Abstract
Recently, the development of skin barrier depend on wound healing, which is one of the most complicated biological processes. As an alternative to conventional antibiotics, nanoparticles (NPs) have become more utilized generally to attack bacteria. Due to their distinct characteristics, potential microbicidal action, and ability to speed up the wound healing process, zinc oxide nanoparticles (ZnO-NPs) have attracted much attention. Biological techniques can solve the restrictions of both physical and chemical approaches for nanoparticles synthesis. Because it does not require expensive chemicals, high temperatures, or a lot of time, biological synthesis is relatively easy, inexpensive, and environmentally benign. The secondary metabolic extract from Escherichia coli was used in this study to biologically synthesize three distinct quantities of ZnO-NPs, which were then assessed for their effectiveness in wound healing and bacterial infection prevention. The biofabricated ZnO-NPs were fully characterized in terms of particle shape, morphology, and stability against aggregation. Depending on the concentration of the utilized zinc salt, three different samples were fabricated biologically, nominated as ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3. The findings of Uv-vis absorption peaks were obtained at 352 nm, demonstrating the preparation of ZnO-NPs. The results demonstrated the formation of ZnO-NPs with an average particle size of 79.19, 79.83 and 91.57 nm for the three prepared samples (ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3), respectively. Additionally, these samples of ZnO-NPs exhibited zeta potential values around −34.3, −33.7, and −33.4 mV, respectively. Energy dispersive X-ray confirmed the successful formation of ZnO-NPs. It was also observed from the obtained results that, ZnO-NP-3 showed superior antimicrobial potential against selected skin infectious microbes. The effective killing dosage of ZnO-NPs-3 was recorded to be 40 mg/L which can eliminate microbial growth. The dysregulation of skin flora significantly influences the etiology of inflammatory skin disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hemostasis, inflammation, proliferation, and maturation are interrelated biological systems contributing to wound healing, leading to minimal damage and eventual recovery [1, 2]. Wound healing aims to reduce patients’ pain and scarring while preventing severe infection and accelerating wound healing [3]. Nanotechnology is evolving as a rapidly expanding field with its use in science and technology to produce effective nanoscale materials [4]. It opens a new era in wound treatment by providing methods that accelerate wound healing and showcasing unique qualities as bactericidal agents [5]. Nanoparticles (NPs) are particles with one dimension ranging from 1 to 100 nm [6]. Due to nanoparticles’ tiny size, they exhibit unique properties that significantly differ from those displayed by their relative bulk materials [7]. These particles, such as zinc oxide nanoparticles (ZnO-NPs), have huge surface areas, which enhances their activities [8]. ZnO-NPs are less toxic and more affordable than other metal oxide nanoparticles. They have biological uses, including drug delivery, wound healing, and anti-inflammatory purposes [9,10,11,12]. Reactive oxygen species (ROS) produced by ZnO-NPs can prohibit cell adhesion, proliferation, and migration [13, 14]. When free Zn2+ ions interact with biomolecules like proteins, all of the bacteria’s essential processes fail [15]. Numerous physical, chemical, and biological processes can be used to synthesize ZnO-NPs. However, physical and chemical procedures have several drawbacks, including using poisonous and dangerous substances, high energy requirements, and expensive costs, which restrict their suitability for biomedicine purposes [16, 17]. Because it is more economical and environmentally friendly than physical and chemical processes, biological ways (green process) are favored for the preparation of nanoparticles [18,19,20]. Moreover, investigations have indicated that prokaryotic bacteria are used as a component in the synthesis of NPs due to their simpler culturing, short development times, and tolerant experimental conditions (pH, pressure, and temperature) [21]. Considering where nanoparticles are biosynthesized, two distinct production processes can be categorized as intracellular and extracellular [22]. Additional procedures, such as ultrasound or interaction with suitable detergents, are needed to release the synthesized NPs after intracellular production [23]. Because it is less expensive and does not require a complicated downstream stream technique, the extracellular approach to producing NPs is preferred over the intracellular method [24]. Microbial enzymes and proteins reduce metal ions to create NPs in the extracellular process [25].
