Abstract
The urgency for reducing the dependence on fossil-based materials is increasing the interest in the utilization of renewable feedstocks. Lignocellulosic residual biomass can be used as feedstock to produce chemicals and energy without generating food security problems. Wheat straw (WS) has a clear potential for developing sustainable processes in a circular bioeconomy context. However, the development of processes requires a strategy for utilizing the hemicellulosic, cellulosic, and lignin fractions. This work covers the utilization of the hemicellulosic fraction as the first stage of a wheat straw biorefinery. The aim was to evaluate the hydrolysis of WS by using liquid hot water (LHW) treatment, the detoxification of the produced wheat straw hydrolysate (WSH), and the cultivation of Trichoderma reesei using it as the only carbon source as proof of detoxification. LHW treatment was performed at 160 °C and 90 min and yielded a WSH rich in monomeric and oligomeric saccharides (~ 14 g/L) and containing degradation products in low concentration (furfural, HMF, and acetic acid). As part of the development of the extraction and detoxification strategy, we determined the specific inhibition thresholds for T. reesei for the mentioned degradation products. Detoxification was carried out by evaporation by modifying the % of volume evaporated and the pH of the solution. Approximately 55.9% of acetic acid and 100% of furfural were removed from the WSH. The fungal biomass obtained in the medium containing WSH was equivalent to 98% of the biomass obtained in the control medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the United States Department of Agriculture (USDA), approximately 778.6 million metric tons of wheat were produced globally in 2021 [1]. The European Union (EU), followed by China and India, are the worldwide leaders with 18%, 18%, and 14% of the total production, respectively [1]. In the EU, wheat species (common and spelt) are considered strong agricultural-drivers representing 46.9% of the cereals cultivated in the EU [2, 3]. During the harvesting stage, high amounts of straw are generated, becoming the primary residue of this crop. Animal bedding and energy production are some of the applications implemented for this residue; however, a surplus can still be used for biorefining purposes to reduce the environmental impact and contribute to a circular bioeconomy.
The significant amount of structural saccharides makes wheat straw an interesting feedstock for obtaining several products under biorefinery schemes. These biorefinery schemes can be designed based on C6 and C5 mono- and oligo-saccharides and lignin-based platforms [4]. Recalcitrance represents the main drawback of utilizing the oligosaccharides from the different lignocellulosic fractions [5,6,7,8,9,10]. It is given by the complex structures formed by the interlinkage of hemicellulose, cellulose, and lignin [5,6,7,8,9,10]. Therefore, overcoming recalcitrance is one of the main challenges in developing sustainable biorefineries to use lignocellulosic residues efficiently. Hemicellulose is the second most abundant polysaccharide in nature. It is an amorphous, branched heteropolymer composed of a xylan backbone bonded to pentoses (D-xylose and D-arabinose), hexoses (D-mannose, D-glucose, and D-galactose), and organic acids as acetic, ferulic and coumaric acid [11, 12]. Hemicellulose hydrolysis is favored because of its structure, high solubility, low molecular weight, and crystallinity.
Several pretreatments have been used to deconstruct the lignin-hemicellulose complex matrix, such as hydrothermal, steam explosion, organic solvents, and acid pretreatments, among others [13,14,15,16,17,18]. After pretreatment, two fractions are obtained: a liquid fraction rich in monomeric and oligomeric sugars, also called hydrolysate, and a solid fraction with high cellulose content available for further processing stages [12, 19,20,21,22]. The severity factor is the main parameter used to describe the intensity of a given pretreatment; this parameter is estimated considering the temperature, pH, and treatment time [12]. High severity factors increase hydrolysis yields, a positive aspect that contrasts with the generation of undesired degradation products such as furans (furfural and hydroxymethylfurfural (HMF)), phenolic compounds, and weak organic acids (acetic, formic, levulinic, and succinic) [23, 24].
Liquid hot water (LHW) is a hydrothermal treatment used for biomass deconstruction. It is considered an environmental-friendly and economically viable pretreatment due to the absence of additional catalysts considering water exclusively as a solvent. In LHW, pressurized water at high temperatures above 120 °C promotes autoionization of water and other in-situ generated compounds (uronic, phenolic, and acetic acid), releasing hydronium ions. These ions catalyze the hydrolysis of lignin-hemicellulose bonds simultaneously to the depolymerization of hemicellulose. Hemicellulose hydrolysis releases monomeric and oligomeric sugars mainly from xylan fractions and small portions from glucan fractions. A reduced amount of degradation products are generated due to the dehydration of the produced sugars, caused, among other reasons, by the severity of the treatment.
Degradation products, such as acetic acid, furfural, and HMF, cause stress to the microorganism through diverse mechanisms turning into the main drawback for the utilization of the sugars in a LHW hydrolysate [23, 25,26,27,28,29]. In its undissociated form (pKa 4.76), the acetic acid can easily permeate cellular membranes by diffusion, affecting the inner cellular pH. Consequently, the maintenance coefficient increases, and the cells are forced to use glucose to preserve cell integrity, reducing biomass growth and yield [25, 26]. HMF damages cellular components by inhibiting metabolic enzymes and forming reactive oxygen species [27]. Furfural damages cell membranes and DNA, inhibits glycolysis, impairs sugar consumption, and reduces the production efficiency of primary metabolites [28, 29].
Two conditions need to be met to utilize hemicellulosic hydrolysates as carbon sources. First, the microorganism needs to be able to metabolize monomeric and oligomeric sugars. And second, the amount of degradation products with inhibitory effects should not impair or inhibit the growth and development of the microorganism. Regarding carbon sources, filamentous fungi can use various carbon sources and convert many residues into several products. The features mentioned earlier confer filamentous fungi an important role as industrial hosts in a circular bioeconomy context [27, 33, 34]. The filamentous fungi are currently the main route to produce extracellular cellulases, being the host of 11% of the enzyme manufacturers [30]. This remarkable characteristic as an enzyme-producer makes T. reesei an appealing partner for co-cultivation to produce different metabolites [31, 32].
Several detoxification strategies have been formulated using physical, chemical, or biological means to reduce the content of inhibitory compounds in solution [24, 35,36,37,38,39,40]. However, the implementation of many of the detoxification strategies leads to long treatment times, low efficiencies, sugar losses of up to 20%, the demand of chemicals increasing the environmental impact, and the generation of byproducts like gypsum [35,36,37, 39,40,41,42,43].
