Abstract
In this study, the clay was interwoven with cellulose to change its structure. The product clay/cellulose was used to assess the efficacy of the methylene blue (MB) dye removal from aqueous solutions (CC25). The response surface methodology and Box-Behnken design were used to optimize the influence of crucial parameters (cellulose load, adsorbent dosage, solution pH, temperature, and contact duration) (RSM-BBD). The greatest removal effectiveness was 98.76% for a cellulose loading of 25.0% and the following working conditions, i.e., adsorbent dosage of 0.06 g/L, pH 7, temperature of 45 °C, and contact length of 20 min. At the time, the maximum adsorption capacity was 254.8 mg/g. The pseudo-second-order adsorption model, according to the adsorption kinetics research, was used to describe the process. The MB adsorption process was endothermic and spontaneous, according to computed thermodynamic functions. The developed composite material, according to our results, has a very high capacity for the color absorption and removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nobody can deny that water is necessary for the human life. Life is drastically improved as technology has advanced; nonetheless, the pollution of water and its shortages are a real problem that is more and more exacerbated. The water pollution is indeed a severe environmental problem that jeopardizes the global economic growth and the health of billions of people [1]. Although there are no immediate solutions to the issue of the water scarcity, water contamination remains a major global concern that has to be solved. Among the impurities that contaminate water are oil, heavy metal species, organic dyes, and other toxic chemicals [2]. As long as dyes, finishing chemicals, and auxiliary agents are ubiquitously used, the dyeing industries are now considered the most polluting businesses [3]. The release of these dyes and pigments without their prior treatment endangers the environment to be poisoned and consequently the deterioration of human health [4]. Nearly 15% of dyes discharged into the environment, without being initially cleaned, enters groundwater, increasing the risk of various types of cancer among people even at low amounts. Getting into the environment in uncontrolled way, dyes and pigments may additionally cause allergies, dermatitis, and skin irritation [5]. A bulk of the industries employ methylene blue (MB). It is difficult to remove this dye from water due to its flat and intricate structure. According to [6], MB prevents the sunlight from reaching water and hinders photosynthesis. As a consequence, it is critical to get rid of this dye before it is released into the environment [7]. The advanced oxidation methods [8], photocatalysis [9], coagulation [10], biodegradation, and ion exchange [11] in addition to membrane filtering and adsorption are typically used to accomplish this goal [12].
Among all methods listed above, adsorption is quite promising, simple, economical, efficient, and without any secondary pollution. To eliminate dyes and pigments, many types of adsorbents are available, including natural clays, nanocomposites, activated carbons, gels, and porous polymers [13]. Despite its strong adsorption capacity, activated carbons remain expensive and their production leads to the emission of greenhouse gases. Therefore, the search for low-cost adsorbents based on natural and agricultural products provides a range of new materials with different possibilities [14]. Clay and cellulose are two groups of natural and largely abundant materials that can be used as adsorbents in modified or unmodified forms [15]. The annual production of cellulosic biomass is approximately 1 billion tons, so this raw material is considered to be an inexhaustible natural polymer. Different organic and inorganic pollutants are attracted by the clays. The elimination of water pollutants by clay minerals is well affirmed in the literature. The clays are used in the purification of water, as well as the production of medicines and detergents, cosmetics, and paints [16]. Several authors claim that the adsorption capacity of clays could by improved by their purification, chemical treatment, or modification [17]. Clays and clay-based materials have a good mechanical stability and a high capacity to absorb heavy metals and wastewater dyes [18].
Cellulose and its modified forms have recently been discovered to efficiently sorb the dyes and pigments. Because each hydrogel unit in cellulose contains hydroxyl groups, it may efficiently adsorb such compounds from contaminated waters used alone or combined with other materials [15, 19, 20]. Considering that cellulose has a large specific surface area and chirality, its nanocomposites are possible to be formed. In the case of clay-polymer nanocomposites (CPNS), they combine the benefits of clay materials (cheapness, availability, ecological safety, big surface, and durability) with those of polymers (high adsorption efficiency, large area) to meet the adsorption constraints [20]. These days, it appears that clays and biopolymers work together to make the intelligent nanoparticles for the environmental applications. Pesticides, heavy metals, reactive dyes, and even emerging pollutants can all be removed thanks to such unique biohybrid materials [21]. Many hybrid inorganic–organic composites, such as cellulose-based hydrogels and clays, being microstructures based on the cellulosic biomass of carbon and montmorillonite, and bentonite/biopolymer composites, have been previously demonstrated to have good adsorption properties [22]. Argil-hydrogel nanocomposites are a class of the complex adsorbent materials made up of a unique combination of polymers and clay minerals. Clay leaves and polymers are intercalated and exfoliated to significantly improve the physicochemical properties of these nanocomposites [23].