ZnO-NPs have broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. The antibacterial mechanism of action is thought to involve the release of zinc ions and the generation of reactive oxygen species (ROS) on the surface of NPs. These zinc ions and ROS can cause oxidative damage to bacterial cells, leading to cell death. In comparison, some other metal and metal oxide nanoparticles, such as silver nanoparticles (AgNPs) [26] and copper oxide nanoparticles (CuO NPs) [27], also exhibit antibacterial activity by releasing metal ions and ROS [28]. Some other metal and metal oxide nanoparticles, such as titanium dioxide nanoparticles (TiO2-NPs) and gold nanoparticles (AuNPs), have also been shown to promote the wound healing [29, 30]. ZnO-NPs have relatively low toxicity compared to other metal and metal oxide nanoparticles. This is thought to be due to the formation of a protective protein corona around NPs, which can reduce their interaction with cells and tissues [31]. ZnO-NPs have been shown to promote wound healing by stimulating cell proliferation, migration, and differentiation. The mechanism of action is thought to involve the release of zinc ions, which can activate signaling pathways involved in wound healing. ZnO-NPs can also enhance angiogenesis and extracellular matrix synthesis, which are important for tissue regeneration. A significant advantage of biosynthesized ZnO-NPs is their eco-friendliness and sustainability. Biosynthesis method typically uses natural and renewable sources, such as plant extracts, and microorganisms (fungi, actinomyctes and bacteria). Another advantage of biosynthesized ZnO-NPs is their potential for enhanced biocompatibility and reduced toxicity level. Biosynthesis methods can produce nanoparticles with controlled sizes and shapes, improving their biocompatibility and reducing their potential toxicity. In addition, the presence of natural compounds in the synthesis process can provide additional benefits, such as antioxidant or anti-inflammatory properties, which can be beneficial for wound healing applications [32]. Biosynthesis method can be considered as an affordable strategy owing to the utilization of natural and green resources, reduced energy and equipment costs, and potential for large-scale production [33]. On contrary, the chemically synthesized nanoparticles often require toxic solvents and reducing agents, which can be harmful to the environment and human public health. The microbiota of the skin plays a significant role in the development and homeostasis of cutaneous immunity. The skin is home to a diverse community of microorganisms, including bacteria, fungi, and viruses, collectively known as the skin microbiota [34]. The skin microbiota interacts with the host immune system and helps to maintain skin health and protect against pathogens. The skin microbiota can influence the development of the immune system in early life. Studies have shown that the skin microbiota composition in infants is associated with the development of the immune system and the risk of allergic diseases later in life. For example, infants with a higher abundance of Staphylococcus aureus on their skin have been found to have a higher risk of developing atopic dermatitis [35]. The skin microbiota can also modulate the immune response in the skin. The skin microbiota can interact with immune cells in the skin, such as Langerhans cells and T cells, and can influence the production of cytokines and other immune molecules. This interaction can help to maintain immune homeostasis in the skin and protect against infection and inflammation [36]. Other prevalent bacterial microflora species are found on the epidermis include Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes species.
This study aimed to investigate the wound-healing properties of green synthesized ZnO-NPs with three different concentrations using E. coli metabolic filtrated extract. The biofabricated ZnO-NPs were characterized to ensure their synthesis in nanoform. The antimicrobial performance of prepared biogenic synthesized ZnO-NPs was explored against some skin-causing bacterial infection.
2 Materials and methods
2.1 Materials
Zinc acetate (Zn(CH3COO)2) and sodium hydroxide (NaOH) were obtained from Sigma Aldrich Co. (Germany). Deionized water was used for dilution, analysis, and application.
2.2 Production of biomass
The bacterial strain E. coli ATCC 25922 was cultured in LB medium to produce the biomass for biosynthesis. The culture flask was incubated on an orbital shaker at 37 °C and agitated at 200 rpm. The biomass was harvested after 24 h of growth and centrifuged at 10000 rpm for 10 min. The supernatant was collected for further reaction [37].
2.3 Biological synthesis of ZnO-NPs
Three different concentrations of ZnO-NPs were synthesized by adding 12.5 mL of zinc acetate Zn(CH3COO)2 [0.11,0.23,0.35 M] to 12.5 mL of E. coli metabolic filtrated extract. After that, 1.25 mL of NaOH (10 g NaOH in 50 mL of distilled water) was slowly added drop by drop into the beaker containing Zn(CH3COO)2 and E. coli metabolic filtrated extract solution under stirring conditions for 30 min. Then, the mixture was centrifuged at 10000 rpm for 40 min; the white precipitate was washed with distilled water H2O and dried in an oven at 150 °C for 24 h. The obtained three concentrations were coded as ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3. ZnO-NPs-1 refers to the low concentration of ZnO-NPs prepared using 0.11M of zinc acetate. Meanwhile, the sample ZnO-NPs-1 was defined for the nanoparticles prepared using zinc acetate (0.23 M). Finally, ZnO-NPs-3 is the code for ZnO-NPs synthesized using a high concentration (0.35 M) of zinc acetate.
2.4 Physical characterization of ZnO-NPs
The optical characteristics of the biosynthesized ZnO-NPs were evaluated using UV-visible spectroscopy (UV-vis) in the wavelength range of 250–500 nm. The particle shape of ZnO-NPs was examined using TEM analysis. A small amount of ZnO-NPs was deposited on a coated copper grid to generate thin films, and any extra solution was blotted off using paper towels. Then, the grids were left for drying in the air before TEM investigation. Additionally, using dynamic light scattering (DLS), the particle size, polydispersity index, and zeta potential were determined via Malvern Instruments (UK). Scanning electron microscopy (SEM) was used to examine the ZnO-NPs’ surface morphology. With the application of energy-dispersive X-ray analysis (EDX), The elemental analysis of the scanned samples can be detected.
2.5 Microorganisms used
Six different types of skin infectious pathogens, including Pseudomonas aeruginosa and Acinetobacter baumannii as a model for Gram-negative (G-ve), Staphylococcus aureus and Staphylococcus epidermidis as a model for Gram-positive (G+ve) and were donated by the Rhizopus oryzae and Aspergillus niger as a model for fungal species were selected and inoculated aerobically in nutrient agar for bacteria and Sabouraud dextrose agar (SDA) for fungal species. The stock solution of each NPs was made by dissolving the appropriate mass of the tested ZnO-NPs (50 mg) into a tube having 5 mL of DMSO/deionized water and placed for sonication for 30 min.