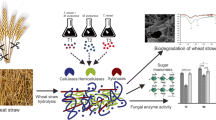
The development of a detoxification strategy applicable to hemicellulosic hydrolysates must integrate the analysis of the initial conditions and the requirements for further applications (sugar levels and inhibitory thresholds). In this paper, we developed a detoxification strategy of a WSH for cultivating T. reesei QM6a∆tmus53 as a contribution to the wheat straw biorefinery. The development of this strategy included the determination of the inhibition threshold for the degradation products acetic acid, furfural, and HMF. And considering these thresholds, the characterization of the performance of detoxification strategy for the growth of T. reesei biomass. The manuscript is developed in two sections (see Fig. 1). The first section of the manuscript (Fig. 1a) covers the LHW extraction, detoxification, and posterior culture of T. reesei in synthetic solutions and hydrolysates. Based on a preliminary work [24], the LHW extraction of wheat straw was carried out at 160 °C for 90 min; under these conditions, approximately 39% and 4% of the hemicellulose and cellulose content in the feedstock were hydrolyzed. Afterward, the detoxification stage targeted furfural and acetic acid removal through evaporation. The unit operations were selected considering furfural volatility and the equilibrium of acetic acid species. The second section (Fig. 1b) covers the determination of the inhibition thresholds for acetic acid, furfural, and HMF, at which the biomass growth was impaired. Finally, the detoxified synthetic solutions and hydrolysates were used as the carbon source for the growth of T. reesei. The approach herein focuses on biomass growth as proof of the efficiency of the detoxification strategy; no metabolite or product was followed in this work.
A deep analysis of the material flows coupled to the maximization of the services provided by a material are crucial elements to be considered in the conceptual development of biorefineries in a circular bioeconomy context [44, 45]. The contribution of this work to the circular-based bioeconomy is the proof of concept of the valorization of the hemicellulosic fraction of wheat straw as one of the main residues from wheat production. This work can be integrated to other projects that target cellulose and lignin valorization to get a maximization in the use of this residue.
2 Materials and methods
2.1 Raw materials and reagents
The wheat straw used in this work was collected in Margarethen am Moos in Lower Austria in 2019. The straw belongs to the same batch reported and characterized in previous works [24, 36]. It had a moisture content of 7.2%wt and composition (%wt, dry basis) of 2.13% arabinan, 0.67% galactan, 35.31% glucan, 21.94% xylan, and 0.72% mannan as the main structural saccharides; added to 17.35% lignin, 20.45% extractives, and 1.09% ash [24]. The straw was milled and sieved for further processing, ensuring a particle size below 2 mm. Standards HPLC grade used in analytics (D-(+)-xylose, D-(−)-arabinose, D-(+)-glucose, D-(+)-galactose, D-(+)-mannose, L-(−)-fructose, furfural, HMF, and acetic acid) were purchased from Merck (Darmstadt, Germany). Microbiological grade glucose, urea, NaOH, H2SO4, MgSO4.7H2O, KH2PO4, NaCl, peptone, tween 80, FeSO4.7H2O, MnSO4.H2O, ZnSO4.7H2O, and CaCl2.2H2O were purchased from Merck (Darmstadt, Germany). Type I ultrapure water produced in a Startorius device (Goettingen, Germany) was used for the experiments.
2.2 LHW pretreatment of wheat straw
LHW was carried out in a 1 L Zirbus HD 9/18 pressurized autoclave (Bad Grund, Germany) equipped with a stirring system inside the reaction vessel, covered by a jacket grouping the heating and cooling system. A feedback on-off control system controlled the whole autoclave, being the temperature of the mantel the variable to be controlled by switching on/off the resistance. Consequently, the reaction temperature inside the vessel was indirectly controlled by controlling the mantel temperature. About 30 g dry basis of wheat straw was loaded to the reactor, and water was added in a 1:11 dry biomass to water ratio (equivalent to 8.3%wt solid load). The reactor was heated to 160 °C, holding this temperature for 90 min, and the biomass was stirred at 200 rpm during the pretreatment. At the end of the pretreatment, the biomass and the hydrolysate were recovered and separated using a hydraulic press (Hapa HPH 2.5, Achern, Germany). The hydrolysate liquid fraction was centrifuged at 12,000 rpm for 15 min to remove residual solids (Sorvall RC 6+, Schwerte, Germany).
2.3 Hydrolysate detoxification
The detoxification was done by evaporation with and without pH modification. The operational conditions, namely temperature and pressure, were selected based on simulation in the software ASPEN Plus V10.0 (AspenTech, Bedford, USA). The removal of degradation products from a solution containing xylose, glucose, acetic acid, HMF, and furfural was simulated (data not shown). This analysis evaluated evaporation fractions of the degradation products at different temperature and pressure conditions using the electrolyte Non-Random Two-Liquid (NRTL) thermodynamic model. The electrolyte-NRTL model is a tool used for the prediction of the thermodynamic properties of the liquid and vapor phases in the equilibrium considering the non-ideality of the liquid phase. Finally, a temperature and pressure of 55 °C and 140 mbar were selected to evaporate the WSH, and an initial 60% evaporated fraction was selected for developing the detoxification at the lab scale.
Detoxification tests were performed using a rotary evaporator R-220 EX (Buchi, Flawil, Switzerland) equipped with a heating bath. To improve acetic acid removal, the influence of two parameters was evaluated on the detoxification performance. The first parameter was the evaporated fraction, considering the evaporation of 60% and 90% of the initial volume. The second parameter evaluated was the modification of the distribution of acetic acid species in equilibrium. For this specific task, the undissociated acetic acid concentration (AcH, pKa = 4.76) and its conjugate base (dissociated acetic acid, Ac−) were estimated. The estimation was done based on the total concentration of acetic acid determined by HPLC, the current pH, and the pKa value assuming the ideality of the solution (Eq. 1 and Eq. 2). Then, this equilibrium was altered by adding NaOH and H2SO4 to affect the volatility and removal of acetic acid species.
2.4 Toxicity assays
2.4.1 T. reesei QM6a∆tmus53
The strain QM6a∆tmus53 used for the cultivation experiments is a wild-type phenotype that is utilized as a parental strain in molecular biology [46]. This is a strain with a deletion of the gene tmus53, leading to a deficiency in the Non-Homologous End joining repair system. This modification improves transformation efficiency.
2.4.2 Spore suspension and inoculum
T. reesei QM6a∆tmus53 was seeded on fresh malt extract agar plates about a week before the experiment. Plates were incubated for three to four days at 30 °C without light, followed by 2 to 3 days at room temperature under natural light. The spore suspension was prepared on the day of the experiment. Spores scratched from the surface of the plates were resuspended by vortexing in a 0.8% NaCl/0.05% Tween 80 sterile solution. The absorbance of the spore solution was measured at 700 nm.
To ensure the reproducibility of the experiments, every flask was individually inoculated, targeting the same initial spore content. All the flasks were seeded with a target absorbance of 0.05. The volume of spore suspension to add per flask was calculated using Eq. 3, where x corresponds to the volume of spore suspension to use per flask, and Absorbance700 corresponds to the absorbance measured at 700 nm.
