Abstract
Recovery of protein is one of the important variables to optimise enzymatic hydrolysis of fish processing by-products. This study investigated the role played by emulsion formation on protein recovery and the molecular weight distribution of the protein hydrolysates at different solids concentration of fish processing by-products with a high oil content. Solids concentration of fish processing by-products was varied between 26 and 50% at constant pH of 7.8, 60 °C hydrolysis temperature and 4% enzyme dosage using Alcalase 2.4L. The results showed that emulsion formation, more than inhibition of enzyme activity, at high solids concentration contributed to the reduction in yield of dry solids and protein recovery. Emulsion formation also led to the presence of high molecular weight lipo-protein complexes in the protein hydrolysates, which caused an increase in the average molecular weight of the hydrolysates. The findings show the negative relationship between solids concentration, protein recovery and emulsion formation in enzymatic hydrolysis, and the need for methods that eliminate or reduce emulsion formation at high solids concentration without increasing process complexity or reducing protein recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Substrate concentration during enzymatic protein hydrolysis of complex substrates that contain significant amounts of lipids is an important processing condition that impacts hydrolysis efficiency and downstream processing, but its impacts remain poorly described. During the hydrolysis step, substrate concentrations affect the kinetics of enzymatic hydrolysis through mass and energy transfer, and non-competitive inhibition of enzyme activity [1,2,3]. Downstream processing costs increase when the amount of water, resulting from low substrate concentration, in the hydrolysed material is high [4,5,6].
On an industrial scale, ideal operating conditions would be to carry out enzymatic hydrolysis at the lowest water content possible, i.e., without the addition of any water. This, however, is not practical since it results in poor mass and energy transfer due to poor mixing, in turn resulting in low protein recovery [7,8,9,10,11,12]. On the other hand, adding water to the material to be hydrolysed results in protein loss due to emulsion formation, as Šližyte et al. [7] found. Naturally, oil and water do not mix to form a stable phase. An emulsion, which is a stable phase of oil-in-water or water-in-oil, is formed in the presence of a stabilizer under suitable pH conditions. Most studies have shown that protein hydrolysates generally form stable emulsions between pH 6 and 8, but there are cases where stable emulsions have been formed at pH 2 and pH 10, showing that this is heavily dependent on the solubility of the protein hydrolysates at that pH and the degree of hydrolysis [13,14,15,16,17,18,19]. However, it is important to note that studies that investigated the emulsion formation and stabilisation properties of protein hydrolysates did so using protein hydrolysates that have already been separated from other products of hydrolysis, under conditions that also differ from the enzymatic hydrolysis process. The extent to which enzymatic hydrolysis temperature and the presence of solid particles in the hydrolysis media affect emulsion formation and stability is not fully known. The current understanding is that, during emulsion formation, the interaction between intact and hydrolysed proteins, water, and oil happens between common non-polar and polar functional groups found in both peptide and fatty acid chains [20]. Hydrolysed proteins reduce the surface tension at the oil–water interface, thus acting as emulsifiers that facilitate emulsion formation through the formation of lipo-protein complexes that have different physico-chemical properties from the oil and water phases. Lipo-protein complexes can be less or more dense than water [20, 21]. That means during separation, some lipo-protein complexes will settle together with other heavy materials like undigested protein and bones, while lighter fractions form an emulsion phase above the aqueous phase. This also implies that there are lipo-protein complexes that remain dissolved in water in the form of micelles (spherical amphiphilic structures that have a hydrophobic core and a hydrophilic shell [22]). Visualizations of the different layers formed after separation of the hydrolysed material are found in Kristinsson et al. [23] and Siddik et al. [24].
There are several studies that have shown that large molecular weight proteins and peptides (> 2 kDa) from fish protein hydrolysates have high emulsifying activity and emulsion stabilising indices because of the presence of hydrophobic functional groups [25,26,27,28,29,30]. These emulsifying and stabilising properties were shown to be comparable, and in some cases even better than casein (molecular weight — 20 to 25 kDa) [31], which is a protein with well-known emulsifying and stabilizing properties [25, 32,33,34,35]. Low molecular weight peptides have low emulsifying activity indices because they do not possess sufficient hydrophobic groups to allow them to unfold and reorient to stabilize the emulsion, but they have high emulsifying stability indices [25, 36]. Emulsifying activity and stability properties are therefore a function of degree of hydrolysis. That is, mild degree of hydrolysis (3 to 10%) were found to produce hydrolysates with high emulsifying properties [10, 25, 29, 37,38,39]. Consequently, during enzymatic hydrolysis of substrates containing oil, large molecular weight proteins and peptides play the role of emulsifiers while small molecular weight peptides act as emulsion stabilisers [25].
Sardines are oily pelagic fishes with a high protein and oil content compared to other types of fish [40]. They make up over 80% of the catch in South Africa, with approximately 85% of the catch going through the canning process [41]. Since the canning process does not use heads and tails, these end up as low-value fish processing by-products that are normally used to make fish meal and fish oil [42]. FAO [43] estimates that 45–50% of the material fed into the canning process ends up as by-products. Due to the high oil content of these sardine processing by-products and the relatively high mixing speeds used in most enzymatic hydrolysis processes, emulsion formation is inevitable. Quaglia and Orban [44] and Vieira et al. [45] recovered protein from sardines without any emulsion formation by using defatted sardines. However, pretreatments such as chemical defatting using organic solvents or cooking and pressing to remove oil have been shown to result in lower degree of hydrolysis and protein recovery [15, 46]. Our previous study using sardine processing by-products without any pretreatment showed protein loss to emulsion that ranged from 1.52 to 15.5%, on a dry basis, depending on solids concentration, mixing speed, and enzyme dosage [1]. That study showed that solids concentration had the strongest influence on protein loss to emulsion and to sludge. The effect of solids concentration was affected by the levels of mixing speed and enzyme dosage, which resulted in reduced protein loss to emulsion and increased protein loss to sludge as solids concentration increased. There is need for understanding the effect of solids concentration without the influence of the levels of other processing variables such as mixing speed or enzyme dosage.