2.6 Evaluation of antimicrobial behavior of ZnO-NPs
2.6.1 Inhibition zone assay
The antibacterial effects of biologically synthesized ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 were evaluated using the agar well diffusion experiment. The turbidity of the bacterial culture was modified to correspond with the 0.5 McFarland standards solutions after 24 h incubation in a nutrient broth medium. The microbes were then dispersed using streaking techniques over the upper layer of the Mueller-Hinton agar (MHA) and sabouraud dextrose agar (SDA) plate to induce sporulation. An aseptic cork borer was utilized to punch wells of 6 mm diameter on the MHA and SDA agar plates. The wells already inoculated with the tested bacterial/fungal cultures were then treated with different concentrations of the treatment solution (5, 15, 25, and 50 mg/L). Positive control substances were augmentin and voriconazole (500 mg/mL) for bacteria and fungi. While sterile distilled water was applied as a negative control. For an additional hour, the culture plates were held at 4 °C to give the NPs an adequate period to disseminate. The plates were after that maintained in the incubator for growth at the standard conditions of each type of microbe. Following the growth condition, the inhibition zones were measured to determine the antibacterial performance [38].
2.6.2 Estimation of minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC)
The macrodilution (broth) technique used for the MIC assay was approved by the Clinical and Laboratory Standards Institute (CLSI) [39]. For the bacteria and fungi, the constant volume (5 mL) each of sterile Mueller-Hinton broth (MHB) and Sabouraud dextrose broth (SDB) were poured separately into the sterile test tubes. The evaluated ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 stock solutions served as the basis for making different concentrations (0–65 ppm). Following that, a standardized microbial inoculum resulted in the final approximation of log 6.2 CFU/mL microbial cell densities in a separate tube. After that, 5 mL of the broth medium containing the respective concentration of each NPs was applied successively along with 0.1 mL of the inoculum (3.6 × 106 CFU/mL). As positive control samples, broth media (MHB and SDB) having bacterial or fungal organisms were introduced into a tube. The fungal cultures were incubated for 5–7 days at 28°C, whereas the bacterial cultures were incubated for a day at 37 °C [40]. After the suitable incubation span of all tubes, each tube received 100 μL of a resazurin solution (0.1% w/v), which was allowed to incubate at 37 °C for approximately 2 h.
The term “MBC” or “MFC” means the smallest dosage of the antimicrobial agent, which can eradicate at least 99.9% of the microbes. In order to calculate the MBC/MFC, 100 mL of treated samples from MIC tubes that were withdrawn but had no observable microbial growth on them were streaked onto sterile plates of MHA for bacteria and SDA for fungi. The streaked plates were then incubated. The MBC/MFC levels were those at which no discernible proliferation was witnessed [41].
2.6.3 Time-kill kinetics assays
The time-kill assay was employed to determine the sufficient killing duration of NPs (ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3) against explored microbes. In essence, the cell suspension was modified to 1×106 CFU/mL. The estimated concentration levels that are MIC-equivalent, twice-MIC-equivalent, and four-MIC-equivalent of each NPs were utilized by inoculation in the 5 mL of PBS with 10 μL of each explored microbes. All mixtures were set in a frequent shaking incubator at 200 rpm. At frequencies of 0, 5, 15, 30, 45, 60, 75, 80, 105, and 120 min, aliquots (1.0 mL) of the medium mixture were withdrawn and aseptically seeded into MHA and SDA agar in sterile Petri dishes. The inoculated Petri dishes were incubated at optimal conditions for each type of bacteria and fungi. In parallel, another trial was run as a control using only the organisms. The test organisms’ colony-forming unit (CFU) was calculated, and the procedure was carried out three times. Log CFU/mL was displayed against time on a graph. Furthermore, the nonlinear dose-response modeling method was used in GraphPad Prism to obtain the IC50 values and log the IC record for the MIC values of each NPs [42].
3 Results and discussion
3.1 Biological synthesis of ZnO-NPs and their physical characterizations
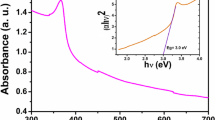
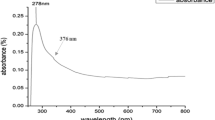
Because of their straightforwardness, affordable prices, and advantages of preservation of the environment when compared to traditional chemical and physical paths, green synthesis approaches of metal and metal oxide nanoparticles have recently been brought to the interest of investigators [43, 44]. In the current investigation, ZnO-NPs were observed firstly by the naked eye via the appearance of a white precipitate that formed after 20 min of mixing the E. coli metabolic filtrated extract with zinc acetate solution. The appearance of this white precipitate is owing to the formation of zinc hydroxide coated by the metabolic filtrate of E. coli. In the second step, zinc hydroxide was converted to ZnO-NPs via a drying process. Figure 1 shows ZnO-NPs with absorbance peaks at 352 nm. As demonstrated in Fig. 1, the absorption of ZnO-NPs increased with the concentration of the utilized zinc acetate. The obtained data are in accordance with another study that affirmed that the absorption peaks of ZnO-NPs were around 340–385 nm [45].