2.4.3 Preparation of medium for inhibition threshold tests
The cultivation medium contained 70 g/L glucose, 80 mM urea, 1 g/L MgSO4.7H2O, 4 g/L KH2PO4, 0.5 g/L NaCl, 0.1 g/L peptone, and 0.5 g/L tween 80, 5 mg/L FeSO4.7H2O, 17 mg/L MnSO4.H2O, 14 mg/L ZnSO4.7H2O, and 2mg/L CaCl2.2H2O. Glucose and urea solutions were sterile filtered (VacuCap 0.8/0.2 μm, PALL Corporation, New York USA); the other components of the media were autoclaved at 121 °C for 1 h for sterilization.
Degradation products were added individually to the flasks at different concentrations for acetic acid (0.05, 0.1, 0.2, 0.5, 0.625, 0.75 g/L), furfural (100, 250, 400, 550, 660, 1500 mg/L), and HMF (0.4, 0.6, 1.0, 1.5, 2.0 g/L). The tests were performed in triplicates using 250 mL shake flasks containing a total culture volume of 100 mL. Flasks were incubated at 30 °C at 180 rpm for 72 h, and the initial pH was 5. Dry biomass measurement was performed at the end of the cultivation using Whatman quantitative filter papers previously dried and stored in a desiccator after drying.
2.5 Cultivation of T. reesei on detoxified hydrolysate
The composition of the cultivation medium included 6–8 mM urea, 1 g/L MgSO4.7H2O, 4 g/L KH2PO4, 0.5 g/L NaCl, 0.1 g/L peptone, and 0.5 g/L tween 80, 5 mg/L FeSO4.7H2O, 17 mg/L MnSO4.H2O, 14 mg/L ZnSO4.7H2O, and 2 mg/L CaCl2.2H2O. The final urea concentration was lower than for the toxicity tests, as high urea concentration had not been tested by the time of the tests with hydrolysates. WSH was diluted in Mili-Q water to get a final sugar concentration (monomeric and oligomeric) between 22.0 and 32.5 g/L. Glucose, as the sole carbon source, was used as the control medium. WSH and urea solutions were sterile filtered (VacuCap 0.8/0.2 μm, PALL Corporation, New York USA); the other components of the media were autoclaved at 121 °C for 1h for sterilization.
The tests were performed in triplicates using 100-mL shake flasks containing a total culture volume of 25 mL. The spore suspension was added to the flasks and was incubated at 30 °C and 180 rpm for 4 to 7 days. The initial pH of the culture medium was adjusted to 5 with concentrated NaOH. Dry biomass measurement was performed at the end of the cultivation using Whatman quantitative filter papers previously dried and preserved.
2.6 Sugars and degradation products analytics
Monomeric and oligomeric sugars (D-(+)-xylose, D-(−)-arabinose, D-(+)-glucose, D-(+)-galactose, D-(+)-mannose, L-(−)-fructose) were determined by high-performance anion-exchange chromatography with a pulsed amperometric detection HPAEC-PAD on a Dionex ICS 5000 system (Thermo Scientific, Walhman, USA) equipped with a guard column and an analytical column of the reference Dionex CarboPac PA1 and analytical column from the same reference (2 × 250 mm) (Thermo Scientific, Walhman, USA). The utilized method was described by Lindermann, Friedl, and Srebotnik [47] using isocratic flow mode with a preconditioning and measurement routine employing 200 mM NaOH/65 mM sodium acetate, water, and 67 mM NaOH as eluents. Oligomeric sugars were hydrolysed into monomeric sugars using 4% sulfuric acid solution at 121 °C for 60 min, according to the Laboratory Analytical Procedure from the National Renewable Energy Laboratory (NREL) [48].
Degradation products (furfural, HMF, and acetic acid) were determined by High-Performance Liquid Chromatography equipped with a refractive index detector and a UV detector on an LC-20A HPLC-RID-UV (Shimadzu, Kyoto, Japan). A total of 10 μL of the sample were injected into the system and eluted with 5 mM H2SO4 (isocratic flow 0.6 mL/min). A guard and an analytical column arrangement of Sugar SH-G and Shodex SH1011 (Showa Denko Europe, Munich, Germany) were used to determine the mentioned compounds. All the eluents involved in chromatography were filtered through a 0.2-μm Nylon 66 membrane (Supelco, St. Louis, USA) and degassed before utilization.
3 Results and Discussion
3.1 Sugars and degradation products in WSH
Many authors have described the stages involved during the breakdown of hemicellulose into monomeric and oligomeric sugars as arabinose, galactose, glucose, and xylose [13, 49, 50]. The first stage involves the creation of radical products on the surface of biomass. The second stage is the dissolution of products like oligomeric sugars and acetic acid, among others in water. The oligomeric fractions released are mainly composed by xylans (linear xylose units with a backbone of different small carbohydrates) as the main fraction; mannans (heterogyclans linked to cellulose), xyloglucans (short poly or oligosaccharides), and glucans (linear glucose fractions), among other components [13]. Finally, the last stage considers a further decomposition of the products generating xylose, glucose, furfural, and acetic acid, among other compounds [50]. In this paper, the extension of the hydrolysis is limited to an intermediate stage where the conversion of oligomeric sugars towards monomeric sugars is low. Therefore the further degradation of monomers into inhibitory compounds is limited compared to other scenarios with a higher concentration of monomeric sugars and degradation products [24].
LHW extractions of wheat straw were performed in triplicate according to the protocol described in the materials and methods section. Table 1 presents the characterization of sugars and degradation products in the WSH. The sugar fraction is described by the concentration of monomeric and oligomeric sugars expressed as C5 sugars as arabinose and xylose; and C6 sugars as glucose, galactose, mannose, and fructose. The standard deviation values shown in Table 1 were associated with the indirect control strategy of the reaction vessel, affecting the accuracy in the control of temperature in the system. However, all the extractions showed the same trends in composition at different concentration levels.
Table 1 shows an average production of 14.6 g/L of total sugars, with 9.8 times more oligomeric than monomeric sugars. Xylose oligomers were the main sugars detected in the solution with an approximate concentration of around 9.9 g/L, followed by oligomeric glucose and galactose in lower concentrations. The oligomeric to monomeric ratio is a measurement of the extension of the LHW treatment for the hydrolysis of hemicelluloses.
These results agree with those presented by Beisl et al. (2019), who reported an increase in the obtention of C5 sugars while increasing the temperature to 170 °C [36]. In a previous work, Serna-Loaiza et al. reported similar concentrations of sugars for WSH obtained at 160 °C and 90 min treatment; at these conditions, minimum lignin solubilization was found [24].