Šližyte et al. [7] suggested enzymatic hydrolysis without the addition of water for maximal lipid recovery as this did not result in emulsion formation. However, this was accompanied by a reduction in degree of hydrolysis and protein recovery. de Moura et al. [47, 48] suggested additional enzymatic hydrolysis or pH shift steps on the recovered emulsion to separate the oil from the protein. Despite enzymatic hydrolysis or pH shift showing promise to increase protein and oil recovery by destabilizing the emulsion, these methods have been shown to add complexity and cost to the process. Therefore, there is a need to prevent or control emulsion formation during enzymatic hydrolysis, which may be achieved by understanding and manipulating the variables that encourage emulsion formation during enzymatic hydrolysis.
The aim of this study was to evaluate the effect of substrate concentration on protein recovery, loss of protein due to emulsion formation, and protein loss to sludge during enzymatic hydrolysis without the interaction effects of other processing variables. The effect of substrate concentration on the molecular weight distribution of the hydrolysates formed was also determined.
2 Materials and methods
Sardine (Sardina pilchardus) by-product, consisting of off-specification material (muscles free of heads, tails, and viscera), was obtained from West Point Processors (Pty) Ltd, a canning factory located in St Helena Bay, South Africa.
2.1 Sample preparation and characterisation
Samples were prepared and characterised using equipment and methods described by Chiodza and Goosen [1]. The raw material was minced using a Trespade no. 12 mincer (Tre Spade, Italy) and then stored at − 26 °C until use. Proximate analysis was performed to determine moisture, ash, protein, and lipid content of the minced samples. Moisture content was analysed using 1-g sample sizes on a Kern DBS 60–3 moisture analyser (KERN & SOHN GmbH, Germany) set to auto drying at 180 °C. Ash analysis was performed on the dried samples by incineration at 600 °C for 3 h (AOAC Official Method 942.05) in a Nabertherm muffle furnace Lt 3/11/B180 L-030H1CN (Nabertherm GmbH, Germany). Protein analysis was carried out according to the Kjeldhal method (AOAC Official Method 979.09) using a DK8 Kjeldhal digestion system (VELP Scientifica Srl, Italy).
Total lipid content was measured according to the method by Lee et al. [49], with a slight modification. A 2:1 (v/v) chloroform–methanol solution at a solvent-to-sample ratio of 10:1 was used. Five grams of representative samples was mixed with 50 mL of 2:1 chloroform–methanol solution and homogenised. The filtration stage was skipped to minimise solvent loss to evaporation. The accuracy of the method is dependent on the amount of solvent used to extract lipids, and any loss would result in concentrating the lipids thus giving an inaccurate result. The equation presented by Lee et al. [49] has a term to account for solvent loss, but working at such small volumes, it was difficult to accurately determine how much would be lost during the filtration process. Twenty millilitres of 0.5% sodium chloride solution was added to the homogenised sample to prevent formation of emulsion. Five-millilitre aliquots of the chloroform phase were extracted and dried in a pre-weighed beaker on a hot plate set at 60 °C in a well-ventilated fume hood. Lipid content was calculated using Eq. 1, modified from Lee et al. [49].
2.2 Enzymatic hydrolysis
Commercial food grade Alcalase 2.4L from Sigma-Aldrich was used for the enzymatic hydrolysis experiments. Alcalase was selected for this study because of its broad specificity and mild operating conditions (pH 7–10 and temperature 50–60 °C) that results in high degree of hydrolysis and better functional properties compared to the use of other enzymes [45, 50,51,52].
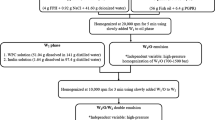
Enzymatic hydrolysis experiments were done in two phases, first to determine the optimum pH and temperature, and second, to determine the effect of solids concentration. A rotatable central composite design (CCD) with four centre point replicates was used to determine the optimum levels of hydrolysis temperature and pH that gave the maximum degree of hydrolysis. The ranges of independent factors were chosen based on preliminary one-factor-at-a-time experiments. An alpha value of 1.4142 was employed to maintain rotatability of the CCD (Table 1). Mixing speed, solids concentration, and enzyme dosage were fixed at 200 rpm, 16.66%, and 2%, respectively, based on previous studies [44, 53].
Enzymatic hydrolysis experiments were carried out in a 400-mL temperature-controlled batch reactor. Minced sardine muscles were homogenised with water to produce a suspension of 16.66% (w/v) solids on a wet basis with a total volume of 150 mL. The homogenised material was allowed to acclimatise to the operating conditions before enzyme addition. pH was adjusted using 2 M sodium hydroxide (NaOH). After the required pH and temperature were reached, Alcalase was dosed to start the hydrolysis reaction, which was allowed to run for 120 min. Enzymatic hydrolysis pH was maintained constant using 0.5 M NaOH that was automatically dosed into the reactor using Neon PR (Kuntze Instruments GmbH, Germany) PID controller connected to a PULSAtron K4VCT1 electronic pump (PULSAFEEDER Inc., USA). The reaction was quenched by increasing temperature to 90 °C for 15 min.
Once the optimum temperature and pH were determined, hydrolysis experiments were performed using solids concentration as the independent variable (26%, 30.86%, 38%, 44.14%, and 50% solids (w/v) on a wet basis) at 4% enzyme dosage, which was based on a previous study [1]. Time-series hydrolysis experiments used reactor contents volume of 350 mL, with 10-mL samples taken at 0, 2, 5, 10, 20, 30, 60, and 120 min during enzymatic hydrolysis.
Degree of hydrolysis (DH) was calculated using the pH–Stat method (Eq. 2) with α determined using the method described by Camacho et al. [54].
where VB (mL) is the volume of the base consumed, cB (mol/L) is the base concentration, α is the average degree of dissociation of the alpha-amino groups (α-NH2 groups), mP (g) is the mass of protein in the substrate, and htot (mmol of peptide bonds per g of protein) is the total peptide bonds in the substrate.
After enzymatic hydrolysis, reactor contents were centrifuged at 5000 rpm for 20 min (LASEC ISLXTG16.5, Lasec, South Africa). The centrifuged material was then separated into three main fractions: emulsion (top layer) containing light lipo-protein complexes, aqueous layer containing protein hydrolysates (middle layer), and solid sludge (bottom layer) containing heavy lipo-protein complexes and undigested material. The oil content of the sample and the small quantities used in each experiment were not sufficient to form a free oil layer that could be separated and quantified. Oil droplets reported mainly to the emulsion phase, with some remaining in the liquid FPH after separation. Each fraction was analysed for moisture and protein content.