The present research was focused on the development of new clay/cellulose and clay/cellulose nanofiber composite sorption materials that could remove methylene blue (MB) from water. The dye concentration, pH, the contact time, the adsorbent dose, and the temperature were studied as the parameters that affect the composites’ adsorption efficiencies. The researchers used kinetics, equilibrium modeling, and thermodynamics to figure out how the colors got onto the composites; this study will provide a practical basis for the use of clay/cellulose materials for real-life wastewater treatment application.
2 Materials and methods
2.1 Materials
2.1.1 Dye used
A cationic dye methylene blue (see its structure in Fig. 1) was used without any previous purification. The dye was dissolved in distilled water to prepare its 1000 mg/L bulk solution. The working solutions of MB were prepared by appropriate dilutions of the bulk solution. The chemical formula of MB is C16H18N3ClS, with pKa of 3.5 and maximum absorption at 664 nm. Because MB is highly adsorbed by different materials, it was selected for this study. Its dispersed positive charge might help in its sticking to the clay’s surface (Allam et al. 2018).
2.1.2 Raw materials and pretreatment methods
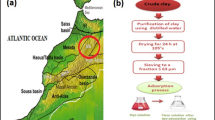
The raw clay sample was taken in the Djelfa region (southern Algeria). It was purified to select the material having the particles with the diameter lower than 2 μm. The purification was as follows:
-
(a)
Physical: the raw clay was crushed at first. The fine powder obtained was then sieved at 20 μm. This preliminary sieving operation significantly reduced the amount of impurities by eliminating large particles. This also facilitated the continuation of the purification operation [24].
-
(b)
Chemical: 20 g of clay was crushed and sifted at 20 μm before being placed in a 500 mL Erlenmeyer with 200 mL of a buffer solution (16.4 g sodium acetate and 11 mL of acetic acid). The mixture was agitated at room temperature for 1 h and then centrifuged and rinsed several times with distilled water to remove any leftover acid. Then, 60 mL of a 6% hydrogen peroxide solution was added and shaken for 10 min before being heated at 80 °C for 5 min to remove organic compounds. The decarbonated clay (20 g) was dispersed in 200 mL of water, to which a 2 mol/dm3 NaCl solution in order to exchange the cations. The resulting solution was transferred to a graduated tube and allowed to decant for 7 h and 45 min, after that float layer is collected at a depth of 10 cm to produce particles smaller than 2 μm. The particles were rinsed multiple times in the centrifuge. After that, it is dried at 105° C [24].
2.1.3 Clay activated
The acid activation of a clay is the chemical process that boosts its adsorption ability. The chemical parameters such as the Cation Exchange Capacity (CEC) and the acidity rise, as do the surface quality and the porosity. Because H2SO4 for the clay activation is less costly and simpler to handle than HCl, it was preferred [25]. Accordingly, in a 500 mL round flasks, H2SO4 (100 g, 20% w) and 20 g of the pretreated clay were refluxed for 4 h at 80 °C [22]. Such activated clay was separated by filtration and dried at 108 °C. Finally, it was stored after being washed multiple times with distilled water until it was neutral (pH 6.8–7).
2.1.4 Synthesis of clay/cellulose composite
The adsorption capacity of the clay can be improved by intercalating polymers into its intercut zones by the physical adsorption, the chemical grafting, or the ion exchange. Polyesters, polypropylene, chitosan, and polystyrene are some of the polymers that are currently used to make the polymer clay composites. The latter materials have much better physical and chemical properties than the individual components [25]. A clay/cellulose composite in this study was developed by combining 4 g of cellulose and 12 g of the activated clay in 100 mL of deionized water. In this way, a 25% modification of the clay was achieved, labeled as CC25. After that, the prepared mixture was stirred for 7 h at 130 °C under reflux. Before washing with an ethanol/water solution, the mixture was centrifuged. Next, it was dried at 50 °C. Finally, the resultant clay/cellulose composite was finely ground and used for the characterization and the adsorption experiments [22]. The whole scheme of the clay/cellulose composite material is given in Fig. 2. The same procedure was used to prepare a 50% modification of the clay with cellulose, which was labeled CC50.
2.2 Characterization of materials
To characterize the clay it seals and the composite materials produced, a variety of methods were used. An Agilent Technologies Cary FTIR spectrometer, model 630, was used to perform the FTIR analysis in the range of 400–4000 cm−1 before and after the MB dye removal. The X-ray diffraction was used to determine the materials’ crystalline structural characteristics (XRD, Mini Flex 600 Rigaku). The morphology of the prepared materials was examined before and after the adsorption of the MB dye using a desktop Phenom ProX scanning electron microscope (SEM). This microscope enabled to carry out the scanning and the electron microprobe examination, utilizing energy-dispersive X-ray spectroscopy (EDS). With the ASAP 2020 V4.03 (V4.03 J) analyzer and 77 K nitrogen adsorption/desorption isotherms, the surface area and the pore volume of the produced materials were measured. This method was also used to perform the zero charge point (pHpzc) test.