As a consequence of sugars degradation and the hydrolysis of hemicellulose, acetic acid, furfural, and HMF were formed. The major contributor in concentration was acetic acid, released during hydrolysis due to the rupture of the acetyl-hemicellulose bonds. The second degradation product in concentration was furfural, generated by the dehydration of pentoses, the main sugars produced during hemicellulose hydrolysis. Finally, HMF was present in a significantly lower concentration than acetic acid and furfural. Differences in concentration levels presented between furan compounds are related to the very limited amount of available glucose in WSH that can be isomerized to fructose as an intermediate, which is then dehydrated to HMF [51].
The concentration and distribution of the sugars in the hydrolysate elucidate the main criteria for its utilization as a carbon source in further cultivation stages as a C5-based oligomeric sugar solution (oligomeric sugars represent 90% of the total produced sugars). The growing interest in this type of substrate is supported by the increasing development of policies promoting a circular-based bioeconomy [44, 52]. Consequently, microorganisms able to metabolize pentoses and hexoses substrates are gaining interest in biotechnology. Some of these microorganisms include yeasts as Pichia stipitis and Kluyveromyces marxianus for the production of ethanol [53] and xylitol [54]; bacteria as Shingomonas sanxanigenens for the production of an exopolysaccharide named Sanxan and Corynebacterium glutamicum for glutamate and succinate production [55]; and filamentous fungi as T. reesei for the production of enzymes and other metabolites [56]. However, hemicellulosic hydrolysates must meet the requirements of each microorganism in terms of providing the necessary sugars concentration without exceeding the degradation product thresholds. This remark is strongly connected to the detoxification strategy presented in this work, which allows concentrating the sugars in solution simultaneously to remove degradation products from the hydrolysate.
3.2 Detoxification of WSH
The conceptual development of the detoxification strategy was performed in two stages, considering evaporation as the core unit operation. The first stage was carried out using synthetic solutions to understand the removal of degradation products better. Then, the second stage aimed to transfer and assess the outcomes from the first stage to the WSH.
3.2.1 Removal of degradation products in the synthetic hydrolysate (SH)
Synthetic hydrolysate (SH) solutions were prepared by adding xylose, glucose, acetic acid, furfural, and HMF to ultrapure water; an initial pH value of 3.36 was measured. All the SH solutions were prepared at a lower concentration than the WSH (Table 1). These concentrations were based on preliminary extractions with lower yields. However, the results were interpreted and presented as a proof-of-concept for the removal of degradation products validated in subsequent experiments. In this stage, the SH solution was evaporated at 55 °C and 135-140 mbar. The volume to be evaporated was set to 60% of the initial volume; this parameter was visually controlled, and variations were presented among the samples. Two fractions were obtained after evaporation: a condensate fraction containing the removed degradation products and a concentrate also called detoxified fraction.
Table 2 presents the concentrations of the main compounds in the SH, concentrate and condensate fractions when 66.7% of the initial volume was evaporated. About 70% of the initial acetic acid, 100% xylose, glucose, and HMF remained in the concentrate fraction, whereas 100% of furfural was removed to the condensate fraction. The resulting concentrate fraction contained approximately 34 g/L of sugars, 2.7 g/L of acetic acid, and 30 mg/L HMF. The aggregated results describe a concentration factor for xylose and glucose of 3.1 times, 2.5 times for acetic acid, and 5 times for HMF. The low vapor pressure explains the concentration effect observed for two of the three degradation products at standard conditions of acetic acid (20 mbar at 21 °C) and HMF (7 mbar at 25 °C) [57].
The same conditions (55 °C and 140 mbar) were implemented to WSH, evaporating approximately 70.6% of the initial volume. A similar trend (shown in Table 3) was observed for acetic acid, furfural and HMF. In this case, only 25% of the initial mass of acetic acid was removed by evaporation. No HMF was removed accordingly to the results presented in the evaporation of SH. Monomeric and oligomeric sugars remained in the concentrate fraction, and their concentration increased at different levels. Xylose and glucose were considered the reference components to determine a total concentration factor of 3.5 times for all sugars except fructose. Fructose is widely recognized as an unstable and easily degradable sugar. The resultant concentrate fraction contained approximately 46.8 g/L of total sugars, 3.5 g/L of acetic acid, and 85.8 mg/L of HMF. The first experiments demonstrated that evaporation at the mentioned conditions is suitable for the removal of furfural, avoiding sugar losses. Considering the small amounts of HMF produced, the removal of acetic acid was defined as the next step to be explored.
3.2.2 Removal of acetic acid
The removal of acetic acid (AcH) was studied considering the equilibrium with its conjugate base, the acetate ion (Ac−), described by Eqs. 1 and 2 (Section 2.3). To study the effect of pH in the equilibrium, a SH solution containing 1.42 g/L of acetic acid, 0.41 g/L furfural, and 21.1 mg/L HMF was prepared in ultrapure water, giving an initial pH value of 3.36. This solution was distributed into 16 beakers, adding different volumes of sulfuric acid and sodium hydroxide to each beaker to obtain three different pH levels (alkaline, acid, and initial). pH and acetic acid concentrations were measured, and equilibrium calculations were performed as described in Section 2.3.
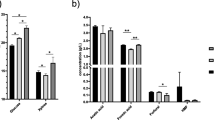
The addition of sulfuric acid and sodium hydroxide did not affect the concentration of the species in solution since the same value was measured for all of them after pH modification. The main effect was observed in the distribution of the acetic acid species in equilibrium, and Figure 2 presents the distribution of acetic acid equilibrium species at different pH values. Lower pH values favored the equilibrium towards AcH, whereas high pH values favored it towards Ac-. Three SH solutions at different pH levels were evaporated to evaluate the impact of acetic acid species distribution over the total acetic acid removal. The SH solutions were prepared by adding sugars and degradation products (see Fig. 2); two of the SH were alkalinized with sodium hydroxide up to different pH levels (SH + NaOH (1) and SH + NaOH (2)) The third SH solution was acidified with sulfuric acid (SH + H2SO4).
Table 4 presents the removal results and the final concentration of acetic acid according to the distribution of the species in the equilibrium. As observed, the addition of sulfuric acid led to the removal of 44.8% of the acetic acid. This value was 1.8 times higher than the removal without pH modification and 2 times and 6.9 times higher than the values presented by the alkalinized samples. Consequently, the SH + H2SO4 solution presented the lowest concentration of acetic acid observed (2.8 g/L) and the lowest concentration factor in the solution (1.9 times). In summary, it was evidenced that the removal of acetic acid is favored at low pH values when AcH is the only acetic acid specie in solution.
After acidification with sulfuric acid, a second WSH sample was detoxified through evaporation (55 °C and 140 mbar). Considering the higher complexity of the WSH compared to the SH solutions, evaporation was performed until an average of 87.7% of the initial volume was removed. The initial pH of 4.4 was reduced to 2.7, ensuring that 99% of the acetic acid species remained in this undissociated form. Results are presented in Table 5; high deviation values were presented due to the different evaporation ratios obtained during the triplicates evaluation. However, mass balances were checked in all cases, and differences below 12% were reported in every case except fructose, a highly degradable sugar.