2.3 Material balance
The results from moisture analysis were used to complete a material balance, which was subsequently used to determine the yield of dry solids according to Eq. 3:
The protein content of each fraction from the separation process was determined to calculate protein recovery in FPH, protein in emulsion, and protein in sludge, as a percentage of the protein in the initial sample before hydrolysis. The formulae for determining these variables are presented in Eq. 4, Eq. 5, and Eq. 6, respectively.
2.4 Size exclusion chromatography (SEC)
Molecular weight distribution of protein hydrolysates was determined using size exclusion chromatography (SEC) on a Dionex UltiMate 3000 HPLC System (Thermo Fisher Scientific, USA) mounted with an 8 × 300 mm Suprema column (PSS Polymer Standards Service GmbH, Germany). The SEC equipment used a Varian (Agilent Technologies, USA) evaporative light scattering (ELS) detector to detect molecules in the protein hydrolysates. The column was calibrated using Pullulan standards (PSS Polymer Standards Service GmbH, Germany) with a molecular weight range of 0.342 to 708 kiloDalton (kDa).
Hydrolysate samples were prepared by filtration into 2-mL HPLC vials using 0.26-µm FilterBio® nylon syringe filters. Samples were injected into the SEC column at a volume of 4 µL, for an elution rate of 1 mL/min using ultrapure water as the mobile phase for 45 min per sample.
Average molecular weight was determined based on the area under the chromatogram. Data was grouped into the following molecular weights: > 500 kDa, 100–500 kDa, 10–100 kDa, 3–5 kDa, 1–3 kDa, 0.5–1 kDa, and < 0.5 kDa.
2.5 Statistics
For the determination of the optimum pH and temperature, enzymatic hydrolysis data was analysed using Statistica v13.5.0.17 (TIBCO Software Inc, USA). Results of the experiments were fitted to a second-order regression model (Eq. 7), and the resulting model was employed to predict the degree of hydrolysis based on different levels of the independent variables.
Analysis of variance (ANOVA) was performed on the standardised effects to determine factors that were statistically significant in explaining the variation observed in the response variable. Factors were standardised by scaling the original factors such that the values of the low and high factors are transformed − 1 and + 1, respectively. This makes the interpretation of effect estimates standardised and comparable in size [55].
Desirability analysis was used to determine the optimum pH and temperature that gave the maximum degree of hydrolysis. A full description of the method is found in Derringer [56]. For response variables that were to be maximised (DH, dry solids yield and protein recovery), the desirability function was set as follows:
where di(Yi) is the desirability, with a value between 0 and 1 mapped to all the possible values of Yi. di(Yi) = 0 represents the most undesirable value of the response variable and di(Yi) = 1 represents the most desirable value of the response variable Yi, and Yi(X) is the value of response variable i at independent factor X. Li is the lower target (response) value and Ti is the desired value for the response variable. Equation 7 was used as Yi(X) for Eq. 8.
3 Results and discussion
Raw material proximate composition is shown in Table 2. The results obtained here are within the range of previously published studies [57,58,59,60]. This sample had a high oil content (4.39%), on a wet basis, compared to what has been reported elsewhere in literature [59]. Bandarra et al. [40] reported that the oil content of sardines varies seasonally, ranging from 1.2 to 18.4% on a wet basis.
3.1 Optimum enzymatic hydrolysis pH and temperature
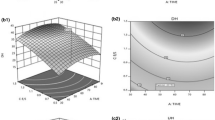
The results for degree of hydrolysis (DH) at different levels of pH and temperature are provided in Table 4 in the supplementary material. DH ranged from 9.55 to 20.3% for pH ranging from 7.09 to 9.91 and temperature ranging from 54.86 to 74.14 °C. Figure 1 shows that pH between 7.2 and 8.7, and temperature between 53 and 62 °C resulted in DH greater than 20%. This range of pH and temperature agrees with the optimum conditions that were obtained by other researchers using Alcalase with different substrates [61,62,63]. For the substrate used in this study, the optimum pH and temperature that gave the highest DH were pH 7.80 and 60 °C, respectively (Fig. 2). Analysis of variance (Table 5, supplementary material) showed that both the linear and quadratic terms of the second-order regression equation were statistically significant and had a negative effect (Table 6, supplementary material) on the variation observed in DH at different values of pH and temperature. That is, increasing pH or temperature or both results in an increase in DH that reaches a maximum (at pH 7.795 and 60 °C in this study) then a decrease in DH as pH or temperature continues to increase. The regression coefficients for the equations that were used in performing the desirability analysis are in Table 7 in the supplementary material.
Contour plot showing the effect of pH and temperature (°C) on degree of hydrolysis during enzymatic hydrolysis of sardine processing by-products. Mixing speed, solids concentration, and enzyme dosage were fixed at 200 rpm, 16.66% (w/v), and 2% (v/w dry protein), respectively, based on previous studies [44, 53]
Desirability profile of predicted values of degree hydrolysis from enzymatic hydrolysis of sardine processing by-products using Alcalase. Mixing speed, solids concentration, and enzyme dosage were fixed at 200 rpm, 16.66% (w/v), and 2% (v/w dry protein), respectively, based on previous studies [44, 53]. Blue continuous horizontal lines show the ± 95% confidence interval of the predicted value
3.2 Effect of solids concentration
3.2.1 Degree of hydrolysis
DH varied from 24.5 to 27.4%, which showed a general decrease as solids concentration increased (Fig. 3). This observation confirms the results of a previous study where DH was shown to decrease with increase in solids concentration [1]. However, that study showed that the effect of solids concentration also depended on the level of mixing speed used. The reasons for the decrease in DH with increasing solids concentration that have been cited in various studies are enzyme inhibition, and poor mass and energy transfer at high solids concentration [2, 3, 64].
3.2.2 Yield of dry solids and protein recovery
Yield of dry solids and protein recovery are, in most cases, codependent variables. This is because of the specificity of the enzymes used during enzymatic extraction of protein. Yield of dry solids, which ranged from 56.7 to 68.7%, decreased with increase in solids concentration (Fig. 4A). This observation agrees with previous studies where low solids concentration consistently resulted in higher yields [1, 2, 65, 66]. Protein recovery showed the same trend where there was a decrease from 75.6% protein recovery at 26% solids concentration to 67.0% at 50% solids concentration (Fig. 4B).
