Abstract
Effective in-site treatment of medical waste has become a weak link in hospitals. Pyrolysis technology is a treatment method for medical waste that can enable rapid disposal in hospital settings and relieve environmental pressure, while also producing high-value products and reducing disposal costs. In this work, the effects of feedstock ratio and temperature on product yield and components of gauze (GA) and medical bottles (MB) co-pyrolysis have been investigated. The higher yield of solid products was obtained by co-pyrolysis of GA and MB at 400 ℃. With the addition of MB and an increase in temperature for the co-pyrolysis of GA and MB in a similar ratio, the pyrolysis oil and gas yields gradually increased. According to GC–MS analysis, co-feeding 75% MB to GA improved the alcohol content from 33.21% to a maximum yield of 59.8% at a pyrolysis temperature of 700 ℃. The content of aliphatic hydrocarbon reached 38.68% when the pyrolysis temperature and MB addition ratio were 700 °C and 75%, respectively. The GC data shows that the main gas components of co-pyrolysis of GA/MB were CH4 and H2, while the pyrolysis of pure GA or MB resulted in CO or CO2. Additionally, the solid carbon products obtained have an excellent pore structure. This strategy can benefit medical waste control and resource utilization for the low-cost disposal of medical waste and the acquisition of high-value resource products.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, with the development of the social economy, the improvement of living conditions, and the increase in medical institutions or visits, a growing number of medical plastic waste has been generated [1,2,3]. In particular, there were about 817,000 tons of medical waste generated in 200 large and medium-sized cities in China in 2018 [4]. On the one hand, medical waste is different from other urban garbage due to its infectious, toxic, and other hazards as it carries a lot of germs [5]. Therefore, germs and viruses must be eliminated during treatment to avoid their pathogenicity. On the other hand, epidemics such as the coronavirus disease (COVID-19) in 2019 have been frequent in recent years [6]. Medical waste increases rapidly in a short time and piles up in the hospital area. It needs to be transported out for treatment over a long distance, which greatly increases the risk of secondary infection for medical staff. Hence, it is urgent to find a clean, safe, rapid, and suitable method for hospitals to dispose of medical waste in situ.
At present, the widely used disposal and treatment methods for medical waste are incineration, secure landfill, and chemical disinfection [7]. These technologies can achieve the harmless treatment of medical waste in a short period of time and also have significant economic advantages and wide applicability [8, 9]. Still, each technology has its drawbacks. The incineration process produces some toxic metals, toxic gases, and dioxins, which can pose a serious threat to human health and the environment [10]. Secure landfill and chemical disinfection cannot achieve waste reduction, and a lot of land space is occupied [11]. Moreover, these technologies are difficult to apply widely in situ, in urban districts, and in an emergency due to a series of problems such as site requirements, technical restrictions, or environmental pollution [12]. The adaptation of the portable application of these techniques has been investigated in recent years. At the same time, this was the weak link of the hospital. The clean and safe pyrolysis treatment technology has attracted the attention of various fields. It is mainly associated with the production of high-value-added products, waste reduction, and environmental friendliness [13, 14]. In particular, it is more suitable for hospitals to deal with medical waste due to its advantages of small area, high efficiency, and low environmental pollution. For example, China Zhejiang ECO Environmental Protection Technology Co., Ltd is a company that develops pyrolysis technology and equipment. Many cases have also been developed and run, such as the “Municipal sewage plant biochemical sludge pyrolysis and carbonization treatment project” and the “Hazardous organic waste recycling disposal project,” etc. The World Health Organization (WHO) requirements state that hazardous waste should be treated and disposed of as close to its source as possible. The application of pyrolysis technology not only achieves the harmless, reduced, and resourceful treatment of medical waste but also facilitates the on-site treatment of medical waste in the hospital [15, 16].
