surface area, as well as surface functional groups and hydrophobicity.
3.1 Activated carbon
Activated carbon (AC) is an amorphous form of carbon that has a surface area between 600 to 1600 m2/g. Depending on its activation or impregnation method, it can have an acidic or basic feature [19]. Having a non-polar nature, it favors the adsorption of siloxane [86]. In addition to its high porosity and large surface area, activated carbon has shown high adsorption capacity ranging from 100 to 1732 mg/g as well as cost efficiency, availability, and stability. However, one of the drawbacks of AC is its incapability for a selective removal of siloxane. For instance, it has demonstrated affinity toward the adsorption of methane, a highly negative phenomenon. Another obstacle is its probable pore blockage due to siloxane polymerization [19]. Eventually, the regeneration of the adsorbent has not been demonstrated to be efficient enough, which makes the process economically weak [12]. In this regard, Gilson et al. [38] carried out the adsorption of L2-siloxane on a number of AC, and the results corresponding to the best adsorbent confirmed a percentage of adsorption capacity loss around 25–30% after three cycles of adsorption as shown in Table 4.
Various factors impact the performance of siloxane adsorption on AC, among which activated carbon surface properties and biogas water content are the most significant ones [40, 43]. For instance, it was reported that the adsorption of siloxane suffered a tenfold decrease when the relative humidity increased from 50 to 70%, which was related to the competitive adsorption of water molecules into the adsorbent sites [43]. For this reason, the adsorption process has been used together with a pretreatment process such as refrigeration or condensation to reduce the water content and to prevent the probable saturation of AC. Besides, the importance of adsorbent surface structure on adsorption efficiency can be accounted by the fact that the principal mechanism of adsorption is physical, which can be attributed to the hydrophobicity of both materials. In this sense, Yu et al. [40] investigated the effect of AC characteristics on the adsorption capacity of D4-siloxane (octamethylcyclotetrasiloxane) and demonstrated that large micropores and small mesopores (1.7–3 nm of diameter) were the most favorable ones for D4 adsorption. Codony et al. [41] carried out dynamic adsorption of D4 on twelve different ACs and confirmed the significant relationship between the textural properties of AC and the adsorption efficiency of siloxane. Additionally, they demonstrated that the humidity of biogas adversely affects the adsorption capacity. However, the range of adsorption capacity they achieved for these twelve activated carbons were reported to be from 250 to 1732 mg/g as shown in Table 3. Regarding the regenerability of AC, Tran et al. [42] investigated the role of surface chemistry on the regeneration ability of activated carbons and showed that the ACs containing alkali metal cations are the ones favoring the polymerization of siloxanes, which brings about the hindrance of the regeneration of the adsorbent. Most recently, Yang et al. [80] managed to enhance the adsorption capacity of AC up to 500 mg/g by the incorporation of copper oxide. Indeed, they demonstrated that the strong hydrogen bond is responsible for the adsorption of siloxane on the CuO surface (C-H-Cu hydrogen bond). However, the regeneration study of the adsorbents was not carried out, which questions its further industrial applicability.
3.2 Silica and silica gel
Silica gel ((SiO2).nH2O) (SG) is an amorphous porous material as a result of polymerization of silicic acid using a strong acid like hydrochloric or sulfuric acid. The monomer of SG, silica (SiO2), has also been utilized for siloxane adsorption, but not as frequently as SG [18]. In fact, the weak adsorption forces between SG and siloxane causes the desorption of siloxane molecules to require less dissociation energy for regeneration, which makes SG a proper adsorbent for siloxane adsorption with the adsorption capacity ranging from 200 to 916 mg/g. For instance, it was reported that the regeneration of SG was 95% effective under the same conditions (250°C and 20 minutes) than that of AC [31]. As revealed in this study, the characteristics of SG affecting its adsorptive efficacy are the specific surface area, pore volume, pore size, and hydrophobicity, which can be manipulated to achieve better adsorption and regeneration performance. Following this strategy, Jafari et al. [46] attempted to regulate the textural properties of mesoporous silica by means of silica synthesis and post-treatment modifications and proved higher adsorption capacity (686 mg/g) for octamethylcyclotetrasiloxane (D4) than that of commercial silica (642 mg/g). The modified mesoporous silica also demonstrated better regeneration stability as well as more resistance to humidity, a positive point due to the competitive effect of water [43]. In another study, Liu et al. [47] acetylated SG using acetic anhydrite, which showed enhancing effects on the adsorption efficiency of SG, due to a significant increase of micropore specific surface area, total pore volume, and better hydrophobicity. Additionally, thermal regeneration of the acetylated SG proved to be less energy consuming as observed in Table 4. Notably, they achieved an adsorption capacity of 304 mg L2/g and 916 mg D4/g at low temperatures (Table 3). The best performance of the adsorbent at lower temperatures was accounted for by the exothermic nature of micropores adsorption. They also demonstrated that the efficiency of methyl-functionalized SG was within 15 to 18 times higher than unmodified SG in terms of adsorption capacity of hexamethyldisilane (L2) [44]. Furthermore, it was also attempted to regulate the micropores of SG using polyethylene imine, obtaining similar results in siloxane removal [81]. Jung et al. [82] also proposed the surface modification of silica using NaOH for the removal of D5. Although they obtained four times more adsorption capacity than SG or unmodified silica, the regeneration of the adsorbent was carried out only for two cycles with a slight loss as shown in Table 4.