Figure 3 and Fig. 4 show that DH, yield of dry solids, and protein recovery are decreasing with increase in solids concentration. The relationship between these three variables is linked to two important phenomena that exist in enzymatic reactions in aqueous solutions, (1) solubility and (2) the mechanism of enzymatic reactions. Higher recoveries are expected at low solids concentrations since this is below the solubility limit of proteins and peptides in solution. As solids concentration increases, solubility decreases as the solubility limit of the solute is reached, resulting in low recoveries. According to Sarma et al. [67], proteins and peptides aggregate at concentrations near, at, or above the solubility limit due to the strong van der Waals interactions and hydrogen bonds between the backbones and side chains of the protein or peptide, resulting in phase separation. These aggregated protein and peptide molecules, being insoluble in this state, are separated out of the solution during centrifugation. Workman et al. [68] also showed phase separation for short peptides with five amino acids under saturated conditions. This shows that the effect of solubility may be contributing to the reduction in solids yield and protein recovery as the solids concentration increases.
On the mechanism of enzymatic reactions, Mohr [9] showed that enzyme activity occurs in two phases, the solid phase (at the solid–liquid interface) and the liquid phase. At the start of the enzymatic reaction, most of the protein is in the solid phase, resulting in high enzyme activity at the solid–liquid interface. This provides soluble protein for the liquid phase reactions, causing an increase in enzyme activity in this phase. Although it is difficult to measure the kinetics of the two reactions individually, the expectation would be to have the yield of dry solids or protein recovery proportional to DH since it is the breakdown of peptide bonds that is resulting in the solubilisation of insoluble protein. DH measures the percentage of peptide bonds broken down, regardless of whether the reaction occurred at the solid–liquid interface, resulting in the increase of protein in solution, or in the liquid phase, resulting in no increase in the protein concentration in solution. On the other hand, yield of dry solids or protein recovery only quantifies protein that ended up in solution. Consequently, there are cases where DH will not be directly proportional to yield of dry solids and protein recovery.
Figure 5A and B are time-series data for enzymatic hydrolysis carried out using 30.86% and 50% solids concentration, respectively. Protein recovery reached 87.3% for 30.86% solids concentration and 86.0% for 50% solids concentration at 60 min of hydrolysis. DH at 60 min was 23.7% and 22.4% for 30.86% and 50% solids concentration, respectively. The final protein recoveries at 120 min of hydrolysis were lower than at 60 min (81.4% and 82.9% protein recovery for 30.86% and 50% solids, respectively) while DH was slightly higher (26.7% and 24.7% DH for 30.86% and 50% solids, respectively). Figure 5B also shows that, in addition to protein recovery, yield of dry solids decreased from 76.8% to 73.8% for the enzymatic hydrolysis process that used 50% solids concentration. These results show that there are times during an enzymatic hydrolysis process where DH is not directly proportional to yield of dry solids or protein recovery. Although enzyme inhibition is acknowledged as a factor contributing to the reduction in yield of dry solids and protein recovery in enzymatic hydrolysis processes with high solids concentration, Fig. 5 clearly shows that it is more than enzyme inhibition that is causing this decrease. The possible explanation for this observation, in addition to the solubility-induced aggregation and phase separation, is the removal of protein in solution by emulsion formation, which is shown in Fig. 6 and later discussed in Sect. 3.2.3. Between 60 and 120 min of hydrolysis, the rate of protein addition into solution by enzyme activity on the solid–liquid interfaces is lower than the rate of protein removal by emulsion formation, resulting in the trend observed in Fig. 5A and B. An oil-in-water emulsion requires the presence of an emulsifying agent from the emulsion and a stabilizer to keep the emulsion stable. Hydrolysed proteins have been found to have both emulsifying and stabilising properties [25, 34, 36, 50].
3.2.3 Protein loss to emulsion and sludge
Protein loss to emulsion, which ranged from 2.01 to 10.6% (Fig. 6A), showed a correlation with the amount of emulsion recovered from the hydrolysed material (Fig. 7). The correlation can be explained as being a result of the impact of solids concentration, where at low solids concentrations, the oil concentration is low such that only a small amount of emulsion is being formed, and hence only a small proportion of protein participates in emulsion formation. Increasing solids concentration increases both the oil and hydrolysed protein concentrations. Hydrolysed proteins have been shown to have better emulsion formation and stabilisation properties compared to intact protein [25, 30, 34, 69, 70]. It is suspected that these hydrolysed proteins, together with the increased oil content at high substrate concentrations, contribute to the increased protein loss to emulsion at high solids concentration, resulting in the decrease in yield of dry solids and protein recovery shown in Fig. 4. This also supports the reason why there was no free oil layer on top of the emulsion layer after centrifugation. Šližyte et al. [30] showed that emulsion formation can be prevented by adding no water to the substrate to be hydrolysed. However, this only maximises oil recovery at the expense of protein recovery.
Protein loss to sludge, which ranged from 10.5 to 28.9% (Fig. 8), increased with increase in solids concentration. This verifies a previous study using central composite experiments that showed that solids concentration was a significant factor which increased protein loss to sludge as it increased [1]. The reason for this may be attributed to inhibition of enzyme activity both by substrate and product when the solids concentration is high [2, 3, 65], inaccessible peptide bonds during enzymatic hydrolysis, even at low solids concentration [2, 71], and solubility-induced aggregation and phase separation of proteins and peptides at high concentrations [67, 68]. Araujo et al. [72] also showed that collagen (a structural protein with an average molecular weight of 300 kDa commonly found in skin, bones, and organs [73]) settles to the bottom during centrifugation, which contributes to protein loss to the sludge. This also contributes to protein loss to sludge as the solids concentration increases.
Although previous studies have reported protein loss to sludge and the reasons associated with those losses, what has not been clearly highlighted in literature is the role of emulsion formation on protein loss to sludge and to emulsion. During enzymatic hydrolysis, lipo-protein complexes with different densities are formed [20, 21, 74]. Separation of the hydrolysed material by centrifugation causes high-density lipo-protein complexes to settle to the bottom, where they form part of the sludge. This was confirmed by visual observation of the centrifuged material, where bones and scales formed the bottom layer of the sludge followed by an oily paste on top. Therefore, emulsion formation did not only contribute to protein loss through the emulsion phase (Fig. 7), but also through the sludge.