Removal of 55.9% of acetic acid was achieved (Table 5), representing an improvement of nearly 30% compared to the first condition evaluated. Taking into account the high concentration of the hydrolysate, small amounts of furfural were detected. No HMF was removed accordingly to the results presented in the evaporation of the synthetic solution. Monomeric and oligomeric sugars, except for fructose, remained in the concentrate fraction, and their concentration increased at different levels. Xylose and glucose were considered the reference components to determine a total concentration factor of 10 times. The resultant concentrate fraction contained approximately 167.4 g/L of total sugars, 7.4 g/L of acetic acid, 12 mg/L of furfural, and 338 mg/L of HMF.
Further utilization of hydrolysates detoxified under acidic conditions as carbon sources in cultivation might require pH adjustment. This is the case of T. reesei cultivation which is developed at pH 5.0. Therefore, the utilization of detoxified hydrolysate requires the addition of alkali, causing a displacement in the equilibrium of acetic acid species towards acetate. The increase of acetate reduces the harmful potential caused by acetic acid since the undissociated species are more likely to permeate the cell membrane by diffusion affecting the inner cellular pH and forcing the cells to use ATP in pH regulation [25, 26]. The target pH value is specific and characteristic of cultivations. The following sections cover the determination of the toxicity effect of degradation products over biomass production and the cultivation of T. reesei in detoxified solutions. For this purpose, the solutions mentioned in Tables 2, 3, 4, and 5 were preserved and tested.
3.3 Determination of inhibition thresholds for T. reesei
Furfural, acetic acid, and HMF were added to a medium containing glucose as the carbon source in different concentrations to determine inhibition thresholds able to impair biomass growth. Figure 3 presents the relative biomass growth in the media containing furfural, acetic acid or HMF. The result is expressed as the percentage of relative biomass compared to the control without inhibitory compounds. For all the cases, standard deviation values were lower than 15%, always maintaining the same inhibition trend on the maximum and minimum error values.
Regarding furans, furfural (Fig. 3a) impaired the growth of T. reesei the most. It required a relative low concentrations of furfural (400 mg/L) to provide a strong inhibition effect with a reduction of 53% in biomass formation. On the other hand, relatively high concentrations of HMF (above 1.5 g/L, Fig. 3c) must be present in the solution to reduce by 78% the total biomass produced. Finally, acetic acid (Fig. 3b) showed two trends, a slight increase or a negligible effect in biomass production at concentrations around 50 mg/L and a marked reduction at concentrations above 625 mg/L. The overall results indicate a more substantial inhibition effect from furfural than HMF and acetic acid.
Five additional conditions were assessed to determine the synergistic effect of furfural, acetic acid, and HMF. Table 6 describes the study cases evaluated and the corresponding concentration of the inhibitory compounds. Study case 1 assessed the impact of the combined effect of the threshold concentrations. Study cases 2, 3, and 4 were evaluated respectively at the concentration of inhibitory compounds measured in the WSH after the extraction, after detoxification, and after detoxification under acidified conditions. Besides, study case 5 assessed the impact when 30% of the threshold concentration.
Figure 4 shows the results of the biomass growth for the five evaluated study cases. The impairment effect of degradation products in study case 1 showed a synergistic effect of the inhibitory compounds, with a strong inhibition of biomass growth up to 99.1%. This value was significantly higher than the individual compounds (Fig. 3). Study cases 2, 3, and 4 represent the different stages of WSH during the development of the detoxification strategy presented two behaviors. Study cases 2 and 3 showed a strong inhibition with a reduction in biomass growth of 98.4% and 95.3%, whereas the study case 4 reported a slight decrease of 12.2%. These values suggest a main contribution of acetic acid, which is potentialized by the presence of furfural on inhibition of the growth of T. reesei. Finally, study case 5, with 30% of the threshold concentrations, did not represent a negative impact on the growth of biomass.
Similar responses to furans have been reported by studies developed for T. reesei and other fungi like Trametes versicolor and Mortierella isabelina [26, 27, 29]. Kudahettige Nilsson et al. (2016) reported that furfural had an impairing effect on sugar consumption and ethanol production by T. versicolor [29]. In the same direction, He et al. (2020) studied the impact of furfural and HMF on biomass production and cellulase activity from T. reesei. The authors observed a reduction of 46.2% in biomass compared to the control in presence of 480 mg/L furfural, causing a substantial impact on cellulases production. In contrast to furfural, 630 mg/L of HMF presented a lower impact causing a reduction of 15.4% in biomass [28]. These studies support the conclusion of this work concerning the lower toxicity of HMF compared to furfural for the growth of T. reesei.
Cavka and Jornsson. (2014) assessed the utilization of Norway spruce hydrolysate produced under acidified conditions to cultivate T. reesei. The acidified hydrolysate contained a high concentration of acetic acid (5 g/L), formic acid (0.4 g/L), HMF (2 g/L), and furfural (1.7 g/L) which limited its direct use in cultivation as the main source of carbon [58]. For this reason, different culture medium containing different percentages of Norway spruce hydrolysate and commercial carbon sources were tested. The study reported good carbon source assimilation in a culture medium containing 25% Norway spruce hydrolysate and a reduction of 90% biomass when the culture medium was composed of 50% hydrolysate, linked to a high concentration of inhibitory products in the hydrolysate and their interaction [58].
Ivančić Šantek et al. (2021) studied the effect of the concentration of acetic acid and the age of the inoculum (1-day-old vegetative mycelium or spores) on M. isabelline cultivation. For this purpose, corn cobs hydrolysate produced under acidified conditions was used as culture medium; and biomass growth and lipid accumulation were quantified [26]. The results reported a significant reduction in biomass coupled with a total inhibition of lipid production in the presence of acetic acid at concentrations of 3 g/L. Besides, a strong influence of the age of the microorganism was noted on the capability to overcome growth inhibition. Vegetative mycelium showed a higher capability to overcome inhibition linked to the enzymatic activity of the 1-day-old microorganism and the exposition to a higher concentration of inhibitory compounds faced by the spores [26].
According to the literature, the presence of inhibitory compounds triggers a cellular response by promoting the production and activity of different enzymes or the use of reducing enzymes equivalents like NADH or NADPH that can, among other functions, catalyze the reduction of furans to produce furfuryl alcohols and furoic acids [26, 29, 59, 60]. This enzymatic response represents itself a detoxification of the culture medium to preserve the microorganism viability. However, this response of T. reesei reduces the amount of carbon source and energy available for the growth and production of metabolites, supporting the necessity to develop a detoxification strategy for the utilization of lignocellulosic hydrolysates [59].