3.3 Aluminosilicate adsorbents
Aluminosilicates are minerals mainly composed of aluminum, silicon, and oxygen. Although all aluminosilicates present the same basic chemical composition, the variety of atoms and molecular arrangements lead to different structures with different physico-chemical characteristics. Among the types of aluminosilicates, clays, zeolites, and mesoporous aluminosilicates stand out for their wide range of applications in catalysis, wastewater treatment, gas purification, and storage [48]. Among aluminosilicates, zeolites and mesoporous families have been used for the removal of siloxane from biogas with the adsorption capacity ranging from 100 to 150 mg/g. The first work presented was by Ortega et al. [21] (2009) who investigated the adsorption performance of zeolite (type DAY 40) for the removal of siloxanes D4 and L2 and experimentally compared with AC and silica gel. The results demonstrated that the adsorption capacity of zeolite was quite lower than that AC, which was attributed to its lower surface area (607 m2/g) compared to AC (1220 m2/g). However, zeolite, due to its high hydrophobicity, showed better performance in terms of less sensitivity to water as it had no capacity loss with levels of moisture of 70%, a content that AC cannot tolerate [19]. Silica gel also presented negative results due to its hydrophilic nature. Nonetheless, no study of regeneration of zeolite was presented. Later, Montanari et al. [87] studied NaX zeolite in comparison with AC and SG for the adsorption of D3 siloxane. The zeolite used was reported to show neither comparable adsorption performance nor any advantages compared to the AC in terms of the regeneration process. Recently, Caberra-Codony et al. [83] investigated the regeneration of seven types of zeolites using a wet oxidation process including ozonation and Fenton-like treatment. The results revealed that Fe-BEA zeolite had the highest efficiency of 143 mg/g as shown in Table 3. However, they only could achieve a complete regeneration of the zeolite Fe-BEA in the first cycle (Table 4); the recyclability of the adsorbent for the successive cycles was unsuccessful due to the accumulation of C-containing products in the adsorbent. As pointed out before, textural properties of the adsorbents including pore volume and size play a constructive role in the adsorbent adsorption potential. In this respect, Jiang et al. [49], in an attempt to obtain tunable textural properties, synthesized different mesoporous aluminosilicates manipulating the aluminum dopant and the calcination temperatures, which proved to significantly affect the adsorbent pore size and volume and, consequently, the adsorption efficiency. They concluded that the volume of the mesopores and the external surface area of the adsorbent improved as the aluminum dopant ratio and the calcination temperature increased. In fact, the mesopores in this adsorbent are the space between the nanoparticles, and when more atoms of aluminum are added, they can hinder the aggregation of nanoparticles during the calcination process. Therefore, the volume of the space between nanoparticles (mesopores) will increase.
3.4 Waste-derived adsorbents
It is worth mentioning that the utilization of waste-derived materials for different applications such as wastewater treatment and gas purification such as siloxane removal has recently appeared in literature [1, 60, 61].
In this regard, Silva et al. [61] synthesized porous silica using the residual sand from a WWTP. This waste-derived adsorbent silica was used for removal of D4 and D5 siloxanes. However, the cost and energy intensity of the purification and modification process was a critical issue to be considered. For instance, the sand waste, which was used for synthesis of silica, was reported to pass through extreme pretreatments to be well prepared for the synthesis process. Contrarily, a waste wood-derived biochar was utilized without any further modification for the adsorption of siloxane D4 and compared with commercial AC in terms of adsorption capacity. The adsorption capacity of this biochar (3.5 mg/g) proved to be much lower than the commercial ones (37.5 mg/g). However, this shortcoming is supposed to be overcome through activation methods that improve the physical properties of the adsorbent [45]. For instance, a lignocellulosic waste generated in food and wood industry was activated by different agents and then tested for siloxanes L2 and D4 adsorption and compared with commercial AC [64]. Interestingly, it demonstrated an adsorption capacity (438–512 mg/g) comparable to commercial AC. Notably, the temperatures at which the activation process of the waste-derived adsorbent was carried had a significant effect on the development of porosity and thus on the adsorbent adsorption performance. The range of temperature used for the activation process was 800 to 900 °C, which are costly. Most recently, Meng et al. [65] introduced the modification of biochar with iron and accomplished comparable results in terms of adsorption capacity of L2 siloxane from biogas (356 mg/g). Moreover, the pretreatment and activation process that was done in this study is less energy consuming in terms of temperature (600 °C). In addition, the adsorbent remained effective when recycled 5 times with the adsorption capacity practically stable as shown in Table 4. Anyway, more research is required to investigate more and different waste-derived adsorbents without energy-intensive treatments and yet having adsorption capacities comparable to commercial or traditional adsorbents. In addition to this adsorption performance, regeneration of the adsorbent is essential to prove its industrial applicability.