2.3 Design of experiments
The Box-Behnken design (BBD) along with the response surface methodology (RSM) was used to assess the individual and combined effects of the operational adsorption parameters on the efficiency of the MB removal (in %) and the optimization of the process. For the adsorption of the MB on the clay (C), and the biocomposites, being the clays modified with 25 and 50% of cellulose (CC25, CC50), five independent variables (parameters) were considered. Table 1 illustrates these independent variables’ levels and ranges, as well as their codes. Then, the response Y of the system, being the efficiency of the MB dye adsorption, was approximated with the following equation, i.e., a second-order polynomial model, where Xi and Xj are the considered independent parameters, which codes are given in Table 1, while β0, βi, βii, and βij are the respective regression coefficients for the intercept, linear, quadratic, and interaction terms among these input parameters:
Table 2 shows the complete matrix for the BBD experiments of the MB dye as well as the optimization of this process. Before shaking using a thermostat water tube shaker, a portion of the adsorbent was put into a 250 mL Erlenmeyer flask with 100 mL of a 100 mg/L MB solution. A spectrophotometer set at 664 nm was used to track the MB concentration. The equation given above (Biswas et al., 2020) was used to compute the MB removal performance or the % adsorption:
where C0 is the initial MB concentration in mg/L, while Ce is the MB equilibrium concentration in mg/L.
2.4 Batch adsorption studies
Executing the experiments for the BBD, 46 tests were done according to the run order and experimental conditions given in Table 2 using the clay alone as well as its composite clay/cellulose modifications. Table 2 displays the BBD results, which were used to find the optimal operating parameters, enabling the highest MB dye removal. These parameters were as follows: the cellulose loading 25% (CC25 composite), the adsorbent dosage 0.06 g, solution pH = 7, the temperature 45 °C, and the contact time 20 min, and they were responsible for the quantitative removal of MB (98.76%).
For the batch MB adsorption, the optimum conditions found on the basis of the DBB approach were used but the MB dye concentration varied within the 50–250 mg/L range while the contact time changed in the interval of 0–30 min. Using the equation below, the adsorption capacity (qe) was assessed in this way (Slatni et al., 2020):
where C0 is the initial concentration of the MB dye (mg/L), Ce is the equilibrium concentration of the MB dye (mg/L), m is the mass of the adsorbent (g), and V is the volume of the MB dye solution (L).
2.5 Determination of point of zero charge
The original pH of adsorbent solution (pHi) was adjusted from 3 to 11 using H2SO4 or NaOH solutions, and the mixtures were agitated at room temperature for 24 h [6]: pHpzc is an analytic test, it is often used for pinpointing the pH value at which the surface charge of an adsorbent equal zero as per Dalvand et al.’s report. Regarding pzc (point zero charge), it is only concluded from the curve intersection of the change in pH (Δ pH) with x-axis (pHi), which it can be expressed in equation: Δ pH = f (pHi).
where pHf is the pH solution after 24 h and pHi is the pH solution at the start of the test.
3 Results and discussion
3.1 Sample characterization
The findings of the BET surface area study are summarized in Table 3. These results indicate that the highest surface area was achieved in the case of CC25 (90.18 m2/g) that was higher than for the other materials, i.e., C (52.60 m2/g) and CC50 (43.53 m2/g). These findings suggested that a reasonable number of cellulose particles added to the clay framework is the best way to expand its surface area. The CC25 composite material has an amesoporous structure, according to the IUPAC classification. It was also established that the pore diameter of the CC25 composite material, being 0.0477 nm, was higher than these assessed for the unmodified (C—0.0332 nm) and modified (CC50—0.0261 nm) clays. As such, it was expected that the MB dye molecules could diffuse more easily into the CC25 inner pores.
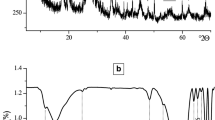
The X-ray diffraction analysis was used to assess the amorphous and crystalline nature of the prepared materials. The XRD patterns of (a) C and (b) CC25 are shown in Fig. 3. It can be seen that C (Fig. 3a) has a strong diffraction peak at the diffraction angel (2theta) of 26.6°, as well as less intense peaks at 2theta of 24.8°, 19.8°, 21.9°, 48.0°, 37.8°, 39.2°, and 50.1°, all of which are linked with kaolinite (k). The quartz diffraction (Q) peaks were discovered at 2theta of 20.9 and 26.4°. At 2theta = 30.2° and 42.4°, the illite (I) was found. Dickite was discovered at 2theta = 35.66° [26]. Diffraction peaks of montmorillonite (M) were detected at 2theta values of 5.91°, 20.9°, and 34.74°. At 2theta = 29.18°, an albite (A) peak was discovered [22]. Figure 3b shows the XRD pattern of the CC25 composite. This spectrum shows a considerable drop in the band intensities after loading the cellulose particles into the clay framework due to the integration of cellulose. There were also new diffraction peaks appeared due to the presence of cellulose. The crystalline structure (as indicated by the X-ray diffraction pattern) of the clay was altered by the cellulose molecules that were attached on its surface. These findings supported the clay structure intercalation and the homogeneous distribution of the cellulose particles.