3.4 Use of hydrolysates for T. reesei growth
A control medium with glucose as carbon source and five detoxified solutions (SH, SH + NaOH (2), SH + H2SO4, WSH, and WSH + H2SO4) described in previous sections (Tables 2, 3, 4, and 5) were inoculated with a spore solution of T. reesei (Section 2.5). Before cultivation, all the solutions were diluted when rare, trace elements and culture medium were added to the detoxified solutions. The initial concentration of sugars in the culture medium (C0) was 22.0–32.5 g/L of total sugars. The results were analyzed based on the yield of biomass to total sugars. For each case, the term total refers to the sum of all the possible monomeric and oligomeric sugar species in the control medium (glucose), detoxified SH (xylose and glucose) and WSH solutions (xylose, arabinose, glucose, galactose, and mannose).
Acetic acid and HMF were the inhibitory compounds detected in the five detoxified culture mediums; only acetic acid was present at potentially harmful concentrations. However, the effective level of hazard of acetic acid was reduced by the adjustment of pH before cultivation to a 5.0 value. In consequence, the acetic acid equilibrium was shifted towards acetate for those solutions with pH value < 5 (SH, SH + H2SO4, WSH, and WSH H2SO4) and towards the undissociated form for those solutions with pH value > 5 (SH + NaOH (2)).
Table 7 presents the results of sugar consumption, initial (C0) and final (Cf) concentrations of acetic acid, HMF, and total biomass to glucose yield. Different levels of sugar consumption were observed; the highest sugar consumption (98.1%) was presented in the control medium. Among the five detoxified solutions, only those treated with sulfuric acid showed significant utilization by the microorganism. WSH treated with sulfuric acid reported 60.3% sugar consumption, followed by SH + H2SO4 with 34% sugar consumption. However, only the WSH treated with sulfuric acid presented similar levels of biomass production compared to the control. The highest yield was presented by the glucose control (0.430 g/g), followed by the WSH treated with sulfuric acid (0.420 g/g), and SH with a lower yield (0.078 g/g). The differences in sugar consumption and biomass yield presented between SH + H2SO4 and the acidified WSH are related to the effect of acetic acid species and their concentrations in the culture medium. This behavior allows inferring that high energy investment is required to counteract the negative effect caused by the acetic acid species, reducing the substrate available to be converted to biomass and other metabolites (Table 7).
Remarkable differences were found between the WSH samples. WSH culture medium led to significantly lower sugar consumption and biomass production than acidified WSH. At the same time, the acidified WSH showed a similar performance to the control medium despite the lower consumption of sugars. These differences are supported by the complexity of WSH and the wide variety of compounds in the solution. These compounds include other organic acids and phenolic fractions, among other compounds that might be present in levels that the microorganism was capable of dealing with, favoring biomass production in acidified WSH. It is worth mentioning that the control medium consists exclusively of monomeric glucose, whereas the WSH + H2SO4 is mainly oligomeric sugars. Even though the microorganism metabolizes easier sugars in a monomeric form, with the utilization of the sugar mixture in the hydrolysate the fungal biomass produced was 77% of the reported in the control, with 98% of the yield reached in the glucose control. These results indicate and corroborate the potential usage of the WSH after detoxification as carbon source for T. reesei.
4 Conclusions
This work addressed the extraction and detoxification of hemicellulosic WSH for the cultivation of T. reesei as an alternative to commercial bulk sugars used for cultivation. LHW was used to produce a hemicellulosic WSH containing 14.64 g/L of monomeric and oligomeric sugars and degradation products as furfural (0.41 g/L), HMF (0.024 g/L), and acetic acid (1.57 g/L). The development of the detoxification strategy targeted the removal of degradation products without sugar losses. Evaporation was proven as an efficient alternative for removing inhibitory compounds below the determined inhibition thresholds (furfural > 250 mg/L, acetic acid > 750 mg/L, HMF > 1 g/L). The set-up for the detoxification process included the acidification of WSH followed by the evaporation of 87% of the initial volume at 55 °C and 140 mbar. At these conditions, 100% of furfural and up to 55.9% of acetic acid were removed.
T. reesei was cultivated in detoxified WSH obtaining a fungal biomass to total sugars yield (0.420 g biomass/total sugars in WSH) similar to the control medium (0.43 g biomass/g glucose). The biomass growth here is a good indication for the development of a suitable upstream strategy for utilizing the hemicellulosic fraction of wheat straw as the sole carbon source in fungal cultivation. These results contribute to the formulation of potential routes and strategies for the utilization of lignocellulosic biomass for the development of sustainable biorefineries.
Data availability
Not applicable.
References
USDA (2022) World agricultural production. USDA
Eurostat (2021) Agricultural production- crops. Eurostat, Luxembourg
Helin T et al (2012) BIOCORE. BIOCOmmodity REfinery Deliverable D1.1 : Availability of lignocellulosic biomass types of interest in the study regions. BIOCORE
Serna-Loaiza S, Zikeli F, Adamcyk J, Friedl A (2021) Towards a wheat straw biorefinery: combination of Organosolv and liquid hot water for the improved production of sugars from hemicellulose and lignin hydrolysis. Bioresour Technol Reports 14(February):100667. https://doi.org/10.1016/j.biteb.2021.100667
Daza Serna LV, Orrego Alzate CE, Cardona Alzate CA (2016) Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour Technol 199:113–120. https://doi.org/10.1016/j.biortech.2015.09.078
Lu K, Hao N, Meng X, Luo Z, Tuskan GA, Ragauskas AJ (2019) Investigating the correlation of biomass recalcitrance with pyrolysis oil using poplar as the feedstock. Bioresour Technol 289(May):121589. https://doi.org/10.1016/j.biortech.2019.121589
Phitsuwan P, Sakka K, Ratanakhanokchai K (2013) Improvement of lignocellulosic biomass in planta: A review of feedstocks, biomass recalcitrance, and strategic manipulation of ideal plants designed for ethanol production and processability. Biomass and Bioenergy 58:390–405. https://doi.org/10.1016/j.biombioe.2013.08.027