The morphology of the biosynthesized ZnO-NPs was examined with transmission electron microscopy (TEM). Figure 2a–c represent the particle shape of ZnO-NPs-1, ZnO-NPs-2, and, ZnO-NPs-3. It was observed that ZnO-NPs were prepared with an irregular shape. The aggregation and particle size were increased while the concentration of ZnONPs increased, as shown in Fig. 2c for the sample (ZnO-NPs-3).
The appearance of aggregation particles is mainly attributed to nanoparticles’ reactivity which tends to combine with each other’s to form cluster particles. Thus, the formed nanoparticles need to be stabilized using the external stabilizing agent. In this current study, the metabolic filtrate of E. coli is able to prevent the aggregation of NPs. However, the metabolic filtrate cannot avoid the agglomeration of ZnO-NPs prepared using a high zinc acetate concentration (as shown for the sample ZnO-NPs-3).
The average particle size (nm), PDI, and zeta potential (mv) of the biosynthesized ZnO-NPs were calculated using dynamic light scattering (DLS) and zeta potential analyses and set in Table 1. The average particle size of the biological prepared ZnO-NPs-1, ZnO-NPs-2 and ZnO-NPs-3 recorded 79.19, 79.83 and 91.57 nm, respectively as seen in Table 1. As observed, the size of ZnO-NPs was marginally increased as zinc acetate concentration was increased.
Table 1 presents the PDI values for ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 as 0.265, 0.274 and 0.385, respectively. The homogeneity of ZnO-NPs is indicated by the illustration of nanoparticles with PDI values between 0 and 0.5. Therefore, all biosynthetized ZnO-NPs are assumed to contain homogenous particles. The negative protein groups covering the produced nanoparticles are responsible for any negative charges on their surface. The negative protein groups covering the generated nanoparticles are responsible for any negative charges on their surface [46]. In previous study, ZnO-NPs’ charge at the surface was assessed via zeta potential analysis, which revealed a mean zeta potential of -20.9 mV and suggested they had moderate stability, while the stability is considered as a good when the value is above -30 mV or +30 mV [47]. The zeta potential values of ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 are as follows: −34.3, −33.7, −33.4 mV (Table 1), respectively, indicating the stability of the biosynthesized ZnO-NPs.
3.1.1 Scanning electron microscopy (SEM) analysis
Morphological features of the bio-synthesized ZnO-NPs were examined via SEM. Figure 3 shows SEM of the three prepared samples: ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3. Each sample was scanned at two different magnifications. From the scanned samples, it was observed that the as-prepared ZnO-NPs exhibit rough surfaces with definite structures. Thus, SEM for analyzed samples (ZnO-NPs-1 and ZnO-NPs-2) shows the uniform shape of nanoparticles. Regarding the sample (ZnO-NPs-3), the shape was changed, and there were more aggregated particles. Overall, E. coli metabolic filtrated extract succeeded in forming ZnO-NPs with small size.
The elemental analysis of the bio-synthesized ZnO-NPs (ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3) was determined using energy dispersive X-ray analysis (EDX). In all EDX graphs (Fig. 4), the existence of zinc and oxygen proved the preparation of ZnO-NPs. Meanwhile, the other elements such as nitrogen, carbon, phosphorus, and sulfur are mainly attributed to the E. coli metabolic filtrated extract. As the weight and atomic percentages of each component were recorded in the onset table, the variety of the percentage could be attributed to the scanned sample spot. As known, the surface elements are randomly deposited, so it is normal to find the percentage difference of each component in the scanned three samples. The percentage of zinc and oxygen elements of ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 was 41.52/40.16%, 45.97/34.62%, and 45/36.57%, respectively, affirming the successful biosynthesis of ZnO-NPs.
3.1.2 Antimicrobial properties of ZnO NPs
The Zone of inhibition measurements
ZnO-NPs have been shown to have antibacterial properties, and their use in wound healing applications has been explored due to their ability to promote wound healing and reduce inflammation. Bacterial cell-free metabolites, on the other hand, are compounds produced by bacteria that can have various biological activities, including antimicrobial activity. Six skin-infectious pathogenic microorganisms, namely, P. aeruginosa, A. baumannii, S. aureus, S. epidermidis, R. oryzae, and A. niger, were explored using the agar well diffusion technique to look into the effect of three biogenic synthesized ZnO-NPs with varying concentrations of zinc acetate on the proliferation of the selected potential pathogens (Fig. 5). From the stock solution of each separate ZnO-NPs, six various concentrations (5, 15, 25, 50, 75, and 100 mg/L) were prepared for antimicrobial trials. The highest measured width of ZOI of ZnO-NPs-3 indicated that vigorous antimicrobial action was observed against all selected pathogens, where the diameters of ZOI ranged between 16 and 36 mm for P. aeruginosa, 18 and 39 mm for A. baumannii, 13 and 31 mm for S. aureus, 14 and 33 mm for S. epidermidis, 12 and 28 mm for R. oryzae, and 11 and 25 mm for A. niger (Figs. 5 and 6). Results depicted that the antimicrobial potential of ZnO-NPs against G-ve bacteria was higher than other selected G+ve and fungal species. This phenomenon is attributed to the rigid structure and thick peptidoglycan in the cell wall of G+ve bacteria compared with the cell wall structure of G-ve bacterial which has a thin layer of peptidoglycan [48]. To assess their antibacterial effectiveness, the well-diffusion test was used with biologically produced ZnO-NPs against several kinds of microorganisms. According to the data, P. aeruginosa and A. flavus both exhibited a greatest inhibiting zone, measuring 22 ± 1.8 mm and 19 ± 1.0 mm, respectively [49].