3.5 Emerging adsorbents
3.5.1 Graphene oxide
Graphene oxides (GO) are monomolecular sheets composed of carbon, oxygen, and hydrogen. They have proved to be a suitable candidate for diverse applications such as energy harvesting and storage, gas separation, water treatment, catalysis, and biomedical applications [88,89,90,91]. Notwithstanding this, there has not been any reports of using graphene oxide for siloxane removal until 2020; since then, few studies have been carried out on them for siloxane adsorption and achieved adsorption capacities ranging from 104 to 190 mg/g. Recently, the adsorption of hexamethyldisiloxane (L2) by amine-reduced graphene oxide aerogels (rGOA) has been reported [57], and in comparison to GO, it demonstrated higher adsorption efficiency toward L2 due to a higher surface area and hydrophobicity (Table 3). Although the adsorption capacity of these adsorbents is relatively lower than that of some of the other adsorbents mentioned before (Table 3), the critical point in the use of such adsorbents is the simple and low energy intensiveness of their synthesis and regeneration. These authors also examined the usage of vitamin C as a more environmentally friendly reducing agent for the synthesis of rGOA and obtained practically the same adsorption and regeneration efficiency [58]. Furthermore, in another attempt, they also took advantage of the cross linker β-cyclodextrin for its abundant hydroxyl and amine groups for the modification of the surface properties of rGOA. The adsorbent proved to be consistent after 10 times of recycling while maintaining its adsorption capacity [59] (Table 4). However, they achieved much higher adsorption capacity when they attempted to modify the rGOA using citric acid, which was pointed to be due to the improvement in the textural properties (higher surface area) of the adsorbent and its hydrophobicity. It is worth mentioning that this adsorbent did not show as good reusability as the previous ones, but better than some other adsorbents like AC [84].
3.5.2 Metal organic frameworks (MOFs)
Metal organic frameworks (MOFs) are porous inorganic-organic hybrid constituents. Due to their particular features such as high pore volume, high specific area, shape and pore tailorability, they have been exploited for many applications including biogas upgrading [53, 55, 92]. In this case, MOFs were mainly investigated for the separation of carbon dioxide until recent years [53, 55]. Lately, researchers started the investigation of MOFs for the removal of siloxane. In 2013, for the first time, Mito-oka et al. [93] employed DUT-4 MOF for the D4 siloxane adsorption. They investigated the adsorption of D4 in the presence of water, carbon dioxide, and methane in comparison with a conventional AC, and the results showed better selectivity and adsorption capacity than that of conventional AC. This high efficiency and selectivity were attributed to the strong hydrophobicity of DUT-4 and its optimal chemical interactions with the adsorbate molecules (D4). Later, chromium-based MIL-101 MOF was investigated for the adsorption of D4 by Gargiulo et al. [56] as shown in Tables 3 and 4. They demonstrated high adsorption capacity of 950 mg/g and good reusability. Contrarily to activated carbon and silica gel, adsorption of D4 on MIL-101 did not lead to a polymerization of adsorbate on the adsorbent surface, which is critical for the successful reusability of the adsorbent. In addition, they achieved high adsorption capacity under ambient temperature (Table 3). This is another important point, as traditional adsorbents significantly lose their adsorption efficiency as temperature increases and their best performance is limited to low temperatures (0 °C).
However, as far as industrial application is concerned, MOFs have shown a significant obstacle that is their poor thermo-mechanical stability. To tackle such problems, recently, Pioquinto-García et al. [85] incorporated DUT-4 into polyacrylonitrile fiber (Al-MOF fiber). The results proved faster adsorption kinetics and the diffusion of adsorbate (D4) was roughly 3 times higher than that of DUT-4 powder. This means that MOF particles distribution on the fiber enhances the contact between the adsorbate and MOF particles. Eventually, through an environmental assessment, the adsorbent preparation appeared to have less adverse environmental effects than those derived from the synthesis of DUT-4 powders. Unfortunately, the Al-MOF fiber did not show a good reusability performance, and after one regeneration cycle, the adsorption capacity decreased by 30% (Table 4). In another study, Tiempos-Flores et al. [54] managed to enhance the hydrophobicity of zeolitic imidazolate framework (ZIF-71), which led to an improvement of the adsorption capacity. In general, the adsorption capacities obtained with MOFs are not comparable to other typical adsorbents. ’Unfortunately, there are no systematic reports on the regeneration of MOF adsorbents, which is considered as a downside of MOFs as adsorbents. The authors highly recommend researchers to include the reusability studies to their investigations in order to be able to establish the excellence of MOFs at industrial scale for siloxane removal.