Banu Jamaldheen S et al (2022) A review on physico-chemical delignification as a pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour Technol 346(October 2021):126591. https://doi.org/10.1016/j.biortech.2021.126591
Saravanan A, Senthil Kumar P, Jeevanantham S, Karishma S, Vo DVN (2021) Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour Technol 344(August):2022. https://doi.org/10.1016/j.biortech.2021.126203
Wang W, Lee DJ (2021) Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: a review. Bioresour Technol 339(July):125587. https://doi.org/10.1016/j.biortech.2021.125587
Arzami AN, Ho TM, Mikkonen KS (2022) Valorization of cereal byproduct hemicelluloses: fractionation and purity considerations. Food Res Int 151(November 2021):110818. https://doi.org/10.1016/j.foodres.2021.110818
Rodríguez-Sanz A, Fuciños C, Torrado AM, Rúa ML (2022) Extraction of the wheat straw hemicellulose fraction assisted by commercial endo-xylanases. Role of the accessory enzyme activities. Ind Crops Prod 179:114655. https://doi.org/10.1016/j.indcrop.2022.114655
Scapini T et al (2021) Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour Technol 342(July):126033. https://doi.org/10.1016/j.biortech.2021.126033
Monteiro CRM et al (2021) Hydrothermal treatment on depolymerization of hemicellulose of mango seed shell for the production of xylooligosaccharides. Carbohydr Polym 253(April 2020):1–8. https://doi.org/10.1016/j.carbpol.2020.117274
Gao Y, Wang H, Guo J, Peng P, Zhai M, She D (2016) Hydrothermal degradation of hemicelluloses from triploid poplar in hot compressed water at 180-340 °c. Polym Degrad Stab 126:179–187. https://doi.org/10.1016/j.polymdegradstab.2016.02.003
Borrero-López AM, Masson E, Celzard A, Fierro V (2018) Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment. Ind Crops Prod 124(July):919–930. https://doi.org/10.1016/j.indcrop.2018.08.045
Đorđević T, Antov M (2018) The influence of hydrothermal extraction conditions on recovery and properties of hemicellulose from wheat chaff – a modeling approach. Biomass and Bioenergy 119(February):246–252. https://doi.org/10.1016/j.biombioe.2018.09.030
Gallina G, Alfageme ER, Biasi P, García-Serna J (2018) Hydrothermal extraction of hemicellulose: from lab to pilot scale. Bioresour Technol 247(July 2017):980–991. https://doi.org/10.1016/j.biortech.2017.09.155
Sun D, Lv ZW, Rao J, Tian R, Sun SN, Peng F (2022) Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr Polym 281(August 2021):119050. https://doi.org/10.1016/j.carbpol.2021.119050
Mugwagwa LR, Chimphango AFA (2022) Physicochemical properties and potential application of hemicellulose/pectin/nanocellulose biocomposites as active packaging for fatty foods. Food Packag Shelf Life 31(April 2020):100795. https://doi.org/10.1016/j.fpsl.2021.100795
Lorenci Woiciechowski A et al (2020) Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance – conventional processing and recent advances. Bioresour Technol 304(October 2019):122848. https://doi.org/10.1016/j.biortech.2020.122848
Yang J, Ching YC, Chuah CH (2019) Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers (Basel) 11(5):1–26. https://doi.org/10.3390/polym11050751
Phaiboonsilpa N, Champreda V, Laosiripojana N (2020) Comparative study on liquefaction behaviors of xylan hemicellulose as treated by different hydrothermal methods. Energy Rep 6:714–718. https://doi.org/10.1016/j.egyr.2019.11.143
Serna-Loaiza S, Dias M, Daza-Serna L, de Carvalho CCCR, Friedl A (2022) Integral analysis of liquid-hot-water pretreatment of wheat straw: evaluation of the production of sugars, degradation products, and lignin. Sustain 14(1):362. https://doi.org/10.3390/su14010362
Jourdier E, Poughon L, Larroche C, Ben Chaabane F (2013) Comprehensive study and modeling of acetic acid effect on trichoderma reesei growth. Ind Biotechnol. 9(3):132–138. https://doi.org/10.1089/ind.2013.0002
Ivančić Šantek M, Grubišić M, Galić Perečinec M, Beluhan S, Šantek B (2021) Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem 109(May):46–58. https://doi.org/10.1016/j.procbio.2021.06.021
Troiano D, Orsat V, Dumont MJ (2021) Use of filamentous fungi as biocatalysts in the oxidation of 5-(hydroxymethyl)furfural (HMF). Bioresour Technol 344(August):2022. https://doi.org/10.1016/j.biortech.2021.126169
He J, Liu X, Xia J, Xu J, Xiong P, Qiu Z (2020) One-step utilization of non-detoxified pretreated lignocellulose for enhanced cellulolytic enzyme production using recombinant Trichoderma reesei RUT C30 carrying alcohol dehydrogenase and nicotinate phosphoribosyltransferase. Bioresour Technol 310(April):123458. https://doi.org/10.1016/j.biortech.2020.123458
Kudahettige Nilsson RL, Holmgren M, Madavi B, Nilsson RT, Sellstedt A (2016) Adaptability of Trametes versicolor to the lignocellulosic inhibitors furfural, HMF, phenol and levulinic acid during ethanol fermentation. Biomass and Bioenergy 90:95–100. https://doi.org/10.1016/j.biombioe.2016.03.030
Bischof RH, Ramoni J, Seiboth B (2016) Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Fact 15(1):1–13. https://doi.org/10.1186/s12934-016-0507-6
Kolasa M, Ahring BK, Lübeck PS, Lübeck M (2014) Co-cultivation of Trichoderma reesei RutC30 with three black Aspergillus strains facilitates efficient hydrolysis of pretreated wheat straw and shows promises for on-site enzyme production. Bioresour Technol 169:143–148. https://doi.org/10.1016/j.biortech.2014.06.082
Liu X et al (2022) One-pot fermentation for erythritol production from distillers grains by the co-cultivation of Yarrowia lipolytica and Trichoderma reesei. Bioresour Technol 351(March):127053. https://doi.org/10.1016/j.biortech.2022.127053
Shen L, Gao J, Wang Y, Li X, Liu H, Zhong Y (2022) Engineering the endoplasmic reticulum secretory pathway in Trichoderma reesei for improved cellulase production. Enzyme Microb Technol 152(August 2021):109923. https://doi.org/10.1016/j.enzmictec.2021.109923
Siamphan C et al (2022) Production of D-galacturonic acid from pomelo peel using the crude enzyme from recombinant Trichoderma reesei expressing a heterologous exopolygalacturonase gene. J Clean Prod 331(January 2021):129958. https://doi.org/10.1016/j.jclepro.2021.129958
Andary J, Maalouly J, Ouaini R, Chebib H, Rutledge DN, Ouaini N (2021) Stability study of furans, glucose and xylose under overliming conditions: effect of sugar degradation products. Bioresour Technol Reports 15(March):100722. https://doi.org/10.1016/j.biteb.2021.100722
Beisl S, Quehenberger J, Kamravamanesh D, Spadiut O, Friedl A (2019) Exploitation of wheat straw biorefinery side streams as sustainable substrates for microorganisms: A feasibility study. Processes 7(12):956. https://doi.org/10.3390/PR7120956
do Nascimento BF et al (2020) Detoxification of sisal bagasse hydrolysate using activated carbon produced from the gasification of açaí waste. J Hazard Mater 409(November):2021. https://doi.org/10.1016/j.jhazmat.2020.124494