Furthermore, results have shown that augmentin has only antibacterial action with ZOI of 17 and 18 mm against P. aeruginosa and A. baumannii and 14 and 16 mm against S. aureus and S. epidermidis. On another side, voriconazole has a potent antifungal activity with no significant effect against bacterial species, where the ZOI for R. oryzae and A. niger was 14 and 12 mm, respectively.
3.1.3 MIC and MBC values
The MICs and MBCs/MFCs of three distinct NPs, including ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3, are presented in Table 2. The fact that the estimated MICs and MBCs of the studied ZnO-NPs-3 were much smaller than those of the ZnO-NPs-1 and ZnO-NPs-2 displays that nanoparticles have superior antimicrobial properties. Compared to other G+ve and fungal species, all antimicrobial NPs are considerably diminished in the MICs and MBCs/MFCs in G-ve bacterial species. ZnO-NPs-3 was found to have the most antibacterial capability for all of the chosen microbial species, followed by ZnO-NPs-2 and ZnO-NPs-1. This is likely because the ZnO-NPs-3 was biologically generated with increasing zinc acetate concentrations. The results summarized in Table 2 indicated that the measured MIC values of ZnO-NPs-3 against P. aeruginosa, A. baumannii, S. aureus, S. epidermidis, R. oryzae, and A. niger were 10 ± 0.14, 15 ± 0.06, 30 ± 0.21, 20 ± 0.43, 30 ± 0.12, and 35 ± 0.38 mg/L, respectively, while MBC/MFC values were 15 ± 0.54, 20 ± 0.24, 35 ± 0.18, 25 ± 0.37, 35 ± 0.82, and 40 ± 0.60 mg/L, respectively. Notably, the ZnO-NPs were highly efficient against P. aeruginosa and A. baumannii, compared to the others. The interaction effects of the respective microbes and ZnO-NPs provide a clearer picture of how the ZnO-NPs could interact with selected microbes. The diameter, structure, durability, and quantity of ZnO-NPs all affect their bactericidal properties. Rectangle ZnO-NPs produced using a green-mediated synthesis have efficient antimicrobial action toward S. aureus and E. coli. Findings indicate that the MIC value was 20.0 mg/L and that ZnO-NPs with a rectangular shape had a significant antimicrobial property at lower doses [50]. The results suggested that the size of NPs exhibits more vigorous antibacterial activity than the size of their counterparts [51].
3.1.4 Time-kill kinetics assay
Figure 7 depicts the time-kill kinetics patterns of the MIC dose of biologically synthesized ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 against a variety of microbial pathogens. Findings demonstrate that after treatment with the tested NPs, there was a noticeable decrease in cell viability, followed by a rise for up to 120 min when compared to the control (microbial evolution without NPs). Concerning G-ve bacteria like P. aeruginosa and A. baumannii, time-kill kinetics profiles of investigated ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 at the MIC dosage employed demonstrated a corresponding decrease in the amount of cell viability after time points of 60, 30, and 15 min and 45, 15, and 10 min, respectively, followed by a steady rise up to the 24 h and a consequent decrease against its own control (Fig. 7a, b). Results of the time-kill kinetics profile of investigated ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 at the MIC dosage for G+ve bacteria, including S. aureus and S. epidermidis, revealed a decrease in the amount of cell viability for S. aureus at 60, 45, and 30 min and 60, 30, and 15 min for S. epidermidis, endured constant until 24 h, in comparison to its control (Fig. 7c, d). For R. oryzae and A. niger, the time-kill kinetics profile of investigated ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3 against fungal species revealed a decrease in the amount of cell viability between 90, 60, and 45 min (Fig. 7e, f).
To explain the biosynthesized ZnO-NPs, the calculated values of IC50, Log IC50, and R2 were accomplished for three explored NPs (ZnO-NPs-1, ZnO-NPs-2, and ZnO-NPs-3) toward P. aeruginosa, A. baumannii, S. aureus, S. epidermidis, R. oryzae, and A. niger and are represented in Fig. 8 and Table 3. The estimated values of IC50, Log IC50, and R2 fluctuated according to the as-prepared and concentrated zinc acetate.
Our findings (Table 3) show that the examined bacteria and fungus interacted with produced ZnO nanoparticles as antimicrobial agents in considerably different ways, with varying ZI marginal means. Compared to S. aureus and the other examined fungi, P. aeruginosa was the microbe that was more affected by the ZnO-NPs, which shows that it tended to be resistant to the particles. ZnO-NPs can inhibit the evolution of G+ve bacteria, G-ve bacteria, and fungi. The potency of the antibacterial effect depends on how sensitive the bacteria are [52]. The cell wall of G+ bacteria comprises a thick layer of peptidoglycan with a diameter of 20–80 nm that functions as a defensive barrier to safeguard the cell’s surroundings.