4 Conclusions
Adsorption is a robust method for siloxane removal from biogas and makes a valuable contribution to the biogas upgrading as the presence of siloxanes in biogas causes damage to the equipment used for its conversion to electricity and heat. As discussed in this review, conventional adsorbents such as activated carbon or silica gel need to be more investigated to be optimized in terms of adsorption capacity and especially reusability. However, this study did not aim to cover subjects such as comprehensive comparison of different methods and technologies for siloxane removal and elaborative discussion on studies regarding the source of biogas such as anaerobic digestion process. Furthermore, two main factors affecting the adsorbents regenerability have been found: the adsorbate-adsorbent bonding and the polymerization of siloxane in the pores of the adsorbent. Thus, many studies are carried out to manipulate the surface functional groups of the adsorbents towards the occurrence of physical adsorption in which weak Van der Waals are the bonding forces. In addition to this, water vapor typically exists in the biogas composition, so the hydrophobicity degree of the adsorbents plays an essential role in the adsorption efficiency of the siloxane. Activated carbon, due to its high surface area and availability, has been used commercially, but its low selectivity for siloxane and its complicated regeneration due to the blockage of its pores because of siloxane polymerization question its utilization and cost efficiency. In comparison to activated carbon, silica gel demonstrated better reusability due to the presence of weak forces between adsorbate and the silica gel surface. Zeolites, despite their lower adsorption capacity compared to activated carbon and because of their high hydrophobicity, were found more practical for siloxane adsorption from real biogas with high water content. GO adsorbents showed relatively lower adsorption capacities as well; however, the cost and energy efficiency of their synthesis and regeneration makes them more industrially appealing candidates. Eventually, metal organic frameworks (MOFs) proved to be promising for adsorption of siloxane due to their higher selectivity, high hydrophobicity, high adsorption capacity, and more efficient regeneration owing to lack of the occurrence of siloxane polymerization, contrarily to activated carbon, as well as maintaining high adsorption capacity at ambient temperature, an advantage that other adsorbents did not present. However, limited studies have been published for MOF utilization as adsorbents for siloxane removal. The regeneration was also another issue that needs to be addressed in case of MOFs.
5 Future prospects and challenges
The concluding authors’ recommendation is that more focus is required to be put on the regeneration of adsorbents to establish the industrialization of adsorbents for the removal of siloxanes. In addition, more research should be carried out on the adsorbents derived from waste considering the worldwide increasing significance of circular economy. In this respect, different potential wastes that have not been tested before for the production of adsorbents need to be evaluated. Furthermore, more attempts need to be made on decreasing the cost and energy consumption in the synthesis process of waste-derived adsorbents as well as the optimization studies to improve their efficiency to convince manufacturers and companies to move towards such potential waste materials. As discussed, MOFs were found one of most promising candidates for the selective and highly efficient adsorption of siloxane. However, only limited studies were conducted on MOFs as far as siloxane removal is concerned. In addition, MOFs need to be deeply investigated in terms of regeneration, since this issue is not addressed. Hence, more studies are recommended to be carried out specifically on different types of MOFs, which have proved highly effective for various purposes, and yet not tested for siloxane adsorption. Finally, an important issue to be considered when comparing adsorbents efficiency is the initial siloxane concentration. This is important since the siloxane concentration in the real biogas does not exceed 0.5 mg/L. Therefore, it is highly recommended to conduct adsorption experiments in the ranges of siloxane concentrations close to the real biogas concentrations to have more realistic results for larger and industrial applications.
Data availability
Not applicable.
References
Álvarez-Gutiérrez N, García S, Gil MV et al (2014) Towards bio-upgrading of biogas: Biomass waste-based adsorbents. Energy Proc 63:6527–6533. https://doi.org/10.1016/j.egypro.2014.11.688