Dong JJ, Han RZ, Xu GC, Gong L, Xing WR, Ni Y (2018) Detoxification of furfural residues hydrolysate for butanol fermentation by Clostridium saccharobutylicum DSM 13864. Bioresour Technol 259(February):40–45. https://doi.org/10.1016/j.biortech.2018.02.098
Ruiz HA et al (2021) Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour Technol 342(August):2021. https://doi.org/10.1016/j.biortech.2021.125961
Tian S, Zhang H, Fu S (2022) Improvement of xylo-oligosaccharides dissolution from Caragana korshinskii through liquid hot water pretreatment with tiny choline chloride. Ind Crops Prod 176(December 2021):114418. https://doi.org/10.1016/j.indcrop.2021.114418
Fayet A, Teixeira ARS, Allais F, Bouix M, Lameloise ML (2018) Detoxification of highly acidic hemicellulosic hydrolysate from wheat straw by diananofiltration with a focus on phenolic compounds. J Memb Sci 566(April):112–121. https://doi.org/10.1016/j.memsci.2018.08.045
Liu Z, Natalizio F, Dragone G, Mussatto SI (2021) Maximizing the simultaneous production of lipids and carotenoids by Rhodosporidium toruloides from wheat straw hydrolysate and perspectives for large-scale implementation. Bioresour. Technol. 340(May):125598. https://doi.org/10.1016/j.biortech.2021.125598
Arminda M, Josúe C, Cristina D, Fabiana S, Yolanda M (2021) Use of activated carbons for detoxification of a lignocellulosic hydrolysate: statistical optimisation. J Environ Manage 296(July):113320. https://doi.org/10.1016/j.jenvman.2021.113320
Sherwood J (2019) The significance of biomass in a circular economy. Bioresour Technol 300(November):2020. https://doi.org/10.1016/j.biortech.2020.122755
Rajesh Banu J et al (2021) Lignocellulosic biomass based biorefinery: a successful platform towards circular bioeconomy. Fuel 302(May):121086. https://doi.org/10.1016/j.fuel.2021.121086
Steiger MG et al (2011) Transformation system for hypocrea jecorina (trichoderma reesei) that favors homologous integration and employs reusable bidirectionally selectable markers. Appl Environ Microbiol 77(1):114–121. https://doi.org/10.1128/AEM.02100-10
Lindemann M, Friedl A, Srebotnik E (2017) Enhanced cellulose degradation of wheat straw during aqueous ethanol organosolv treatment. BioResources 12(4):9407–9419. https://doi.org/10.15376/biores.12.4.9407-9419
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars , byproducts, and degradation products in liquid fraction process samples laboratory analytical procedure ( LAP ) Issue Date : 12 / 08 / 2006 Determination of Sugars , Byproducts, and Degradation Products in Liquid Fraction Proce. National Renewable Energy Laboratory
Chen WH et al (2021) Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: a review. Bioresour Technol 344(September):2022. https://doi.org/10.1016/j.biortech.2021.126207
Kim JH et al (2021) Production of succinic acid from liquid hot water hydrolysate derived from Quercus mongolica. Biomass and Bioenergy 150(December 2020):106103. https://doi.org/10.1016/j.biombioe.2021.106103
Weingart E, Tschirner S, Teevs L, Prüße U (2018) Conversion of fructose to HMF in a continuous fixed bed reactor with outstanding selectivity. Molecules 23(7):1802. https://doi.org/10.3390/molecules23071802
Camia A et al (2018) Biomass production, supply, uses and flows in the European Union. First results from an integrated assessment. Joint Research Centre, Ispra, Italy
Zhou M, Lü X (2021) Strategies on simultaneous fermentation of pentose and hexose to bioethanol. Elsevier
Zhang B et al (2021) High temperature xylitol production through simultaneous co-utilization of glucose and xylose by engineered Kluyveromyces marxianus. Biochem Eng J 165(September 2020):107820. https://doi.org/10.1016/j.bej.2020.107820
Jo S, Yoon J, Lee SM, Um Y, Han SO, Woo HM (2017) Modular pathway engineering of Corynebacterium glutamicum to improve xylose utilization and succinate production. J Biotechnol 258:69–78. https://doi.org/10.1016/j.jbiotec.2017.01.015
Ribeiro Correa T, Barreto Roman J, Vieira dos Santos L, Guimaraes Pereira G (2021) Secretome analysis of Trichoderma reesei RUT-C30 and Penicillium oxalicum reveals their synergic potential to deconstruct sugarcane and energy cane biomasses. Microbiol Res no. Ii:1–31. https://doi.org/10.1016/j.micres.2022.127017
National Center for biotechnology information, “PubChem Database,” 2019. [Online]. Available: https://pubchem.ncbi.nlm.nih.gov/compound/Erythritol. [Accessed: 17-Jan-2020].
Cavka A, Jornsson LJ (2014) Comparison of the growth of filamentous fungi and yeasts in lignocellulose-derived media. Biocatal Agric Biotechnol 3(4):197–204. https://doi.org/10.1016/j.bcab.2014.04.003
Guarnieri MT, Ann Franden M, Johnson CW, Beckham GT (2017) Conversion and assimilation of furfural and 5-(hydroxymethyl)furfural by Pseudomonas putida KT2440. Metab Eng Commun 4(December 2016):22–28. https://doi.org/10.1016/j.meteno.2017.02.001
Li H, Chen H (2008) Detoxification of steam-exploded corn straw produced by an industrial-scale reactor. Process Biochem 43(12):1447–1451. https://doi.org/10.1016/j.procbio.2008.05.003
Acknowledgements
The authors want to acknowledge the TU Wien and the Institutional Open Access Program (IOAP) for financial support (Open Access Funding provided by TU Wien).
Funding
Open access funding provided by TU Wien (TUW). The financial support for the development of this research was provided by the Christian Doppler Research Association, the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development, and Conzil Estate GmbH.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.D-S, S.S-L, A.R.M-A, R. M, and A.F; investigation, L.D-S (Sections 3.1, 3.2, and 3.4), A. M (Sections 3.3 and 3.4), J. P (Sections 3.3 and 3.4), and G. S (Sections 3.3 and 3.4); formal analysis, L.D-S; funding acquisition, A.R.M-A; writing original draft: L.D-S. All authors contributed to the review and editing of the manuscript. All authors have read and agrees to the published version of the manuscript. This material is the author’s own original work. It has not been previously published elsewhere.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
Conzil Estate GmbH filed a patent (EPO application no. 21 216 448.7) related to the content of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Daza-Serna, L., Masi, A., Serna-Loaiza, S. et al. Detoxification strategy of wheat straw hemicellulosic hydrolysate for cultivating Trichoderma reesei: a contribution towards the wheat straw biorefinery. Biomass Conv. Bioref. 13, 16495–16509 (2023). https://doi.org/10.1007/s13399-023-04099-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04099-8