In contrast to G-ve bacteria possess a thin layer of cell walls that make these types of bacteria more sensitive to antimicrobial agents [53]. According to Baek and An [54], reported that G-ve bacteria are less resistant to ZnO-NPs than G+ve bacteria. Where it has been shown that the antibacterial capabilities of ZnO NPs directly correlate with their dosage and particle surface area. It has been demonstrated that zinc oxide nanoparticles’ concentration and particle surface area directly connect with their antibacterial properties [55]. Compared to ROS, ZnO-NPs could affect the media surrounding bacterial cells by forming them or altering the microbe’s cell wall due to their electrostatic adhesion to the cell surface, which can cause cellular damage [56]. Many infections, such as E. coli, S. aureus, P. aeruginosa, Bacillus subtilis, and others, can be suppressed by ZnO-NPs because of their exceptional features, including their crystallinity, permeability, particle size, and form [57]. Moreover, under UV and visible light irradiation, ZnO-NPs’ intra and interparticle pores enhance photocatalytic antimicrobial reactions based on ROS [58].
The possible mechanisms of antimicrobial wound healing formulations can depend on the specific ingredients and formulation [59]. Some possible mechanisms include the following: Direct killing of bacteria: Antimicrobial agents in the formulation can directly kill or inhibit the growth of bacteria at the wound site. For example, ZnO-NPs or iodine-containing compounds commonly use antimicrobial agents in wound healing formulations [60]. Modulation of the immune response: Antimicrobial agents can also modulate the immune response to enhance the body’s natural defense mechanisms against infections. For example, some antimicrobial agents can stimulate the production of antimicrobial peptides or enhance the activity of immune cells, such as neutrophils and macrophages, which can help to eliminate bacteria at the wound site. In summary, the hypothesis behind antimicrobial wound healing formulations is that they can expedite wound healing by preventing or treating bacterial infections. The possible mechanisms of these formulations can vary depending on the specific ingredients and formulation but may include the direct killing of bacteria, modulation of the immune response, promotion of tissue regeneration, and reduction of inflammation [61].
4 Conclusion
With the global need not to use chemicals harmful to humans and the surrounding environment, the focus of the current research was on the use of green chemistry in the preparation of zinc oxide nanoparticles (ZnO-NPs) with different concentrations to know the ability of extracellular metabolites of E. coli to stabilize zinc oxide in nanoform. Thus, ZnO-NPs are biologically synthesized from extracellular metabolites of E. coli, which is an inexpensive and environmentally friendly strategy for synthesizing ZnO-NPs with antimicrobial properties for potential use during wound repair and healing. The particles’ enhanced morphologies and extremely small sizes enable them to infiltrate cell membranes readily. Biogenic ZnO-NPs have powerful microbicidal effects against some skin-infectious pathogens when reduced by microbes, and toxicological studies have shown they have biocompatibility features. Therefore, the biogenic ZnO-NPs produced through biosynthesis could be utilized for biological purposes such as wound curing.
Data availability
All the datasets used and/or analyzed in this study are available in the manuscript.
References
Pastar I, Stojadinovic O, Yin NC et al (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3:445–464
Hamdan S, Pastar I, Drakulich S et al (2017) Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci 3:163–175
Wang W, Lu KJ, Yu CH et al (2019) Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology 17:1–15. https://doi.org/10.1186/s12951-019-0514-y
Albrecht MA, Evans CW, Raston CL (2006) Green chemistry and the health implications of nanoparticles. Green Chem 8:417–432
Kalashnikova I, Das S, Seal S (2015) Nanomaterials for wound healing: scope and advancement. Nanomedicine 10:2593–2612
Ebadi M, Zolfaghari MR, Aghaei SS et al (2019) A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium: Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv 9:23508–23525. https://doi.org/10.1039/c9ra03962g
Barzinjy AA, Azeez HH (2020) Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl Sci 2:1–14. https://doi.org/10.1007/s42452-020-2813-1
Kalra K, Chhabra V, Prasad N (2022) Antibacterial activities of zinc oxide nanoparticles: a mini review. J Phys Conf Ser 2267. https://doi.org/10.1088/1742-6596/2267/1/012049
Mishra PK, Mishra H, Ekielski A et al (2017) Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today 22:1825–1834
Zhang Z-Y, Xiong H-M (2015) Photoluminescent ZnO nanoparticles and their biological applications. Materials (Basel) 8:3101–3127