Ryckebosch E, Drouillon M, Vervaeren H (2011) Techniques for transformation of biogas to biomethane. Biomass Bioenerg 35:1633–1645. https://doi.org/10.1016/j.biombioe.2011.02.033
Struk M, Kushkevych I, Vítězová M (2020) Biogas upgrading methods: recent advancements and emerging technologies. Rev Environ Sci Biotechnol 19:651–671. https://doi.org/10.1007/s11157-020-09539-9
Divya D, Gopinath LR, Merlin Christy P (2015) A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew Sustain Energy Rev 42:690–699. https://doi.org/10.1016/j.rser.2014.10.055
Nyamukamba P, Mukumba P, Chikukwa ES, Makaka G (2020) Biogas upgrading approaches with special focus on siloxane removal—a review. Energies 13:6088. https://doi.org/10.3390/en13226088
Atelge MR, Senol H, Mohammed D et al (2021) A critical overview of the state-of-the-art methods for biogas purification and utilization processes. Sustainability 13:11515. https://doi.org/10.3390/su132011515
Farghali M, Osman AI, Umetsu K, Rooney DW (2022) Integration of biogas systems into a carbon zero and hydrogen economy: a review. Environ Chem Lett 20:2853–2927. https://doi.org/10.1007/s10311-022-01468-z
Osman AI, Fawzy S, Farghali M et al (2022) Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: a review. Environ Chem Lett 20:2385–2485. https://doi.org/10.1007/s10311-022-01424-x
François M, Lin K-S, Rachmadona N, Khoo KS (2023) Advancement of biochar-aided with iron chloride for contaminants removal from wastewater and biogas production: a review. Sci Total Environ 874:162437. https://doi.org/10.1016/j.scitotenv.2023.162437
Adnan AI, Ong MY, Nomanbhay S et al (2019) Technologies for biogas upgrading to biomethane: a review. Bioengineering 6:92. https://doi.org/10.3390/bioengineering6040092
Awe OW, Zhao Y, Nzihou A et al (2017) A review of biogas utilization, purification and upgrading technologies. Waste Biomass Valoriz 8:267–283. https://doi.org/10.1007/s12649-016-9826-4
Dewil R, Appels L, Baeyens J (2006) Energy use of biogas hampered by the presence of siloxanes. Energy Convers Manag 47:1711–1722. https://doi.org/10.1016/j.enconman.2005.10.016
Ruiling G, Shikun C, Zifu L (2017) Research progress of siloxane removal from biogas. Int J Agric Biol Eng 10:30–39. https://doi.org/10.3965/j.ijabe.20171001.3043
Parker WJ, Shi J, Fendinger NJ et al (1999) Pilot plant study to assess the fate of two volatile methyl siloxane compounds during municipal wastewater treatment. Environ Toxicol Chem 18:172–181. https://doi.org/10.1002/etc.5620180211
Xu S (1999) Fate of Cyclic Methylsiloxanes in Soils. 1. The degradation pathway. Environ Sci Technol 33:603–608. https://doi.org/10.1021/es980803a
Graiver D, Farminer KW, Narayan R (2003) A review of the fate and effects of silicones in the environment. J Polym Environ 11:129–136. https://doi.org/10.1023/A:1026056129717
Kuo J, Dow J (2017) Biogas production from anaerobic digestion of food waste and relevant air quality implications. J Air Waste Manag Assoc 67:1000–1011. https://doi.org/10.1080/10962247.2017.1316326
Schweigkofler M, Niessner R (2001) Removal of siloxanes in biogases. J Hazard Mater 83(3):183–196. https://doi.org/10.1016/S0304-3894(00)00318-6
Gaj K (2020) Adsorptive biogas purification from siloxanes-a critical review. Energies 13:2605. https://doi.org/10.3390/en13102605
Ajhar M, Melin T (2006) Siloxane removal with gas permeation membranes. Desalination 200:234–235. https://doi.org/10.1016/j.desal.2006.03.308
Ortega DR, Subrenat A (2009) Siloxane treatment by adsorption into porous materials. Environ Technol 30:1073–1083. https://doi.org/10.1080/09593330903057540
Accettola F, Guebitz GM, Schoeftner R (2008) Siloxane removal from biogas by biofiltration: Biodegradation studies. Clean Technol Environ Policy 10:211–218. https://doi.org/10.1007/s10098-007-0141-4
Läntelä J, Rasi S, Lehtinen J, Rintala J (2012) Landfill gas upgrading with pilot-scale water scrubber: performance assessment with absorption water recycling. Appl Energy 92:307–314. https://doi.org/10.1016/j.apenergy.2011.10.011
Liu F, Chen W, Mi J et al (2019) Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures. AIChE J 65:e16574. https://doi.org/10.1002/aic.16574
Bendall E, Aiken RC, Mandas F Selective Absorption of H2S from larger quantities of C02 by absorption and reaction in fine sprays. AIChE J 29:66–72. https://doi.org/10.1002/aic.690290109
Haimourt N, Sandall OC (1987) Absorption of H2S into aqueous methyl diethanolamine. Chem Eng Commun 59:85–93. https://doi.org/10.1080/00986448708911987
Makoś-Chełstowska P, Słupek E, Kramarz A, Gębicki J (2021) New carvone-based deep eutectic solvents for siloxanes capture from biogas. Int J Mol Sci 22:9551. https://doi.org/10.3390/ijms22179551