The different functional groups in the structure of the C and CC25 materials before and after the MB adsorption were determined using the FTIR analysis. Figure 4 shows the absorption bands for these two materials. As can be seen, the Si–O-AlVI bending vibrations, where Al is in the octahedral coordination, are attributed to the infrared spectrum of the raw clay at 470 and 520 cm−1. The H2O stretching vibration was also discovered at 1636 cm−1 [26]. The Si–O quartz bands were established to be present close to 790 cm−1 [27]. A band at 997 cm−1 was also observed and attributed to the Si–O bending vibrations in SiO4. The H–O–H curvature of adsorbed water could be represented by the band at 3411 cm−1 [28]. At 3626 cm−1, the internal hydroxyl groups of Mg–OH–Al, Fe–OH–Al, and Al–OH–Al, likely in montmorillonite, were visible between the octahedral and tetrahedral layers [27, 28]. Due to the intercalation of cellulose in the clay structure, the band at 1300 cm−1 disappeared. On the other hand, new bands between 1200 and 1400 cm−1 were developed (please, see Fig. 4), which could be attributable to the aromatic rings of the MB dye, demonstrating the MB loading on the surface of CC25.
The chemical composition as well as the surface morphology was examined using SEM–EDX. The respective SEM images and the EDX spectra of (a) C, (b) CC25, and (c) CC25 are the MB dye adsorption shown in Fig. 5. As can be seen in Fig. 5a, the clay surface seems to be uneven and heterogonous. The elements O, Si, Ca, Al, Fe, Mg, and Ti, which were found in the composition of diverse minerals such as kaolinite, quartz, and other minerals that make up the clays and were identified by the XRD results, were detected in the EDX spectra of the C material. The SEM of the CC25 material had a surface with different diameters of protrusions (as shown in Fig. 5b). Nevertheless, the clay matrix exhibited a homogeneous distribution of cellulose, according to this investigation. The EDX spectrum also revealed the existence of carbon, indicating that the cellulose particles were efficiently loaded into the clay matrix. Furthermore, the surface structure of the CC25 composite material, following the MB adsorption (Fig. 5c), seemed to be more compact due to the loading of the MB dye molecules onto its surface. The spike in the carbonation rate in the related EDX spectrum indicated that the MB dye is on the surface of CC25.
3.2 BBD data statistical validation
The analysis of variance (ANOVA) was used to corroborate the BBD experimental findings. Table 4 shows an overview of these results. In the ANOVA table, the p value (0.0001) less than 0.05 and the high F value for the whole model indicated that it was statistically valid [29]. The terms B, D, BD, DE, A2, and B2 were established to be the key terms for removing the MB dye. In the polynomial model assessed, the second-order equation elucidating the removal MB (%) and the abovementioned terms looks as follows:
To check the validity of the model, the normal probability plot vs. externally studentized residuals is shown in Fig. 6a. It showed that the points extended in a straight line, suggesting that they were dispersed in a reasonably regular pattern, hence proving the normal distribution of the residuals [30]. The relationship between the actual values and the predicted values is illustrated in Fig. 6b. Based on this figure, it was noted that the predicted and the measured data points were congruent to each other. Hence, the experimental outcomes could be deemed to be acceptable. Finally, Fig. 6c shows a plot of the residuals vs. the run number. The fact that all data were conducted in the residual rings (3 in Fig. 6c) indicated that the model was viable and could be used to establish the optimal working conditions, providing the highest removal of the MB dye [29].