Given its ability to produce various high-value products, such as liquid oil, syngas, and char, the cost of pyrolysis of medical waste has been greatly reduced [17]. The liquid oil and syngas can be upgraded into fuels and chemical feedstock by chemical means [18, 19]. Pyrolysis char can be used as fuel for power generation due to its high carbon content and calorific value [20]. Additionally, pyrolysis char can also be used as adsorbents, fertilizer, and storage materials in agricultural and industrial applications due to its excellent compact and porous microstructure [21, 22]. The quality of pyrolysis products is highly dependent on feedstock characteristics and temperature parameters [23]. In particular, the complex components and unbalanced proportion of medical waste significantly affect the pyrolysis process and the distribution of products [24]. In fact, biomass and plastic products account for the largest proportion of medical waste components, more than 60%. Gauze and medical bottles account for the largest proportion of biomass and plastic, respectively. Many studies have illustrated that co-pyrolysis of various feedstocks has a remarkable promotion on the product’s quality or pyrolysis efficiency [25]. Fahim Ullah et al. [4] conducted mixed co-pyrolysis of five kinds of medical waste and reported that the components of medical waste pyrolysis oil were mostly hydrocarbons or oxygenates. The co-pyrolysis of biomass and plastic had a synergistic effect, which was mainly reflected in the fact that the active free radicals generated by gauze (biomass) pyrolysis can promote the fracture of C–H bonds in medical bottles (plastic). Meanwhile, more hydrogen will be provided by plastics with higher hydrogen content than biomass during co-pyrolysis [26, 27]. Zhou et al. [28] found that polyvinyl chloride and tissue paper have more significant synergies, which inhibit the production of alkyls and alkenes. In addition, the pyrolysis temperature not only affects the product distribution but also affects the cost of pyrolysis. Miskolczi. [29] investigated the co-pyrolysis of different waste at 400 ℃, 450 ℃, and 500 ℃, and found that 500 ℃ and a high proportion of high-density polyethylene (HDPE) could improve the quality level of pyrolysis oils. However, few studies have linked the quality of medical waste pyrolysis products with pyrolysis temperature and feedstock (biomass and plastics types) ratio.
In this work, the effect of operational temperature and feedstock ratio on the products for co-pyrolysis of typical biomass (gauze) and plastics (medical bottle) from medical waste was investigated for the first time. The study aimed to quantify and characterize the syngas, liquid oil, and char products in terms of their micromorphology and components. The relationship between the optimum pyrolysis temperature, the ratio of feedstock, and the quality of pyrolysis products was determined. This work shows a new opportunity for medical waste disposal on-site and provides a theoretical reference for the low-cost and commercial application of medical waste pyrolysis treatment.
2 Materials and methods
2.1 Materials
The GA and MB materials from medical waste were used as biomass and plastic materials, respectively. The materials were obtained from the Reproductive and Genetic Hospital Citic Xiangya in Changsha, Hunan province. Since gauze and medicine bottle samples can contain pathogenic and infectious bacteria and viruses, only unused gauze and medicine bottles were used as experimental material. Before the experiment, all materials were cut into 2–3-mm blocks with scissors. GA and MB were then mixed evenly in target mass proportions (0%, 25%, 50%, 75%, and 100%) of MB in the feedstock.
2.2 Experimental setup and procedure
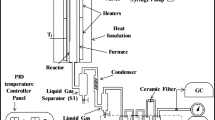
The co-pyrolysis experiments were carried out in a fixed-bed horizontal reactor, as shown in Fig. 1. In each run, a 10 g sample (GA or a mixture of GA and MB) was placed in a quartz boat, and nitrogen gas (N2) was injected for 10 min to remove the air from the pipe. The reactor was operated at temperatures of 400, 500, 600, and 700 °C with a carrier gas of N2 at a flow rate of 100 ml/min. The pyrolysis volatiles passed through a condensate tank, and the condensed gas was collected in an oil collector. Ice water baths and moisture traps were used to remove tar and moisture from non-condensable gases. The purified gas was then collected in a 40-L air bag at the end of the process. The GA and MB were pyrolyzed at different proportions and temperatures, which were denoted as A-B. Here, A represents the pyrolysis temperature, and B represents the proportion of MB in the feedstock. All co-pyrolysis experiments were repeated three times for accuracy.