Kim S, Lee SY, Cho H-J (2017) Doxorubicin-wrapped zinc oxide nanoclusters for the therapy of colorectal adenocarcinoma. Nanomaterials 7:354
Xiong H (2013) ZnO nanoparticles applied to bioimaging and drug delivery. Adv Mater 25:5329–5335
Toduka Y, Toyooka T, Ibuki Y (2012) Flow cytometric evaluation of nanoparticles using side-scattered light and reactive oxygen species-mediated fluorescence–correlation with genotoxicity. Environ Sci Technol 46:7629–7636
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine Nanotechnology, Biol Med 7:184–192
Siddiqi KS, ur Rahman A, Tajuddin HA (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13(1):1–3
Rahman F, Majed Patwary MA, Bakar Siddique MA et al (2022) Green synthesis of zinc oxide nanoparticles using Cocos nucifera leaf extract: characterization, antimicrobial, antioxidant and photocatalytic activity. R Soc Open Sci 9. https://doi.org/10.1098/rsos.220858
Hamrayev H, Shameli K, Yusefi M (2020) Preparation of zinc oxide nanoparticles and its cancer treatment effects: a review paper. J Adv Res Micro Nano Eng 2:1–11
Pandey S, Do JY, Kim J, Kang M (2020) Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr Polym 230:115597
Pandey S, Ramontja J (2016) Sodium alginate stabilized silver nanoparticles–silica nanohybrid and their antibacterial characteristics. Int J Biol Macromol 93:712–723
Parveen K, Banse V, Ledwani L (2016) Green synthesis of nanoparticles: their advantages and disadvantages. In: AIP conference proceedings. AIP Publishing LLC, p 20048
Scientific A, Physics A (2022) Biologically synthesized zinc oxide nanoparticles and its effect - a review. Acta Sci Appl Phys 2:3–10
Singh H, Du J, Singh P, Yi TH (2018) Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J Pharm Anal 8:258–264. https://doi.org/10.1016/j.jpha.2018.04.004
Vosoughian N, Mohammadi A (2021) Microorganisms as biologically safe sources for the synthesis of metal nanoparticles. J Biosaf 13:13–32
Ovais M, Khalil AT, Ayaz M et al (2018) Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci 19:4100
Lahiri D, Nag M, Sheikh HI et al (2021) Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.636588
Shehabeldine AM, Elbahnasawy MA, Hasaballah AI (2021) Green Phytosynthesis of Silver Nanoparticles Using Echinochloa stagnina Extract with Reference to Their Antibacterial, Cytotoxic, and Larvicidal Activities. BioNanoSci 11:526–538. https://doi.org/10.1007/s12668-021-00846-1
Ismail NA, Shameli K, Wong MM, Teow SY, Chew J, Sukri SNAM (2019) Antibacterial and cytotoxic effect of honey mediated copper nanoparticles synthesized using ultrasonic assistance. Mater Sci Eng C Mater Biol Appl 104:109899. https://doi.org/10.1016/j.msec.2019.109899
Mammari N, Lamouroux E, Boudier A, Duval RE (2022) Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 10(2):437. https://doi.org/10.3390/microorganisms10020437
Zhan X, Yan J, Tang H, Xia D, Lin H (2022) Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 14(12):2654. https://doi.org/10.3390/pharmaceutics14122654
Nikpasand A, Parvizi M (2019) Evaluation of the effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; An Animal Model Study. Bull Emerg Trauma 7(4):366–372. https://doi.org/10.29252/beat-070405
Sirelkhatim A, Mahmud S, Seeni A et al (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242
Gharpure S, Yadwade R, Ankamwar B (2022) Non-antimicrobial and non-anticancer properties of ZnO nanoparticles biosynthesized using different plant parts of Bixa orellana. ACS Omega 7:1914–1933. https://doi.org/10.1021/acsomega.1c05324
Mohd Yusof H, Abdul Rahman N, Mohamad R et al (2021) Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: an in vitro study. Anim 11. https://doi.org/10.3390/ani11072093
Chen P, He G, Qian J et al (2021) Potential role of the skin microbiota in Inflammatory skin diseases. J Cosmet Dermatol 20:400–409. https://doi.org/10.1111/jocd.13538
Maguire M, Maguire G (2017) The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309:411–421. https://doi.org/10.1007/s00403-017-1750-3
Hong B, Winkel A, Ertl P et al (2018) Bacterial colonisation of suture material after routine neurosurgical procedures: relevance for wound infection. Acta Neurochir (Wien) 160:497–503. https://doi.org/10.1007/s00701-017-3404-9
Kushwaha A, Singh VK, Bhartariya J et al (2015) Isolation and identification of E. coli bacteria for the synthesis of silver nanoparticles: characterization of the particles and study of antibacterial activity. Pelagia Res Libr Eur J Exp Biol 5:65–70
El Nahrawy AM, Hemdan BA, Mansour AM et al (2021) Integrated use of nickel cobalt aluminoferrite/Ni2+ nano-crystallites supported with SiO2 for optomagnetic and biomedical applications. Mater Sci Eng B Solid-State Mater Adv Technol 274:115491. https://doi.org/10.1016/j.mseb.2021.115491
Clinical and Laboratory Standards Institute (2012) Methods for antimicrobial susceptibility testing of anaerobic bacteria; Approved Standard-Eighth Edition. CLSI Doc M11-A8