3.2.1 Key parameters for solo adsorption
Figure 7 shows the important effect of the model terms (the parameters of the single adsorption), such as the cellulose loading (%) (coded as A), the adsorbent dosage (coded as B), the solution pH (coded as C), the working temperature (coded as D), and the contact timed (coded as E), on the MB dye removal. By increasing the cellulose loading, the MB removal efficiency was lowered (Fig. 7a). This might be explained by the BET data, demonstrating that the specific surface of the modified clay decreased as the cellulose loading raised. However, by increasing the adsorbent dosage from 0.02 to 0.06 g, the MB removal efficiency enhanced, which might be explained by the higher availability of the adsorption sites in the composite material used (Fig. 7b). Furthermore, the adsorption process was unaffected by the medium pH. Figure 7c indicates that by increasing the pH of the solution from 4 to 10, the removal of MB rather slows down. As shown in Fig. 8b, the pHpzc of the CC25 was determined to be 8. The adsorbent’s surface is positively charged at pHpzc, suggesting that the organic dyes with anionic groups might be attached to the CC25 surface. The electrostatic attractions have a lower influence on the adsorbent-adsorbate affinity than other forms of interactions since the MB is positively charged (hydrogen bonding, Yoshida H bonding, n-interactions). Figure 7d shows the increase of the working solution temperature from 30 to 60 °C results in the increase of the MB removal. This points out the endothermic MB elimination mechanism on the CC25 surface. When the contact time of the material with the working solution was prolonged from 5 to 20 min, the MB removal (%) decreased (please, see Fig. 7e), likely because the dye molecules immediately engaged with the adsorption sites.
3.2.2 Collective adsorption essential parameter synergistic effect
Using the three-dimensional (3D) response surface plots of the Design-Expert software, the effect of two parameters on the MB removal was shown, while keeping the remaining operational parameters constant. With a p value of 0.0414, the first significant interaction effect on the MB dye removal, as shown in Fig. 8a, was between the adsorbent dose and the solution temperature. Considering the other parameters, they were as follows: the cellulose loading of 25%, the solution pH of 7, and the contact time of 12.5 min. Increasing the adsorbent dosage from 0.02 g to 0.06 g and the temperature from 30 °C to 60 °C resulted in improving the percentage of MB removed, as shown in Fig. 8a. This was likely due to an increase in the number of active adsorption sites, as well as the influence of the temperature on the collision rate of the dye molecules with the adsorbent surface.
The second interaction effect on the MB removal found, as shown in Fig. 8c, was between the solution temperature and the contact time, with a p value of 0.0139. The relevance of the solution temperature and the contact time in the removal of MB while keeping the other parameters constant (the cellulose loading at 25%, the adsorbent dose = 0.06 g, and the solution pH of 7) is shown by this substantial interaction. It was presumed that the dye molecules moved from the liquid phase of the bulk dye solution to the solid phase of the adsorbent CC25 surface when the contact time between the adsorbent and the adsorbate system was extended from 5 to 20 min and the temperature was raised from 30 to 60 °C. At higher temperatures, the rate at which the MB molecules impacted the adsorbent surface was certainly increased.
3.3 Adsorption study
The adsorption study for the CC25 composite material was carried using the following operating conditions: the CC25 dosage = 0.06 g, the solution pH = 7, the solution temperature = 45 °C, and shaking speed = 100 rpm. The tested MB concentrations in the working solutions were in the range of 50–250 mg/L. In Fig. 9, the adsorption capacity (mg/g) of CC25 plotted versus the contact time (min) is shown. It was found that when the concentration of MB was elevated from 50 to 250 mg/L, the adsorption capacity of CC25 towards this dye increased from 69.6 to 259.3 mg/g. At higher MB concentrations, this behavior might be explained by an increased frequency of the collisions between the MB molecules and those of CC25 (Regioua et al., 2021; Mohammed et al., 2020).
The effect of the initial MB concentration on the CC25 adsorption capacity as a function of the contact time. The following parameters were employed in this experiment: the CC25 dosage = 0.06 g, the solution pH = 7, the solution temperature = 45 °C, the agitation speed = 100 rpm, and the solution volume = 100 mL
3.4 Adsorption kinetics
To further understand the adsorption behavior of the CC25 composite material, the adsorption kinetics was studied in details. The Lagergren pseudo-first-order approach (PFO) (Filho et al., 2020) and the Ho pseudo-second-order (PSO) (Malek et al., 2021) were applied to describe the equilibrium of the system. The nonlinear versions of PFO and PSO models are provided in
where the rate constants in the PFO and PSO models are the amounts of MB adsorbed at time t and equilibrium (mg/g), k1 (1/min), and k2 (g/mg min). The results of both kinetic models are summarized in Table 5. The R2 value for the PSO model was higher than the one for the PFO model. The qe values (qe, cal) predicted with the PSO kinetic model were closer to the observed qe values (qe, exp) than it was in the case of the PFO kinetic model. Based on these kinetic data, it was supposed that the PSO correctly captured the MB adsorption process by the CC25 composite material, depicting that the chemoselective mechanism occurred in this case (Kim et al., 2021).