3.2.4 Molecular weight distribution
Molecular weight of the molecules identified in the samples obtained from the enzymatic hydrolysis of sardine processing by-products at different solids concentration varied between 0.1 and 10,000 kDa (Fig. 9). Only the sample from 26% solids concentration had molecules less than 100 kDa, while the rest of the samples had molecules with molecular weights upwards of 100 kDa (Fig. 9). Vieira et al. [45] reported that unhydrolysed protein extracts from sardines ranged from 29 to 97 kDa, suggesting that the molecules identified in this study with molecular weight > 100 kDa are not unhydrolysed proteins. Many studies have also shown that enzymatic hydrolysis of fish processing by-products produce hydrolysates with molecular weights that are generally in the range up to about 60 kDa [44, 45, 75, 76]. Therefore, the peaks identified on the chromatograms with molecular weights > 100 kDa are possibly lipo-protein complexes that are distributed in the aqueous phase containing FPH. This agrees with the study by Šližyte et al. [74], where triglycerides were mainly found in the free oil and emulsion phase while phospholipids were found both in the aqueous phase containing hydrolysed protein and in the insoluble sludge. Quaglia and Orban [44] obtained protein hydrolysates that had molecular weights that ranged up to 30 kDa using defatted sardines. Defatting removed oil, which is required for emulsion formation, which possibly explains the absence of any molecules with molecular weight > 30 kDa detected. According to Brunner [20], one particle of lipo-protein complexes contains thousands of lipid molecules in association with a large protein molecule and water molecules, which a possible explanation for the presence of compounds with molecular weight as high as 10,000 kDa.
Molecular weight distribution of sardine protein hydrolysates at different solids concentration (26–50%). Hydrolysates were analysed on a Dionex UltiMate 3000 HPLC System mounted with an 8 × 300 mm Suprema column. The equipment used a Varian evaporative light scattering (ELS) detector and the column was calibrated using Pullulan standards with a molecular weight range of 0.342 to 708 kDa
Table 3 shows that the molecular weight of all protein hydrolysates was similarly distributed regardless of solids concentration, except for the sample that used 26% solids concentration, which did not have any molecules above 100 kDa. This could be explained by the low emulsion formation reported in Fig. 7. The 26% solids concentration sample had a significant proportion of hydrolysates in the molecular weight range between 5 and 10 kDa, which accounted for 42.8% of the area under the curve in Fig. 9. This supports the hypothesis that emulsion formation is removing protein hydrolysates of a particular size range since the 5–10 kDa range for solids concentration other than 26% was low, accounting for only 15.6–17.7% of the area under the chromatogram. Previous studies have shown that protein hydrolysates with molecular weight > 2 kDa possess emulsifying properties [25, 36].
Molecular weight is one of the important characteristics of fish protein hydrolysates to consider because it informs the expected physical and bioactive properties of the dried product. More than 50% of the molecules in the protein hydrolysates had molecular weight less than 10 kDa ("SUM<10" row in Table 3). There are studies that have shown that fish protein hydrolysates with molecular weights less than 10 kDa have strong antioxidative properties [77,78,79] while hydrolysates with molecular weights in the range 10 to 30 kDa possess antihypertensive properties [80,81,82]. Thus, Table 3 shows that, with the removal of large molecular weight molecules using methods such as ultrafiltration, the protein hydrolysates obtained in this study have potential application in nutraceuticals because of their bioactive properties. Removal of these high molecular weight lipo-protein molecules will also improve product quality as it removes oil that is responsible for food powders going rancid when it is oxidised during storage.
4 Conclusion
This study has presented the major role that emulsion formation plays in the yield of dry solids and protein recovery during enzymatic protein hydrolysis, where higher solids concentrations result in higher protein losses both to the emulsion and solid phases. The contribution of emulsion formation to protein loss to the solid sludge is through heavy lipo-protein complexes that settle to the bottom during separation. It has also been shown that the amount of emulsion formed is directly proportional to the solids concentration used for the enzymatic hydrolysis process.
Despite most of the peptides in the hydrolysates having molecular weights less than 10 kDa, emulsion formation contributed to the removal of bioactive peptides in the size range 5–10 kDa. This reduces the concentration of peptides with the most desirable bioactive and functional properties. Efficient separation of high molecular weight lipo-proteins from the hydrolysates will reduce oil content of the final product, which improves product quality by preventing the development of off-flavours and rancidity caused by oxidised oils.
Data availability
Data will be available as part of PhD dissertation, on the open-source institutional repository (Stellenbosch University).
References
Chiodza K, Goosen NJ (2023) Influence of mixing speed, solids concentration and enzyme dosage on dry solids yield and protein recovery during enzymatic hydrolysis of sardine (Sardina pilchardus) processing by-products using Alcalase 2.4L: a multivariable optimisation approach. Biomass Convers Biorefinery 1:1–23. https://doi.org/10.1007/S13399-023-03829-2
Valencia P, Pinto M, Almonacid S (2014) Identification of the key mechanisms involved in the hydrolysis of fish protein by Alcalase. Process Biochem 49:258–264. https://doi.org/10.1016/J.PROCBIO.2013.11.012