2.3 Analytical methods and product characterizations
The thermal decomposition process of GA or MB was analyzed using a thermogravimetric analyzer (TGA). Before the actual experiments, a blank test was conducted to avoid systematic errors. To ensure that the error range was ± 2%, the TG test was replicated in triplicate. For each test, a 10-mg sample was heated from 30 to 800 °C at a heating rate of 10 °C/min and a constant N2 flow rate of 100 ml/min. Oil product components were analyzed using a GC–MS (Agilent, 6890N/5975B, America) equipped with a capillary column and compared with the internal library (NIST database). The test parameters were as follows: initial temperature of 50 °C, ramping rate of 5 °C/min, injection temperature of 250 °C, ion source temperature of 200 °C, interface temperature of 250 °C, and split ratio of 30. The gas composition of the permanent gas was analyzed by GC-TCD (GC-9160, Shanghai Ouhua, China). Channel A with molecular sieve 5A was used to measure H2, CH4, and CO, and channel B with packed column TDX-01 was used to measure CO2. Additionally, the pyrolysis char was characterized by a series of methods such as Brunauer–Emmett–Teller (BET) specific surface area analysis, X-ray diffraction spectrometer (XRD), and Fourier transform infrared spectra (FTIR). The BET equation was used to determine the specific surface area of solid products, and the pore size distribution was calculated by the BJH method. The high calorific value (HHV) of the solid produced was measured by an oxygen-bomb calorimeter.
3 Results and discussion
3.1 Thermogravimetric analysis
The TG and DTG curves for different ratios of MB and GA blends are shown in Fig. 2. As shown in Fig. 2a, the significant difference between the decomposition curves of MB and GA may be due to their different compositions. During the pyrolysis process, the initial decomposition temperature of MB was higher than that of GA, indicating that MB appears to be more stable than GA samples. Particularly, the mass loss of samples below 200 °C during pyrolysis is mainly caused by the release of volatile substances or water [30]. Biomass pyrolysis proceeds in two major stages: devolatilization and char formation. The decomposition temperature sequence of the main components of biomass is as follows: lignin > cellulose > hemicellulose. Generally, hemicellulose starts to decompose first (150–300 ℃), which may be due to its unstable structure. The decomposition temperature of long-chain polymers, similar to cellulose, is between 300–400 ℃ [31]. During the decomposition, lignin is more stable than cellulose, and its degradation temperature reached 400 ℃ [32]. The TG curves of MB and GA intersect at about 440 ℃, which is consistent with previous studies [33]. The TG curve of the two samples pyrolyzed alone showed a single step, whereas the MB/GA mixture showed some fluctuation. When comparing the DTG curves of different samples, two mass loss peaks were observed for the mixture of two samples, whereas one peak was observed for the decomposition of one sample (Fig. 2b. In addition, the DTG characteristic curve of the blend shifted slightly to a lower temperature. There were two partially overlapping peaks in the DTG curve of MB and GA, indicating that there may be a synergistic effect between them. Therefore, it can be found that the addition of MB can significantly improve the pyrolysis performance of GA.
The proximate analysis of GA and MB is shown in Table 1. The GA and MB samples had the highest volatile content of 94.28% and 99.86%, respectively. The fixed carbon content of the GA and MB samples varied between 5.57% and 0.09%. Moreover, the ash content of the two samples reached 0.15% and 0.05% in GA and MB, respectively. It is worth noting that both GA and MB have higher volatile content than other biomass or plastic samples [34]. The volatile content of GA was higher than other biomass such as rice husk or herb residues etc. [30]. As the temperature rose, GA was decomposed more than other biomass. This result was similar to the TG data. Combining the thermogravimetric and industrial analysis results, it can be inferred that GA and MB can be pyrolyzed at a lower temperature to produce considerable liquid and gas products.