3.5 Adsorption isotherms
The equilibrium adsorption isotherm is crucial in the design of the adsorption systems. The adsorption isotherms are used to determine the relationship between the amount of the adsorbate and its equilibrium concentration in the solution. Three most often used isotherms (Langmuir, Freundlich, and Temkin) were applied in the evaluation of the adsorption isotherm for the MB dye on the CC25 composite material studied here. The nonlinear equations for these isotherm models are as follows:
The Langmuir, Freundlich, and Temkin constants are Ka, Kf, and KT, respectively, while the heat of adsorption is bT (J/mol). The obtained isotherm parameters for all of the studied isotherm models are shown in Table 6 and Fig. 10.
According to the findings, the Langmuir isotherm much better approximated the adsorption process than the Freundlich and Temkin isotherms; the respective determination coefficient (R2 = 0.93) was the highest for this first model. As such, Langmuir’s isothermal model implied that the adsorption took place on the homogenous surface through the monolayer coverage (Kanani-Jazi & Akbari, 2021). The qmax of MB on CC25 was found to be 254.8 mg/g at 45 °C. This value obtained for the CC25 composite material was compared to that of another previously reported adsorbents (see Table 7), and it was concluded that the developed CC25 adsorbent has reasonably high adsorption characteristics to remove the organic dyes from wastewaters. Certainly, it outperformed other adsorbents in terms of the adsorption capacity and affinity (see Table 7). Furthermore, the newly developed adsorbent is unique, cost-effective, practical, and affordable and could be used as an efficient adsorbent for the treatment of the effluents containing dyes and pigments, such as the effluents from the textile sector, due to its ease of synthesis.
3.6 Adsorption thermodynamics
The following equations were used to calculate the standard enthalpy (ΔH°), the standard entropy (ΔS°), and the Gibbs free energy change (ΔG°) for the MB adsorption on the CC25 composite material [40]:
where Kd is the distribution coefficient of the absorbent at temperature T (K) and R (8.314 J/mol K) is the universal gas constant.
To get the information about the spontaneity of the process, the thermodynamic functions at different temperatures were determined. By plotting lnKd vs. 1/T (Fig. 11), the enthalpy ΔH° and entropy ΔS° values of the MB dye were found by using the slope and the intercept of the linear plot. The values of ΔG°, ΔS°, and ΔH° functions for the studied adsorbent are given in Table 8.
The negative ΔG° values indicated that the CC25 adsorption process occurs spontaneously at all temperatures, but higher temperatures appear to be more favorable. The fact that ΔH° is positive suggested the endothermic sorption process. Finally, the positive ΔS° value indicated a greater disorder at the solid/solution interface [41] and this could be caused by the interaction between the dye and the spots on the material where it can stick.
3.7 Adsorption mechanism
As shown in Fig. 12, the fabricated CC25 composite material has the adsorption sites that initiate the multiple forms of interactions with the MB molecules, including the hydrogen bonds, the electrostatic attractions, the n-interactions, and the Yoshida H bonds. The negative functional groups (e.g., OH−…) on the surface of the CC25 adsorbent are likely responsible for the electrostatic attractions of the positively charged (-N+ =) MB molecules, which are the first type of contact in the MB dye adsorption. The nonbonding doublets on the N and S atoms likely form the hydrogen bonds with the free H atom on the surface of the CC25 composite material in the molecular structure of the MB dye. The Yoshida hydrogen bonds can also be formed between the OH groups of the CC25 adsorbent and the aromatic rings of the MB dye [42]. Finally, the nonbonding electron pairs of the O atoms in the CC25 structure can be responsible for the n-interactions with the aromatic ring orbitals of the MB molecules [29]. All of the above interactions are recognized to well stimulate and enhance the adsorption capacity of the CC25 adsorbent to the MB dye.
4 Conclusion
In this work, a unique, eco-friendly CC25 composite was synthesized and assessed as a green adsorbent for successful MB removal utilizing a simple technique. Cellulose loading, adsorbent dose, solution pH, starting MB concentration, contact duration, and temperature all impacted the composite’s efficacy. By examining the impacts of five independent components, the Box-Behnken design response surface technique was utilized to determine the optimal adsorption parameters. The best MB removal rate (98.76%) was achieved with the following optimal working conditions: 25% cellulose loading, 0.06 g adsorbent dosage, solution pH = 7, temperature = 45 °C, and contact time = 20 min. The Langmuir model was shown to be the most accurate, with qmax (254.8 mg/g) maximum adsorption capacity at 45 °C. The adsorption process was pseudo-second order, according to the kinetic experimental data. The adsorption of MB by CC25 is spontaneous and endothermic, according to the thermodynamic adsorption functions. Hydrogen bonds, Yoshida H bonds, n-interactions, and electrostatic attractions are among the many attraction mechanisms that secure the bonding between MB molecules and the CC25 surface during the adsorption process. As a consequence, the clay-cellulose that resulted may be employed as a stable, ecologically friendly, and effective sewage adsorbent.
Data availability
All data and materials are viable.