Ghorbel L, Tatin R, Couvert A (2014) Relevance of an organic solvent for absorption of siloxanes. Environ Technol 35:372–382. https://doi.org/10.1080/09593330.2013.828778
Price GJ, Hearn MP, Wallace ENK, Patel AM (1996) Ultrasonically assisted synthesis and degradation of poly(dimethyl siloxane). Polymer 37:2303–2308. https://doi.org/10.1016/0032-3861(96)85339-0
Gilbert AR, Kantor SW (1959) Transient catalysts for the polymerization of organosiloxanes. J Polym Sci 40:35–58. https://doi.org/10.1002/pol.1959.1204013603
Shen M, Zhang Y, Hu D et al (2018) A review on removal of siloxanes from biogas: with a special focus on volatile methylsiloxanes. Environ Sci Pollut Res 25:30847–30862. https://doi.org/10.1007/s11356-018-3000-4
Boada E, Santos-Clotas E, Bertran S et al (2020) Potential use of Methylibium sp. as a biodegradation tool in organosilicon and volatile compounds removal for biogas upgrading. Chemosphere 240:124908. https://doi.org/10.1016/j.chemosphere.2019.124908
Wang J, Zhang W, Xu J et al (2014) Octamethylcyclotetrasiloxane removal using an isolated bacterial strain in the biotrickling filter. Biochem Eng J 91:46–52. https://doi.org/10.1016/j.bej.2014.07.003
Li Y, Zhang W, Xu J (2014) Siloxanes removal from biogas by a lab-scale biotrickling filter inoculated with Pseudomonas aeruginosa S240. J Hazard Mater 275:175–184. https://doi.org/10.1016/j.jhazmat.2014.05.008
Santos-Clotas E, Cabrera-Codony A, Boada E et al (2019) Efficient removal of siloxanes and volatile organic compounds from sewage biogas by an anoxic biotrickling filter supplemented with activated carbon. Bioresour Technol 294:122136. https://doi.org/10.1016/j.biortech.2019.122136
Yang L, Corsolini SI (2019) Online removal of volatile siloxanes in solid-state anaerobic digester biogas using a biofilter and an activated carbon filter. J Environ Chem Eng 7:103284. https://doi.org/10.1016/j.jece.2019.103284
Ajhar M, Bannwarth S, Stollenwerk KH et al (2012) Siloxane removal using silicone-rubber membranes. Separ Purif Technol 89:234–244. https://doi.org/10.1016/j.seppur.2012.01.003
Gislon P, Galli S, Monteleone G (2013) Siloxanes removal from biogas by high surface area adsorbents. Waste Manag 33:2687–2693. https://doi.org/10.1016/j.wasman.2013.08.023
Ajhar M, Travesset M, Yüce S, Melin T (2010) Siloxane removal from landfill and digester gas - A technology overview. Bioresour Technol 101:2913–2923
Yu M, Gong H, Chen Z, Zhang M (2013) Adsorption characteristics of activated carbon for siloxanes. J Environ Chem Eng 1:1182–1187. https://doi.org/10.1016/j.jece.2013.09.003
Cabrera-Codony A, Montes-Morán MA, Sánchez-Polo M et al (2014) Biogas upgrading: optimal activated carbon properties for siloxane removal. Environ Sci Technol 48:7187–7195. https://doi.org/10.1021/es501274a
Tran VTL, Gélin P, Ferronato C et al (2019) Siloxane adsorption on activated carbons: Role of the surface chemistry on sorption properties in humid atmosphere and regenerability issues. Chem Eng J 371:821–832. https://doi.org/10.1016/j.cej.2019.04.087
Sigot L, Ducom G, Benadda B, Labouré C (2014) Adsorption of octamethylcyclotetrasiloxane on silica gel for biogas purification. Fuel 135:205–209. https://doi.org/10.1016/j.fuel.2014.06.058
Meng Z, Liu Y, Li X, Ma Z (2020) Removal of siloxane (L2) from biogas using methyl-functionalised silica gel as adsorbent. Chem Eng J 389:124440. https://doi.org/10.1016/j.cej.2020.124440
Papurello D, Gandiglio M, Kafashan J, Lanzini A (2019) Biogas purification: a comparison of adsorption performance in D4 siloxane removal between commercial activated carbons and waste wood- derived char using isotherm equations. Processes 7:774. https://doi.org/10.3390/pr7100774
Jafari T, Jiang T, Zhong W et al (2016) Modified mesoporous silica for efficient siloxane capture. Langmuir 32:2369–2377. https://doi.org/10.1021/acs.langmuir.5b04357
Liu YH, Meng ZY, Wang JY et al (2019) Removal of siloxanes from biogas using acetylated silica gel as adsorbent. Pet Sci 16:920–928. https://doi.org/10.1007/s12182-019-0336-4
Lopes AC, Martins P, Lanceros-Mendez S (2014) Aluminosilicate and aluminosilicate based polymer composites: present status, applications and future trends. Prog Surf Sci 89:239–277. https://doi.org/10.1016/j.progsurf.2014.08.002
Jiang T, Zhong W, Jafari T et al (2016) Siloxane D4 adsorption by mesoporous aluminosilicates. Chem Eng J 289:356–364. https://doi.org/10.1016/j.cej.2015.12.094
Stock N, Biswas S (2012) Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev 112:933–969. https://doi.org/10.1021/cr200304e