Demirhan E, Apar DK, Özbek B (2011) A kinetic study on sesame cake protein hydrolysis by Alcalase. J Food Sci 76:C64–C67. https://doi.org/10.1111/J.1750-3841.2010.01938.X
Balan V, Chiaramonti D, Kumar S (2013) Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod Biorefining 7:732–759. https://doi.org/10.1002/bbb.1436
Janssen R, Turhollow AF, Rutz D, Mergner R (2013) Production facilities for second-generation biofuels in the USA and the EU - current status and future perspectives. Biofuels Bioprod Biorefining 7:647–665. https://doi.org/10.1002/bbb.1451
Beschkov V, Yankov D (2021) Chemical engineering methods in downstream processing in biotechnology. Phys Sci Rev 6(4):20180064. https://doi.org/10.1515/psr-2018-0064
Šližyte R, Daukšas E, Falch E, Storrø I, Rustad T (2005) Yield and composition of different fractions obtained after enzymatic hydrolysis of cod (Gadus morhua) by-products. Process Biochem 40:1415–1424. https://doi.org/10.1016/j.procbio.2004.06.033
Roslan J, Kamal SMM, Khairul KF, Abdullah N (2014) Optimization of enzymatic hydrolysis of tilapia muscle (Oreochromis niloticus) using response surface methodology (RSM). Sains Malaysiana 43:1715–1723
Mohr V (1978) Fish protein concentrate production by enzymic hydrolysis, in: Biochemical Aspects of New Protein Food 53–62. https://doi.org/10.1016/b978-0-08-022625-5.50012-1
Auwal SM, Zarei M, Abdul-Hamid A, Saari N (2017) Optimization of bromelain-aided production of angiotensin I-converting enzyme inhibitory hydrolysates from stone fish using response surface methodology. Mar Drugs 15:104. https://doi.org/10.3390/md15040104
McCabe BK, Antille DL, Birt HWG, Spence JE, Fernana JM, Van Der Spek W, Baillie CP (2016) An investigation into the fertilizer potential of slaughterhouse cattle paunch. 2016 Annu Int Meet ASABE 2016 Am Soc Agric Biol Eng. https://doi.org/10.13031/aim.20162460831
Gaikwad A, Chakraborty S (2013) Mixing effects on the kinetics of enzymatic hydrolysis of Avicel for batch production of cellulosic ethanol. Ind Eng Chem Res 52:3988–3999. https://doi.org/10.1021/ie301234b
Noman A, Xu Y, AL-Bukhaiti WQ, Abed SM, Ali AH, Ramadhan AH, Xia W (2018) Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem 67:19–28. https://doi.org/10.1016/j.procbio.2018.01.009
Kristinsson HG, Rasco BA (2000) Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J Agric Food Chem 48:657–666. https://doi.org/10.1021/jf990447v
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327. https://doi.org/10.1016/j.foodchem.2006.07.016
Ruiz-Álvarez JM, del Castillo-Santaella T, Maldonado-Valderrama J, Guadix A, Guadix EM, García-Moreno PJ (2022) pH influences the interfacial properties of blue whiting (M. poutassou) and whey protein hydrolysates determining the physical stability of fish oil-in-water emulsions. Food Hydrocoll 122:107075. https://doi.org/10.1016/j.foodhyd.2021.107075
Barac M, Cabrilo S, Stanojevic S, Pesic M, Pavlicevic M, Zlatkovic B, Jankovic M (2012) Functional properties of protein hydrolysates from pea (Pisum sativum, L) seeds. Int J Food Sci Technol 47:1457–1467. https://doi.org/10.1111/j.1365-2621.2012.02993.x
Pacheco-Aguilar R, Mazorra-Manzano MA, Ramírez-Suárez JC (2008) Functional properties of fish protein hydrolysates from Pacific whiting(Merluccius productus) muscle produced by a commercial protease. Food Chem 109:782–789. https://doi.org/10.1016/j.foodchem.2008.01.047
Östbring K, Matos M, Marefati A, Ahlström C, Gutiérrez G (2021) The effect of pH and storage temperature on the stability of emulsions stabilized by rapeseed proteins. Foods 10:1657–1674. https://doi.org/10.3390/foods10071657
Brunner JR (1961) Protein-lipid interactions and their relation to the physical-chemical stability of concentrated milk: A review. J Dairy Sci 45:943–951. https://doi.org/10.3168/jds.S0022-0302(62)89532-0
Feingold KR (2021) Introduction to lipids and lipoproteins, in: Feingold KR, Anawalt B, Blackman M (eds) Endotext [Internet], MDText.com, Inc., South Dartmouth (MA). https://www.ncbi.nlm.nih.gov/books/NBK305896/
Aguilar ZP (2013) Types of nanomaterials and corresponding methods of synthesis, in: Nanomaterials for Medical Applications 33–82. https://doi.org/10.1016/B978-0-12-385089-8.00002-9
Kristinsson HG, Rasco BA (2000) Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr 40:43–81. https://doi.org/10.1080/10408690091189266
Siddik MAB, Howieson J, Fotedar R, Partridge GJ (2021) Enzymatic fish protein hydrolysates in finfish aquaculture: a review. Rev Aquac 13:406–430. https://doi.org/10.1111/raq.12481
Steinsholm S, Oterhals Å, Underhaug J, Aspevik T (2021) Emulsion and surface-active properties of fish solubles based on direct extraction and after hydrolysis of Atlantic cod and Atlantic salmon backbones. Foods 10:38–50. https://doi.org/10.3390/foods10010038
García-Moreno PJ, Pérez-Gálvez R, Espejo-Carpio FJ, Ruiz-Quesada C, Pérez-Morilla AI, Martínez-Agustín O, Guadix A, Guadix EM (2017) Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: effect of enzymatic treatment and degree of hydrolysis. J Sci Food Agric 97:299–308. https://doi.org/10.1002/jsfa.7731
Batista I, Ramos C, Coutinho J, Bandarra NM, Nunes ML (2010) Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochem 45:18–24. https://doi.org/10.1016/j.procbio.2009.07.019
Pires C, Teixeira B, Cardoso C, Mendes R, Nunes ML, Batista I (2015) Cape hake protein hydrolysates prepared from alkaline solubilised proteins pre-treated with citric acid and calcium ions: functional properties and ACE inhibitory activity. Process Biochem 50:1006–1015. https://doi.org/10.1016/j.procbio.2015.03.010
Chen C, Chi YJ, Xu W (2012) Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol 5:2342–2352. https://doi.org/10.1007/s11947-011-0606-7
Šližyte R, Rustad T, Storrø I (2005) Enzymatic hydrolysis of cod (Gadus morhua) by-products: optimization of yield and properties of lipid and protein fractions. Process Biochem 40:3680–3692. https://doi.org/10.1016/j.procbio.2005.04.007