3.2 The distribution of co-pyrolysis products
Figure 3 shows the distribution of solid, liquid oil, and gas based on the mass balance. The mass content of MB and GA pyrolysis product distribution was found to be relevant to the mixing ratio and temperature. Pure MB group at the pyrolysis temperature of 700 ℃ produced few solid residues, whereas massive solid products were produced by pyrolysis of pure plastics at low temperature. This may be because it is difficult to pyrolyze plastic at low temperature to produce a large amount of wax residues [35]. The increase in temperature had an inhibiting effect on carbon and promoting effect on volatile products (oil and gas). Previous studies have shown that higher temperatures lead to higher volatile matter production as tar cracking was more likely to occur [36]. The gas yield was highest at 500 ℃, which may be because 500 ℃ is conducive to synergism and promotes bond fracture. In general, the formation of solid char was attributed to lignin in GA, while cellulose or hemicellulose in GA was beneficial to the production of pyrolysis oil and gas [37]. With the increase of MB addition, the liquid oil and gas yields gradually increased, while the char yield gradually decreased. This is because MB has a higher volatile content than GA. Additionally, the solid coke yield obtained in this study does not exclude the contribution of ash. This may be another reason why the solid coke yield with low MB ratio was the highest in all studies. At high temperature, MB can form a large number of hydrogen-free radicals through thermal decomposition, and gradually cause primary depolymerization and secondary cracking reactions to occur, promoting the conversion of oil into gas [38]. Therefore, co-pyrolysis of MB and GA results in a higher gas yield and a lower liquid yield. It is worth noting that the 600–75 and 700–50 ratios had higher gas yields than other ratios, which may be because a similar proportion of GA and MB had a better synergistic effect at higher temperatures [39].
3.3 Pyrolysis oil composition
The chromatogram peak values from the GC–MS analysis of pyrolysis oil were interpreted using the area-normalization method to analyze the pyrolysis oil products. The O-containing compound distributions from each experiment were compared to analyze the reaction process (see Fig. 4a). Alcohol, phenol, aldehyde, ketone, and ester were the major O-containing compounds from the co-pyrolysis of GA and MB, with a small quantity of ether and acid. The alcohol contents from pure GA pyrolysis at 400 ℃ and 500 ℃ reached 48.14% and 31.89%, respectively, while they were almost non-existent at 600 ℃ and 700 ℃. Many alcohols can be obtained by pyrolysis of MB alone at each pyrolysis temperature. More alcohols were produced during the co-pyrolysis process, which may be due to H transfer during the MB chain break, which can easily combine with O in GA. In general, the active hydroxyl from GA was easily combined with the alkyl from MB to form alcohol [40]. Co-pyrolysis of plastics and biomass has been shown to promote the generation of free radicals, especially hydroxyl radicals [41]. With the increase in temperature from 400 to 700 °C during pure GA pyrolysis, the content of phenol increased from 3.76% (400 ℃) and 1.33% (500 ℃) to 16.87% (600 ℃) and 22.24% (700 ℃). With the addition of MB, the content of phenol decreased significantly, which may be because the lignin in GA was the main feedstock of phenol [42]. Increasing the MB ratio leads to an increase in the ester content, reaching 19.87%, 17.66%, and 17.43% for the 400–100, 400–50, and 500–25 groups, respectively. Meanwhile, it can be observed that high temperatures result in a lower ester content, which is attributed to the weakening of the esterification reaction. In addition, aldehydes and ketones also served as the main oxygenated products during pure GA pyrolysis. The content of ketone was lower when the ratio of MB and GA was similar. With the increase of temperature during the co-pyrolysis process, the aldehyde and ketone contents in the pyrolysis oil increased significantly in the 600–0 and 700–0 groups. It was shown that co-pyrolysis and high temperatures promoted the deoxygenation and cracking reaction of oxygen-containing compounds such as phenol, aldehydes, and