Ren HY, Zhang XM (2016) Enhanced selective CO2 capture upon Incorporation of dimethylformamide in the cobalt metal-organic framework [Co3(OH)2(btca)2]. Energy Fuels 30:526–530. https://doi.org/10.1021/acs.energyfuels.5b02393
Termine JD, Eanes ED, Greenfield DJ et al (1973) Hydrogen storage in microporous metal-organic frameworks. Science 300:1127–1129. https://doi.org/10.1126/science.1083440
Chidambaram A, Le DH, Navarro JAR, Stylianou KC (2021) Robust metal-organic frameworks for dry and wet biogas upgrading. Appl Mater Today 22:100933. https://doi.org/10.1016/j.apmt.2020.100933
Tiempos-Flores N, Hernández-Fernández E, Rico-Barragan A et al (2022) Enhanced hydrophobicity of modified ZIF-71 metal-organic framework for biofuel purification. Polyhedron 217:115736. https://doi.org/10.1016/j.poly.2022.115736
Chaemchuen S, Kabir NA, Zhou K, Verpoort F (2013) Metal-organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem Soc Rev 42:9304–9332. https://doi.org/10.1039/c3cs60244c
Gargiulo N, Peluso A, Aprea P et al (2019) Chromium-based MIL-101 metal organic framework as a fully regenerable D4 adsorbent for biogas purification. Renew Energy 138:230–235. https://doi.org/10.1016/j.renene.2019.01.096
Zheng Y, Hou X, Liu Y, Ma Z (2021) Hexamethyldisiloxane removal from biogas using reduced graphene-oxide aerogels as adsorbents. Renew Energy 178:153–161. https://doi.org/10.1016/j.renene.2021.06.043
Zheng Y, Hou X, Ma X et al (2021) Vitamin c-assisted fabrication of aerogels from industrial graphene oxide for gaseous hexamethyldisiloxane adsorption. Appl Sci 11:8486. https://doi.org/10.3390/app11188486
Zheng Y, Hou X, Lv S et al (2022) Efficient removal of siloxane from biogas by using β-cyclodextrin-modified reduced graphene oxide aerogels. Nanomaterials 12:2643. https://doi.org/10.3390/nano12152643
Chen Z, Wei W, Chen H, Ni B-J (2022) Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ Health 1:86–104. https://doi.org/10.1016/j.eehl.2022.05.001
Silva EN, Cantillo-Castrillon M, Dantas TM et al (2021) Siloxane adsorption by porous silica synthesized from residual sand of wastewater treatment. J Environ Chem Eng 9:104805. https://doi.org/10.1016/j.jece.2020.104805
Osman AI, Farrell C, Al-Muhtaseb AH et al (2020) The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci Rep 10:2563. https://doi.org/10.1038/s41598-020-59481-7
Osman AI, O’Connor E, McSpadden G et al (2020) Upcycling brewer’s spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J Chem Technol Biotechno 95:183–195. https://doi.org/10.1002/jctb.6220
Santos-Clotas E, Cabrera-Codony A, Ruiz B et al (2019) Sewage biogas efficient purification by means of lignocellulosic waste-based activated carbons. Bioresour Technol 275:207–215. https://doi.org/10.1016/j.biortech.2018.12.060
Meng Z, Hou X, Liu Y et al (2021) Facile fabrication of iron-modified biochar as a renewable adsorbent for efficient siloxane (L2) removal. J Environ Chem Eng 9:105799. https://doi.org/10.1016/j.jece.2021.105799
Themelis NJ, Ulloa PA (2007) Methane generation in landfills. Renew Energy 32:1243–1257. https://doi.org/10.1016/j.renene.2006.04.020
Abanades S, Abbaspour H, Ahmadi A et al (2022) A critical review of biogas production and usage with legislations framework across the globe. Int J Environ Sci Technol 19:3377–3400. https://doi.org/10.1007/s13762-021-03301-6
Bragança I, Sánchez-Soberón F, Pantuzza GF et al (2020) Impurities in biogas: analytical strategies, occurrence, effects and removal technologies. Biomass Bioenerg 143:105878. https://doi.org/10.1016/j.biombioe.2020.105878
Abdeen FRH, Mel M, Jami MS et al (2016) A review of chemical absorption of carbon dioxide for biogas upgrading. Chin J Chem Eng 24:693–702. https://doi.org/10.1016/j.cjche.2016.05.006
Mulu E, M’Arimi MM, Ramkat RC (2021) A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew Sustain Energy Rev 145:111081. https://doi.org/10.1016/j.rser.2021.111081
Latimer HK, Kamens RM, Chandra G (1998) the atmospheric partitioning of decameteylcyclopentasiloxane(D5) and 1-hydroxynonamethylcyclopentasiloxane(D4TOH) on different types of atmospheric particles. Chemosphere 36:2401–2414. https://doi.org/10.1016/S0045-6535(97)10209-0
Dabrowski A (2001) Adsorption from theory to practice. Adv Colloid Interface Sci 93(1-3):135–224. https://doi.org/10.1016/S0001-8686(00)00082-8
Vali SA, Baghdadi M, Abdoli MA (2018) Immobilization of polyaniline nanoparticles on the polyurethane foam derived from waste materials: a porous reactive fixed-bed medium for removal of mercury from contaminated waters. J Environ Chem Eng 6:6612–6622. https://doi.org/10.1016/j.jece.2018.09.042
Rathi BS, Kumar PS (2021) Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ Pollut 280:116995. https://doi.org/10.1016/j.envpol.2021.116995