References
Somsesta N, Sricharoenchaikul V, Aht-Ong D (2020) Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: equilibrium and kinetic studies. Mater Chem Phys 240:122221
Rong N, Chen C, Ouyang K et al (2021) Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep Purif Technol 274:119120
Laureano-Anzaldo CM, Robledo-Ortiz JR, Manríquez-González R (2021) Zwitterionic cellulose as a promising sorbent for anionic and cationic dyes. Mater Lett 300:130236
Jawad AH, Malek NNA, Abdulhameed AS, Razuan R (2020) Synthesis of magnetic chitosan-fly ash/Fe 3 O 4 composite for adsorption of reactive orange 16 dye: optimization by Box-Behnken design. J Polym Environ 28:1068–1082
Pan Y, Xie H, Liu H et al (2019) Novel cellulose/montmorillonite mesoporous composite beads for dye removal in single and binary systems. Bioresour Technol 286:121366
Malatji N, Makhado E, Ramohlola KE et al (2020) Synthesis and characterization of magnetic clay-based carboxymethyl cellulose-acrylic acid hydrogel nanocomposite for methylene blue dye removal from aqueous solution. Environ Sci Pollut Res 27:44089–44105
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol 142:732–741
Lacerda EHC, Monteiro FC, Kloss JR, Fujiwara ST (2020) Bentonite clay modified with Nb2O5: an efficient and reused photocatalyst for the degradation of reactive textile dye. J Photochem Photobiol A Chem 388:112084
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf Interfaces 18:100422
Igwegbe CA, Mohmmadi L, Ahmadi S et al (2019) Modeling of adsorption of methylene blue dye on Ho-CaWO4 nanoparticles using response surface methodology (RSM) and artificial neural network (ANN) techniques. MethodsX 6:1779–1797
Thirumoorthy K, Krishna SK (2020) Removal of cationic and anionic dyes from aqueous phase by ball clay–manganese dioxide nanocomposites. J Environ Chem Eng 8:103582
Aqdam SR, Otzen DE, Mahmoodi NM, Morshedi D (2021) Adsorption of azo dyes by a novel bio-nanocomposite based on whey protein nanofibrils and nano-clay: equilibrium isotherm and kinetic modeling. J Colloid Interface Sci 602:490–503
Xu X, Yu J, Liu C et al (2021) Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React Funct Polym 160:104840
Rahman MM, Rimu SH (2022) Recent development in cellulose nanocrystal-based hydrogel for decolouration of methylene blue from aqueous solution: a review. Int J Environ Anal Chem 102:6766–6783
Abu-Danso E, Peräniemi S, Leiviskä T et al (2020) Synthesis of clay-cellulose biocomposite for the removal of toxic metal ions from aqueous medium. J Hazard Mater 381:120871
Guiza S, Brouers F, Bagane M (2021) Fluoride removal from aqueous solution by montmorillonite clay: kinetics and equilibrium modeling using new generalized fractal equation. Environ Technol Innov 21:101187
Oussalah A, Boukerroui A (2020) Removal of cationic dye using alginate–organobentonite composite beads. Euro-Mediterr J Environ Integr 5:1–10
Islam MM, Islam MS, Maniruzzaman M et al (2021) Banana rachis CNC/clay composite filter for dye and heavy metals adsorption from industrial wastewater. Eng Sci Technol 2:140–152
Kausar A, Shahzad R, Asim S et al (2021) Experimental and theoretical studies of Rhodamine B direct dye sorption onto clay-cellulose composite. J Mol Liq 328:115165
Amari A, Mohammed Alzahrani F, Mohammedsaleh Katubi K et al (2021) Clay-polymer nanocomposites: preparations and utilization for pollutants removal. Materials (Basel) 14:1365
del Mar OM, Martín J, Santos JL et al (2020) Biopolymer-clay nanocomposites as novel and ecofriendly adsorbents for environmental remediation. Appl Clay Sci 198:105838
Kausar A, Shahzad R, Iqbal J et al (2020) Development of new organic-inorganic, hybrid bionanocomposite from cellulose and clay for enhanced removal of Drimarine Yellow HF-3GL dye. Int J Biol Macromol 149:1059–1071
Khan SA, Khan TA (2021) Clay-hydrogel nanocomposites for adsorptive amputation of environmental contaminants from aqueous phase: a review. J Environ Chem Eng 9:105575
Bouna L (2012) Fonctionnalisation des minéraux argileux d’origine marocaine par TiO2 en vue de l’élimination par photocatalyse de micropolluants organiques des milieux aqueux PhD, National Polytechnic Institute of Toulouse
Barakan S, Aghazadeh V (2021) The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: a review. Environ Sci Pollut Res 28:2572–2599
Meroufel-Zenasni B (2015) Adsorption des polluants organiques et inorganiques sur des substances naturelles: kaolin, racines de Calotropis procera et noyaux de dattes PhD, University of Lorraine