ketones [43]. Figure 5 shows that only aliphatic hydrocarbons and no aromatic hydrocarbons were found at the 400 ℃ temperature pyrolysis. Some aromatic hydrocarbons were gradually generated at high temperatures, which may be due to the fact that aromatic hydrocarbons are more easily formed at high temperatures. Co-pyrolysis promotes the cyclization of hydrocarbons and alcohols to produce more aromatic hydrocarbons [44]. In particular, the aromatic hydrocarbons present in the 600 ℃ pyrolysis are ubiquitous. The 500–50 group had a higher aromatic hydrocarbon content (6.35%). Compared to other pyrolysis temperatures, the content of aromatic hydrocarbons in the 700–0 and 700–100 groups reached 7.92% and 9.04%, respectively. This may be due to the synergistic effect of co-pyrolysis at high temperatures, resulting in the reduction of aromatic hydrocarbons. The hydrocarbon content increased from 400 to 600 ℃. The formation of hydrocarbons may be due to the polymerization of cyclic monomers caused by radical rearrangement or the dehydroxylation of phenols [27]. The GC–MS results show that the pyrolysis oil contains more long-chain hydrocarbons with chain length content from C5 to over C20 (Fig. 6). Similar hydrocarbon chain patterns have been found in previous studies [45]. In some experiments, the C5-C11 (gasoline component) content in the co-pyrolysis groups was higher than that of GA or MB pyrolysis alone. The 400–25 and 500–25 groups had higher C5-C11 content (47.7% and 37.04%). As a result, it can be found that co-pyrolysis at low temperature promoted the formation of C5–C11. Moreover, a high quantity of C5–C11 components was found in high-temperature pyrolysis of pure GA. This may be due to the instability of GA at high temperatures. This result was similar to that of TGA.
3.4 Pyrolysis gas composition
The permanent gas products produced from the GA and MB mixed samples subjected to co-pyrolysis showed that the produced gaseous products mainly consisted of CO, CO2, CH4, and H2. The generation of gas can be attributed to two factors: (1) Thermal decomposition of waste raw materials, and (2) Secondary pyrolysis of primary pyrolysis products. The influence of different temperatures and MB ratios on gas composition is shown in Fig. 7. Increasing the MB content of the feedstock blends led to a decrease in oxygenated gas such as CO, which may be due to the lower oxygen content in plastics. There was more CO content in low MB ratio compared to the pure MB group, probably due to decarbonylation and decarboxylation reactions from biomass pyrolysis promoting the formation of CO and CO2 [30]. The presence of free radicals or the oxidation reaction between fixed carbon and oxygen inhibited the formation of CO and CO2 as the temperature increased [46]. At low pyrolysis temperatures, there were fewer CH4 and other hydrocarbon gases and more CO and CO2 due to the high content of oxygen in biomass. The formation of carbon oxides could be directly related to the cellulose content of biomass samples [47]. Previous studies have reported that cellulose, hemicellulose, and lignin or their mixed forms produce some CO and CO2 [48]. With an increase in temperature (from 500 to 700 ℃), the interaction of hydrogen radicals is promoted, leading to an increasing trend in the content of H2 [49]. Another reason may be that the fracture and reforming reaction of the benzene ring in lignin causes the lignin in the GA to decompose and produce H2. As the pyrolysis temperature increased, the increased CH4 content was attributed to the demethylation of the methoxy group on the benzene ring in GA, which is consistent with the above conclusion [27]. More H2 content was found in the 400 ℃ pyrolysis, which may be due to the low-temperature pyrolysis producing fewer gas products, resulting in an increase in the H2 volume ratio. It is also clear that employing co-pyrolysis is more effective in obtaining higher H2 yield than pyrolysis alone, which is not conducive to the formation of CH4. Therefore, the highest H2 yield of 500–75, 600–50, and 700–50 was 17.2%, 18.3%, and 23.5%, respectively. The above results indicate that co-pyrolysis of GA and MB exhibited a stronger interaction due to the presence of oxygen, which contributed to the formation of hydrogen or oxygen free radicals [48].