Vincent D, Elkins A, Condina MR, Ezernieks V, Rochfort S (2016) Quantitation and identification of intact major milk proteins for high-throughput LC-ESI-Q-TOF MS analyses. PLoS One 11_1–21. https://doi.org/10.1371/journal.pone.0163471
Britten M, Giroux HJ (1991) Emulsifying properties of whey protein and casein composite blends. J Dairy Sci 74:3318–3325. https://doi.org/10.3168/jds.S0022-0302(91)78519-6
Chobert J-M, Bertrand-Harb C, Nicolas M-G (1988) Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin. J Agric Food Chem 36:883–892. https://doi.org/10.1021/jf00083a002
van der Ven C, Gruppen H, de Bont D, Voragen A (2001) Emulsion properties of casein and whey protein hydrolysates and the relation with other hydrolysate characteristics. J Agric Food Chem 49:5005–5012. https://doi.org/10.1021/JF010144C
Dickinson E (1989) Surface and emulsifying properties of caseins. J Dairy Res 56:471–477. https://doi.org/10.1017/S0022029900028958
Padial-Domínguez M, Espejo-Carpio FJ, Pérez-Gálvez R, Guadix A, Guadix EM (2020) Optimization of the emulsifying properties of food protein hydrolysates for the production of fish oil-in-water emulsions. Foods 9:636–652. https://doi.org/10.3390/foods9050636
Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H (2009) The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem 115:238–242. https://doi.org/10.1016/j.foodchem.2008.12.013
Dumay J, Donnay-Moreno C, Barnathan G, Jaouen P, Bergé JP (2006) Improvement of lipid and phospholipid recoveries from sardine (Sardina pilchardus) viscera using industrial proteases. Process Biochem 41:2327–2332. https://doi.org/10.1016/j.procbio.2006.04.005
Tan Y, Chang SKC, Meng S (2019) Comparing the kinetics of the hydrolysis of by-product from channel catfish (Ictalurus punctatus) fillet processing by eight proteases. LWT 111:809–820. https://doi.org/10.1016/J.LWT.2019.05.053
Bandarra NM, Batista I, Nunes ML, Empis JM, Christie WW (1997) Seasonal changes in lipid composition of sardine (Sardina pilchardus). J Food Sci 62:40–42. https://doi.org/10.1111/j.1365-2621.1997.tb04364.x
Coetzee J, de Moor C, Butterworth D (2018) A summary of the South African sardine (and anchovy) fishery, Cape Town. https://open.uct.ac.za/bitstream/handle/11427/30781/MARAM_IWS_2019_Sardine_BG1.pdf?sequence=1. Accessed 24 May 2023
United States Environmental Protection Agency (1995) AP-42, CH 9.13.1: Fish Processing, in: AP-42, Fifth Edition Compilation of air pollutant emissions factors, Volume 1: Stationary Point and Area Sources 9.13.1.1-7. https://www3.epa.gov/ttnchie1/ap42/ch09/final/c9s13-1.pdf. Accessed 24 May 2023
FAO (2021) Processing, in: Myrseth A (ed) Planning and engineering data 2. Fish Canning. https://www.fao.org/3/r6918e/R6918E05.htm. Accessed 24 May 2023
Quaglia GB, Orban E (1990) Influence of enzymatic hydrolysis on structure and emulsifying properties of sardine (Sardina pilchardus) protein hydrolysates. J Food Sci 55:1571–1573. https://doi.org/10.1111/j.1365-2621.1990.tb03571.x
Vieira EF, Pinho O, Ferreira IM (2017) Bio-functional properties of sardine protein hydrolysates obtained by brewer’s spent yeast and commercial proteases. J Sci Food Agric 97:5414–5422. https://doi.org/10.1002/jsfa.8432
Batista I, Ramos C, Mendonca R, Nunes ML (2009) Enzymatic hydrolysis of sardine (Sardina pilchardus) by-products and lipid recovery. J Aquat Food Prod Technol 18:120–134. https://doi.org/10.1080/10498850802581823
De Moura JMLN, Campbell K, Mahfuz A, Jung S, Glatz CE, Johnson L (2008) Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification, JAOCS. J Am Oil Chem Soc 85:985–995. https://doi.org/10.1007/S11746-008-1282-2/TABLES/3
De Moura JMLN, Johnson LA (2009) Two-stage countercurrent enzyme-assisted aqueous extraction processing of oil and protein from soybeans. JAOCS J Am Oil Chem Soc 86:283–289. https://doi.org/10.1007/S11746-008-1341-8/FIGURES/6
Lee CM, Trevino B, Chaiyawat M (1996) A simple and rapid solvent extraction method for determining total lipids in fish tissue. J AOAC Int 79:487–492. https://doi.org/10.1093/jaoac/79.2.487
Saadaoui H, Espejo-Carpio FJ, Guadix EM, Ben Amar R, Pérez-Gálvez R (2019) Bi-objective optimization of tuna protein hydrolysis to produce aquaculture feed ingredients. Food Bioprod Process 115:26–35. https://doi.org/10.1016/j.fbp.2019.03.001
Adler-Nissen J (1976) Enzymic hydrolysis of proteins for increased solubility. J Agric Food Chem 24:1090–1093. https://doi.org/10.1021/JF60208A021
Novozymes (2016) Proteases for biocatalysis, pp 1–6. https://www.novozymes.com/en/solutions/fine-chemicals/biocatalysis/proteases
Vázquez JA, Blanco M, Massa AE, Amado IR, Pérez-Martín RI (2017) Production of fish protein hydrolysates from Scyliorhinus canicula discards with antihypertensive & antioxidant activities by enzymatic hydrolysis & mathematical optimization using response surface methodology. Marine Drugs 15:1–15. https://doi.org/10.3390/md15100306
Camacho F, González-Tello P, Páez-Dueñas MP, Guadix EM, Guadix A (2001) Correlation of base consumption with the degree of hydrolysis in enzymic protein hydrolysis. J Dairy Res 68:251–265. https://doi.org/10.1017/S0022029901004824
TIBCO Statistica (2017) TIBCO StatisticaTM Quick Reference. https://docs.tibco.com/pub/stat/13.3.0/doc/pdf/TIB_stat_13.3_quick_ref.pdf?id=1. Accessed 4 June 2022
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219. https://doi.org/10.1080/00224065.1980.11980968
Benhabiles MS, Abdi N, Drouiche N, Lounici H, Pauss A, Goosen MFA, Mameri N (2012) Fish protein hydrolysate production from sardine solid waste by crude pepsin enzymatic hydrolysis in a bioreactor coupled to an ultrafiltration unit. Mater Sci Eng C 32:922–928. https://doi.org/10.1016/J.MSEC.2012.02.013
Morales-Medina R, Tamm F, Guadix AM, Guadix EM, Drusch S (2016) Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem 194:1208–1216. https://doi.org/10.1016/j.foodchem.2015.08.122. (0308-8146/Ó)