Hajjizadeh M, Ghammamy S, Ganjidoust H, Farsad F (2020) Amino acid modified bentonite clay as an eco-friendly adsorbent for landfill leachate treatment. Polish J Environ Stud 29:4089–4099
Rehman MU, Manan A, Uzair M et al (2021) Physicochemical characterization of Pakistani clay for adsorption of methylene blue: kinetic, isotherm and thermodynamic study. Mater Chem Phys 269:124722
Abd Malek NN, Jawad AH, Ismail K et al (2021) Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: adsorption parametric optimization. Int J Biol Macromol 189:464–476
Reghioua A, Barkat D, Jawad AH et al (2021) Parametric optimization by Box-Behnken design for synthesis of magnetic chitosan-benzil/ZnO/Fe3O4 nanocomposite and textile dye removal. J Environ Chem Eng 9:105166
Kim J-C, Kim J, Park J et al (2021) Highly efficient and sustainable alginate/carboxylated lignin hybrid beads as adsorbent for cationic dye removal. React Funct Polym 161:104839
Jawad AH, Abdulhameed AS, AbdMalek NN, ALOthman ZA (2020) Statistical optimization and modeling for color removal and COD reduction of reactive blue 19 dye by mesoporous chitosan-epichlorohydrin/kaolin clay composite. Int J Biol Macromol 164:4218–4230
Stanly S, Jelmy EJ, John H (2020) Studies on modified montmorillonite clay and its PVA nanohybrid for water purification. J Polym Environ 28:2433–2443
Rahmani S, Zeynizadeh B, Karami S (2020) Removal of cationic methylene blue dye using magnetic and anionic-cationic modified montmorillonite: kinetic, isotherm and thermodynamic studies. Appl Clay Sci 184:105391
Ullah S, Ur Rahman A, Ullah F et al (2021) Adsorption of malachite green dye onto mesoporous natural inorganic clays: their equilibrium isotherm and kinetics studies. Water 13(7):965
Seliem MK, Barczak M, Anastopoulos I, Giannakoudakis DA (2020) A novel nanocomposite of activated serpentine mineral decorated with magnetic nanoparticles for rapid and e ff ective adsorption of hazardous cationic dyes: kinetics and equilibrium studies. Nanomaterials 10(4):684
Abukhadra MR, El-Meligy MA, El-Sherbeeny AM (2020) Evaluation and characterization of Egyptian ferruginous kaolinite as adsorbent and heterogeneous catalyst for effective removal of safranin-O cationic dye from water. Arab J Geosci 13:1–13
Onu CE, Nwabanne JT, Ohale PE, Asadu CO (2021) Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. South African J Chem Eng 36:24–42
Yadav S, Asthana A, Chakraborty R, Jain B (2020) Cationic dye removal using novel magnetic/activated charcoal/β-cyclodextrin/alginate polymer nanocomposite. Nanomaterials 10(1):170
Leshaf A, Ziani Cherif H, Benmansour K (2019) Adsorption of acidol red 2BE-NW dye from aqueous solutions on carboxymethyl cellulose/organo-bentonite composite: characterization, kinetic and thermodynamic studies. J Polym Environ 27:1054–1064
Chopra I, Singh SB (2022) Kinetics and equilibrium study for adsorptive removal of cationic dye using agricultural waste-raw and modified cob husk. Int J Environ Anal Chem 102:7062–7083
Jawad AH, Abdulhameed AS (2020) Facile synthesis of crosslinked chitosan-tripolyphosphate/kaolin clay composite for decolourization and COD reduction of remazol brilliant blue R dye: optimization by using response surface methodology. Colloids Surf A Physicochem Eng Asp 605:125329
Acknowledgements
The authors would like to thank the chemistry department, University of El Oued, for facilitating this research work.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funding for open access charge: Universidade de Vigo/CISUG.
Author information
Authors and Affiliations
Contributions
Abderrazak Hamidi: methodology, formal analysis, investigation, data curation, writing an original draft. Djamal Atia: conceptualization, supervision. Abdelkrim Rebiai: supervision assistant. Abdallah Reghioua: software, validation. Ammar Zobeidi: formal analysis. Bachir Ben Seghir: software, validation. Mohammad Messaoudi: investigation, data curation. Pawel Pohl: writing and editing. Jesus Simal-Gandara: conceptualization, formal analysis, writing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors approved.
Consent for publication
All authors agree to the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamidi, A., Atia, D., Rebiai, A. et al. Investigation of adsorption kinetics and isothermal thermodynamics for optimizing methylene blue adsorption onto a modified clay with cellulose using the response surface approach. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04397-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04397-1