3.5 Characterizations of pyrolysis char materials
The crystal structures of all pyrolysis char were investigated by XRD (Fig. 8). The amorphous structure peaks in XRD patterns of some samples at about 22° and 44° are attributed to the carbon gradually transforming into graphite after pyrolysis [50]. It can be observed from Fig. 8a that there were many different miscellaneous peaks that differed from carbon in the 25, 50, and 75 groups. The peak patterns of these groups were very similar, with several peaks resulting from waxes from incomplete decomposition of MB. Compared with pyrolysis char at 400 °C, there was an apparent increase in amorphous graphite peaks of pyrolysis char at 500 °C, 600 °C, and 700 °C [51]. The XRD patterns of pyrolysis char at higher temperatures seem to have a stronger amorphous graphite peak than those at lower temperatures based on the broad peak at 2θ = 22° of XRD curves. Besides, the intensity of this peak decreased with the increase of MB content, which also indicated a decrease in the char content.
The evaluation of the FTIR spectra of the corresponding pyrolysis char from co-pyrolysis of GA and MB at different temperatures is shown in Fig. 9. For all pyrolysis char samples, the main broadband at 3450 cm−1 represents the stretching vibration of –OH. The peaks at 1580 and 1429 cm−1 were assigned to the C = O or C = C stretching vibration, which became stronger at higher GA content. There were stronger –OH peaks at higher temperature, which may be because high temperature leads to the important carbonization behavior of organic matter in GA [52]. The GA content and temperature have significant effects on pyrolysis char products, which leads to the generation of oxygenated functional groups. A large amount of wax in solid products leads to very weak stretching vibrations of –OH at the 400–50 and 400–75 groups. Meanwhile, it also exhibits the stronger C–H stretching vibration of plastics at 2920, 2856, and 1490 cm−1 [53]. The C–H vibrations disappear at 700 °C, which may be because the solid products were mainly char rather than waxy carbon-hydrogen polymer. The FTIR spectra results show that the carbon materials obtained have rich surface functional groups.
As shown in Table 2, the BET surface area of all samples increased significantly as the co-pyrolysis ratio of MB and GA increased, which could be explained by the release of a large amount of volatile matter from the feedstock that greatly contributed to the surface area. When the ratio of MB and GA feedstock was 1:1, the BET surface area was the highest, and the average pore size was the lowest. This may be because of the collapse of the biochar structure and the accumulation of residue, accompanied by the continuous release of volatile matter at low pyrolysis temperatures. The surface area and average pore size of 75 wt% MB at high temperatures (600 ℃ and 700 ℃) exhibited higher surface area and lower average pore size. A well-developed porous structure was formed at higher pyrolysis temperature, which may be due to more volatile substances escaping from the pyrolysis biochar [54]. Additionally, the average pore size was smaller when the specific surface area was high. As the pore diameter collapses during pyrolysis, smaller pores appeared [55]. Meanwhile, it was confirmed that co-pyrolysis and temperature promoted the formation of the porous structure in mixed biochar. In addition, the HHV of solid char formed by the co-pyrolysis of GA and MB increased with temperature (Table 3). From 400 to 700 ℃, the HHV of solid char increased from 19.2 to 21.3 MJ/kg. The higher calorific value of 400–50 may be due to the presence of wax in the solid products. The research on calorific value reflected the potential for combustion utilization.
3.6 Possible co-pyrolysis pathways of GA and MB
According to the product distribution and composition of pyrolysis oil, gas, and pyrolysis carbon of GA and MB at different temperatures or mixing ratios, possible co-pyrolysis paths of GA and MB were reasonably speculated (Fig. 10). The co-pyrolysis process of GA and MB was extremely complex and can be roughly divided into two stages. Firstly, GA and MB start to thermally decompose and break chemical bonds during the temperature increase. At this stage, the C–C bond in the MB structure is particularly prone to breaking. Structural bond breaking between cellulose, hemicellulose, and lignin components in GA is also common. Secondly, components from GA and MB decomposition fuse with each other to form junior components. Meanwhile, the hydroxyl radical generated from GA combines with the hydrogen radical and alkyl radical generated from MB to form new components. The various free radicals of MB are obtained at higher temperatures than GA, which may be due to MB having a higher decomposition temperature according to the TG curve.