Bentis CA, Zotos A, Petridis D (2005) Production of fish-protein products (surimi) from small pelagic fish (Sardinops pilchardusts), underutilized by the industry. J Food Eng 68:303–308. https://doi.org/10.1016/J.JFOODENG.2004.06.003
Quaglia GB, Orban E (1987) Enzymic solubilisation of proteins of sardine (sardina pilchardus) by commercial proteases. J Sci Food Agric 38:263–269. https://doi.org/10.1002/jsfa.2740380310
Noman A, Qixing J, Xu Y, Ali AH, Al-Bukhaiti WQ, Abed SM, Xia W (2019) Influence of degree of hydrolysis on chemical composition, functional properties, and antioxidant activities of Chinese sturgeon (Acipenser sinensis) hydrolysates obtained by using Alcalase 2.4L. J Aquat Food Prod Technol 28:583–597. https://doi.org/10.1080/10498850.2019.1626523
O’Meara GM, Munro PA (1984) Effects of reaction variables on the hydrolysis of lean beef tissue by alcalase. Meat Sci 11:227–238. https://doi.org/10.1016/0309-1740(84)90039-1
Martin AM, Porter D (1995) Studies on the hydrolysis of fish protein by enzymatic treatment Food Flavors Gener Anal Process Influ 1395–1404
Sierra-Lopera LM, Zapata-Montoya JE (2021) Optimization of enzymatic hydrolysis of red tilapia scales (Oreochromis sp.) to obtain bioactive peptides. Biotechnol Rep 30:e00611. https://doi.org/10.1016/J.BTRE.2021.E00611
Um BH, Hanley TR (2008) High-solid enzymatic hydrolysis and fermentation of solka floc into ethanol. J Microbiol Biotechnol 18:1257–1265
Kristensen JB, Felby C, Jørgensen H (2009) Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol Biofuels 2:1–10. https://doi.org/10.1186/1754-6834-2-11/FIGURES/6
Sarma R, Wong KY, Lynch GC, Pettitt BM (2018) Peptide solubility limits: backbone and side-chain interactions. J Phys Chem B 122:3528–3539. https://doi.org/10.1021/acs.jpcb.7b10734
Workman RJ, Gorle S, Pettitt BM (2022) Effects of conformational constraint on peptide solubility limits. J Phys Chem B 126:10510–10518. https://doi.org/10.1021/acs.jpcb.2c06458
Encina C, Vergara C, Giménez B, Oyarzún-Ampuero F, Robert P (2016) Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci Technol 56:46–60. https://doi.org/10.1016/j.tifs.2016.07.014
Alavi F, Jamshidian M, Rezaei K (2019) Applying native proteases from melon to hydrolyze kilka fish proteins (Clupeonella cultriventris caspia) compared to commercial enzyme Alcalase. Food Chem 277:314–322. https://doi.org/10.1016/j.foodchem.2018.10.122
Sousa R, Lopes GP, Tardioli PW, Giordano RLC, Almeida PIF, Giordano RC (2004) Kinetic model for whey protein hydrolysis by Alcalase multipoint-immobilized on agarose gel particles. Braz J Chem Eng 21:147–153. https://doi.org/10.1590/s0104-66322004000200003
Araujo J, Sica P, Costa C, Márquez MC (2021) Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste Biomass Valor 12:847–855. https://doi.org/10.1007/s12649-020-01029-x
León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G (2019) Hydrolyzed collagen-sources and applications. Molecules 24:4031–4046. https://doi.org/10.3390/molecules24224031
Šližyte R, Van Nguyen J, Rustad T, Storro I (2004) Hydrolysis of cod (Gadus morhua) by-products: influence of initial heat inactivation, concentration and separation conditions. J Aquat Food Prod Technol 13:37–41. https://doi.org/10.1300/J030v13n02
Quaglia GB, Orban E (1987) Influence of the degree of hydrolysis on the solubility of the protein hydrolysates from sardine (Sardina pilchardus). J Sci Food Agric 38:271–276. https://doi.org/10.1002/JSFA.2740380311
Souissi N, Bougatef A, Triki-Ellouz Y, Nasri M (2007) Biochemical and functional properties of sardinella (Sardinetta aurita) by-product hydrolysates. Food Technol Biotechnol 45:187–194
Jeon Y-J, Byun H-G, Kim S-K (1999) Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem 35:471–478. https://doi.org/10.1016/S0032-9592(99)00098-9
Saidi S, Belleville MP, Deratani A, Ben Amar R (2016) Production of interesting peptide fractions by enzymatic hydrolysis of tuna dark muscle by-product using Alcalase. J Aquat Food Prod Technol 25:251–264. https://doi.org/10.1080/10498850.2013.844753
Saidi S, Deratani A, Belleville MP, Ben Amar R (2014) Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: impact on interesting peptides fractions and nutritional properties. Food Res Int 65:453–461. https://doi.org/10.1016/j.foodres.2014.04.026
Je J-Y, Park P-J, Kwon JY, Kim S-K (2004) A novel angiotensin I converting enzyme inhibitory peptide from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. J Agric Food Chem 52:7842–7845. https://doi.org/10.1021/jf0494027
Yathisha UG, Bhat I, Karunasagar I, Mamatha BS (2019) Antihypertensive activity of fish protein hydrolysates and its peptides. Crit Rev Food Sci Nutr 59:2363–2374. https://doi.org/10.1080/10408398.2018.1452182
Huang J, Liu Q, Xue B, Chen L, Wang Y, Ou S, Peng X (2016) Angiotensin-I-converting enzyme inhibitory activities and in vivo antihypertensive effects of sardine protein hydrolysate. J Food Sci 81:H2831–H2840. https://doi.org/10.1111/1750-3841.13508
Funding
Open access funding provided by Stellenbosch University. Research funding was provided by the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 818173.
Author information
Authors and Affiliations
Contributions
Conceptualization: Neill Jurgens Goosen. Methodology: Kudzai Chiodza. Formal analysis: Kudzai Chiodza. Investigation: Kudzai Chiodza. Writing — original draft preparation: Kudzai Chiodza. Writing — review and editing: Kudzai Chiodza, Neill Jurgens Goosen. Funding acquisition: Neill Jurgens Goosen. Resources: Neill Jurgens Goosen. Supervision: Neill Jurgens Goosen. Projection administration: Neill Jurgens Goosen.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiodza, K., Goosen, N.J. Emulsion formation during enzymatic protein hydrolysis and its effect on protein recovery and molecular weight distribution of protein hydrolysates from sardine (Sardina pilchardus) by-products. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04438-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04438-9