Mansour AM, Hemdan BA, Elzwawy A et al (2022) Ecofriendly synthesis and characterization of Ni2+ codoped silica magnesium zirconium copper nanoceramics for wastewater treatment applications. Sci Rep 12:9855. https://doi.org/10.1038/s41598-022-13785-y
El-Naggar ME, Abdelgawad AM, Abdel-Sattar R et al (2023) Potential antimicrobial and antibiofilm efficacy of essential oil nanoemulsion loaded polycaprolactone nanofibrous dermal patches. Eur Polym J 184:111782. https://doi.org/10.1016/j.eurpolymj.2022.111782
Rashdan HRM, Shehadi IA, Abdelrahman MT (2021) Sulfone biscompound containing bioactive. 1–15
Alshameri AW, Owais M (2022) Antibacterial and cytotoxic potency of the plant-mediated synthesis of metallic nanoparticles Ag NPs and ZnO NPs: a review. OpenNano 8:100077. https://doi.org/10.1016/j.onano.2022.100077
Alshameri AW, Owais M, Altaf I, Farheen S (2022) Rumex nervosus mediated green synthesis of silver nanoparticles and evaluation of its in vitro antibacterial, and cytotoxic activity. OpenNano 8:100084. https://doi.org/10.1016/j.onano.2022.100084
Rajan A, Cherian E, Baskar G (2016) Biosynthesis of zinc oxide nanoparticles using Aspergillus fumigatus JCF and its antibacterial activity. Int J Mod Sci Technol 1:52–57
Periyathambi P, Vedakumari WS, Bojja S et al (2014) Green biosynthesis and characterization of fibrin functionalized iron oxide nanoparticles with MRI sensitivity and increased cellular internalization. Mater Chem Phys 148:1212–1220
Agarwal H, Nakara A, Menon S, Shanmugam V (2019) Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J Drug Del Sci Technol 53:101212. https://doi.org/10.1016/j.jddst.2019.101212
El Nahrawy AM, Hemdan BA, Abou Hammad AB et al (2021) Modern template design and biological evaluation of cephradine-loaded magnesium calcium silicate nanocomposites as an inhibitor for nosocomial bacteria in biomedical applications. Silicon 13:2979–2991. https://doi.org/10.1007/s12633-020-00642-8
Jayaseelan C, Rahuman AA, Kirthi AV et al (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc 90:78–84. https://doi.org/10.1016/j.saa.2012.01.006
Khatami M, Alijani HQ, Heli H, Sharifi I (2018) Rectangular shaped zinc oxide nanoparticles: green synthesis by Stevia and its biomedical efficiency. Ceram Int 44:15596–15602. https://doi.org/10.1016/j.ceramint.2018.05.224
Saravanan M, Gopinath V, Chaurasia MK et al (2018) Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog 115:57–63. https://doi.org/10.1016/j.micpath.2017.12.039
Lallo da Silva B, Caetano BL, Chiari-Andréo BG et al (2019) Increased antibacterial activity of ZnO nanoparticles: influence of size and surface modification. Colloids Surfaces B Biointerfaces 177:440–447. https://doi.org/10.1016/j.colsurfb.2019.02.013
Bhattacharyya S, Majhi S, Saha BP, Mukherjee PK (2014) Chlorogenic acid–phospholipid complex improve protection against UVA induced oxidative stress. J Photochem Photobiol B Biol 130:293–298. https://doi.org/10.1016/j.jphotobiol.2013.11.020
Baek Y-W, An Y-J (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608. https://doi.org/10.1016/j.scitotenv.2011.01.014
Raghupathi KR, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028. https://doi.org/10.1021/la104825u
Zhong L, Liu H, Samal M, Yun K (2018) Synthesis of ZnO nanoparticles-decorated spindle-shaped graphene oxide for application in synergistic antibacterial activity. J Photochem Photobiol B Biol 183:293–301. https://doi.org/10.1016/j.jphotobiol.2018.04.048
Lallo da Silva B, Abuçafy MP, Berbel Manaia E et al (2019) Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: an overview. Int J Nanomedicine 14:9395–9410. https://doi.org/10.2147/IJN.S216204
Sánchez-López E, Gomes D, Esteruelas G et al (2020) Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10(2):292. https://doi.org/10.3390/nano10020292
Pino P, Bosco F, Mollea C, Onida B (2023) Antimicrobial nano-zinc oxide biocomposites for wound healing applications: a review. Pharmaceutics 15. https://doi.org/10.3390/pharmaceutics15030970
Rayyif SMI, Mohammed HB, Curuțiu C et al (2021) ZnO nanoparticles-modified dressings to inhibit wound pathogens. Mater (Basel, Switzerland) 14. https://doi.org/10.3390/ma14113084
Liang Y, Liang Y, Zhang H, Guo B (2022) Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci 17:353–384. https://doi.org/10.1016/j.ajps.2022.01.001
Acknowledgements
The authors acknowledge the National Research Centre (NRC), Egypt, for the technical support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Bahaa A. Hemdan, Mehrez E. El-Naggar, Sh. E. Abd-Elgawad, Nessma A. El Zawawy, Yehia A.-G. Mahmoud: methodology, data curation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hemdan, B.A., El-Naggar, M.E., Abd-Elgawad, S.E. et al. Bacterial cell-free metabolites-based zinc oxide nanoparticles for combating skin-causing bacterial infections. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04313-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04313-7