Various gas, liquid, and solid products are generated after the formation of junior components and free radicals. The feedstock (GA/MB mixed) and temperature have a considerable impact on this process. The intermediate alkyl radical from GA low-temperature cracking reacts with hydroxyl to form alcohols, while high-temperature cracking favors the cyclization of hydrocarbons and alcohols to produce phenol. The thermal decomposition of MB tends to form linear alcohols and aliphatic hydrocarbons, probably due to its polymer linear chain structure. In addition, more free radicals and primary components are involved in the co-pyrolysis process of GA and MB, which may have a synergistic effect and promote more reactions, such as Diels–Alder, esterification, cyclization, or decarboxylation, conducive to the formation of alcohols and aliphatic hydrocarbons. High pyrolysis temperature on pure MB and GA/MB mixed group produces more aromatic hydrocarbon content, possibly because high temperature promotes the aromatization of MB. High-temperature co-pyrolysis promotes the generation of H2 and CH4, while single-component pyrolysis facilitates the generation of CO (GA) or CO2 (MB). The formation of char generally comes from the lignin decomposition in GA. The solid product carbon obtained from co-pyrolysis has a more excellent pore structure, making it more suitable for the application of adsorption materials. It is possible that the low phenol content in the co-pyrolysis product results in some aromatic structures in GA being transferred to the coke.
In summary, liquid, gas, and char are the main products of GA and MB co-pyrolysis. The diversity of pyrolysis products proves that the degradation rate of medical waste is high. The pyrolysis performance of co-pyrolysis and middle temperature of GA and MB appeared to be better than the others. However, the selectivity of products is difficult to control, such as unstable hydrocarbon content, low hydrogen content, and small specific surface area of carbon. Therefore, the reaction selectivity of GA and MB needs appropriate catalysts to match the corresponding co-pyrolysis ratio and temperature.
4 Conclusion and future research directions
4.1 Conclusion
To improve the economic viability and sustainability of pyrolysis treatment of medical waste, this study proposes a co-pyrolysis process of typical medical waste. Specifically, GA and MB in medical waste were used as biomass and plastics, respectively. This paper explores co-pyrolysis parameters, such as feedstock ratios and pyrolysis temperature, for the co-production of high-quality pyrolysis oil, char, and pyrolysis gas, and investigates their production pathways. The product distribution analysis reveals a synergistic effect on a distinct enhancement of total gas yields, especially for the 600–75 and 700–50 groups. This result was contributed by the depolymerization and secondary cracking of MB, which promotes the formation of free radicals, and consequently converts oil into gas. The co-pyrolysis is distinguished by the generation of aldehydes and ketones in the pyrolysis oil, while the content of alcohol yield increases. High temperature is conducive to the release of aromatic hydrocarbons. The content of CH4 and H2 increases under the conditions of higher MB ratio or high pyrolysis temperature, while pyrolysis alone produces more CO. Additionally, the char prepared from co-pyrolysis of GA and MB has a better pore structure. The characterizations of gas, pyrolysis oil, char, and investigation of blend ratio, pyrolysis temperature, and reaction pathway can provide theoretical and practical insights into how to generate clean energy and dispose of medical waste efficiently from the co-pyrolysis of GA and MB.
4.2 Challenges and prospects
Many countries worldwide have declared a goal of non-incineration treatment of medical waste. Therefore, this work is in line with the direction of social development. However, this work still has the following limitations: (1) the utilization and outlet of solid, gas, and liquid products; (2) at present, the research has only focused on clean gauze and medical bottles, and some other pollutants, such as blood, drugs, and microorganisms in the co-pyrolysis process, have not been considered; (3) lack of consideration of economic costs. Based on pyrolysis technology, combined with economic and environmental perspectives, may be a promising direction for the comprehensive utilization of medical waste may be a promising direction.
Data availability
The data and materials during the current study are available from the corresponding author on reasonable request.