Tagliabue M, Farrusseng D, Valencia S et al (2009) Natural gas treating by selective adsorption: material science and chemical engineering interplay. Chem Eng J 155:553–566. https://doi.org/10.1016/j.cej.2009.09.010
Sircar S, Golden TC (2000) Purification of hydrogen by pressure swing adsorption. Sep Sci Technol 35:667–687. https://doi.org/10.1081/SS-100100183
Soreanu G, Beíand M, Falletta P et al (2011) Approaches concerning siloxane removal from biogas-a review. Canad Biosyst Eng 53:8.1–8.18
Bui M, Adjiman CS, Bardow A et al (2018) Carbon capture and storage (CCS): the way forward. Energy Environ Sci 11:1062–1176. https://doi.org/10.1039/C7EE02342A
Alves CMAC, Abreu FOMS, Araújo RS, Oliveira MLM (2022) Recent advances in siloxanes removal from biogas and their efficiency: a short review. Chem Papers 77:1–9. https://doi.org/10.1007/s11696-022-02460-1
Yang Z, Chen Z, Gong H, Wang X (2022) Copper oxide modified activated carbon for enhanced adsorption performance of siloxane: an experimental and DFT study. Appl Surf Sci 601:154200. https://doi.org/10.1016/j.apsusc.2022.154200
Meng ZY, Liu YH, Ma ZC, Hou XF (2020) The regulation of micro/mesoporous silica gel by polyethylene imine for enhancing the siloxane removal. Inorg Chem Commun 112:107754. https://doi.org/10.1016/j.inoche.2019.107754
Jung H, Jurng J (2020) Purification of wastewater digester biogas from siloxanes via adsorption-desorption with NaOH-reformed SiO2 adsorbent. Renew Energy 156:459–468. https://doi.org/10.1016/j.renene.2020.03.189
Cabrera-Codony A, Georgi A, Gonzalez-Olmos R et al (2017) Zeolites as recyclable adsorbents/catalysts for biogas upgrading: removal of octamethylcyclotetrasiloxane. Chem Eng J 307:820–827. https://doi.org/10.1016/j.cej.2016.09.017
Hou X, Zheng Y, Lv S et al (2022) Effective removal of hexamethyldisiloxane using a citric acid modified three-dimensional graphene aerogel. Renew Energy 199:62–70. https://doi.org/10.1016/j.renene.2022.08.135
Pioquinto-García S, Álvarez JR, Rico-Barragán AA et al (2022) Electrospun Al-MOF fibers as D4 Siloxane adsorbent: synthesis, environmental impacts, and adsorption behavior. environmental impacts, and adsorption behavior. Microporous Mesoporous Mater:112327. https://doi.org/10.1016/j.micromeso.2022.112327
Barton TJ, Bull LM, Klemperer WG et al (1999) Tailored porous materials. Chem Mater 11:2633–2656. https://doi.org/10.1021/cm9805929
Montanari T, Finocchio E, Bozzano I et al (2010) Purification of landfill biogases from siloxanes by adsorption: a study of silica and 13X zeolite adsorbents on hexamethylcyclotrisiloxane separation. Chem Eng J 165:859–863. https://doi.org/10.1016/j.cej.2010.10.032
Obayomi KS, Lau SY, Danquah M et al (2022) Advances in graphene oxide based nanobiocatalytic technology for wastewater treatment. Environ Nanotechnol Monit Manag 17:100647. https://doi.org/10.1016/j.enmm.2022.100647
Chung C, Kim YK, Shin D et al (2013) Biomedical applications of graphene and graphene oxide. Acc Chem Res 46:2211–2224. https://doi.org/10.1021/ar300159f
Zhu Y, Murali S, Cai W et al (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924. https://doi.org/10.1002/adma.201001068
Wang Y, Li Z, Wang J et al (2011) Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29:205–212. https://doi.org/10.1016/j.tibtech.2011.01.008
Khan A, Qyyum MA, Saulat H et al (2021) Metal–organic frameworks for biogas upgrading: recent advancements, challenges, and future recommendations. Appl Mater Today 22:100925. https://doi.org/10.1016/j.apmt.2020.100925
Mito-Oka Y, Horike S, Nishitani Y et al (2013) Siloxane D4 capture by hydrophobic microporous materials. J Mater Chem A 1:7885–7888. https://doi.org/10.1039/c3ta11217a
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. This study was financially supported by the Spanish Ministerio de Ciencia e Innovación in the call Proyectos de Transición Ecológica y Transición Digital 2022. Squeezer project, ref. TED2021-130407B-I00.
Author information
Authors and Affiliations
Contributions
Seyed Alireza Vali: Writing, original draft; conceptualization; methodology; investigation; writing, review and editing. Javier Moral-Vico: Writing — review and editing. Antoni Sánchez: Writing, review and editing; supervision; project administration; funding acquisition. Xavier Font: writing, review and editing; supervision; project administration; funding acquisition
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vali, S.A., Moral-Vico, J., Font, X. et al. Adsorptive removal of siloxanes from biogas: recent advances in catalyst reusability and water content effect. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04478-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04478-1