References
Kargar S, Pourmehdi M, Paydar MM (2020) Reverse logistics network design for medical waste management in the epidemic outbreak of the novel coronavirus (COVID-19). Sci Total Environ. 746:141183. https://doi.org/10.1016/j.scitotenv.2020.141183
Patricio Silva AL, Prata JC, Walker TR, Campos D, Duarte AC, Soares A et al (2020) Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci Total Environ. 742:140565. https://doi.org/10.1016/j.scitotenv.2020.140565
Mazzei HG, Specchia S (2023) Latest insights on technologies for the treatment of solid medical waste: a review. J Environ Chem Eng. 11(2). https://doi.org/10.1016/j.jece.2023.109309
Ullah F, Zhang L, Ji G, Irfan M, Ma D, Li A (2022) Experimental analysis on products distribution and characterization of medical waste pyrolysis with a focus on liquid yield quantity and quality. Sci Total Environ. 829:154692. https://doi.org/10.1016/j.scitotenv.2022.154692
Lee S, Kim T, Lee E, Lee C, Kim H, Rhee H et al (2020) Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med 180(11):1447–1452. https://doi.org/10.1001/jamainternmed.2020.3862
Klemes JJ, Fan YV, Tan RR, Jiang P (2020) Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew Sustain Energy Rev. 127:109883. https://doi.org/10.1016/j.rser.2020.109883
Qin L, Han J, Zhao B, Wang Y, Chen W, Xing F (2018) Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J Anal Appl Pyrol 136:132–145. https://doi.org/10.1016/j.jaap.2018.10.012
Zhao W, van der Voet E, Huppes G, Zhang Y (2008) Comparative life cycle assessments of incineration and non-incineration treatments for medical waste. Int J Life Cycle Assess 14(2):114–121. https://doi.org/10.1007/s11367-008-0049-1
Okunola A A, Kehinde I O, Oluwaseun A, Olufiropo EA (2019) Public and environmental health effects of plastic wastes disposal: a review. J Toxicol Risk Assess. 5(2). https://doi.org/10.23937/2572-4061.1510021
Selvan Christyraj JRS, Selvan Christyraj JD, Adhimoorthy P, Rajagopalan K, Nimita Jebaranjitham J (2021) Impact of biomedical waste management system on infection control in the midst of COVID-19 pandemic. The Impact of the COVID-19 Pandemic on Green Societies. Springer, Germany, 235–62
Windfeld ES, Brooks MS (2015) Medical waste management - a review. J Environ Manage 163:98–108. https://doi.org/10.1016/j.jenvman.2015.08.013
Deng N, Cui W-Q, Wang W-W, Zhang Q, Zhang Y-F, Ma H-T (2014) Experimental study on co-pyrolysis characteristics of typical medical waste compositions. J Central South Univ 21(12):4613–22. https://doi.org/10.1007/s11771-014-2468-4
Chen R, Zhang D, Xu X, Yuan Y (2021) Pyrolysis characteristics, kinetics, thermodynamics and volatile products of waste medical surgical mask rope by thermogravimetry and online thermogravimetry-Fourier transform infrared-mass spectrometry analysis. Fuel. 295. https://doi.org/10.1016/j.fuel.2021.120632
Jung S, Lee S, Dou X, Kwon EE (2021) Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem Eng J 405:126658. https://doi.org/10.1016/j.cej.2020.126658
Rahbar-Shamskar K, Aberoomand Azar P, Rashidi A, Baniyaghoob S, Yousefi M (2020) Synthesis of micro/mesoporous carbon adsorbents by in-situ fast pyrolysis of reed for recovering gasoline vapor. J Clean Prod. 259. https://doi.org/10.1016/j.jclepro.2020.120832
Omoriyekomwan JE, Tahmasebi A, Dou J, Wang R, Yu J (2021) A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process Technol. 214. https://doi.org/10.1016/j.fuproc.2020.106686
Chai Y, Gao N, Wang M, Wu C (2020) H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem Eng J. 382. https://doi.org/10.1016/j.cej.2019.122947
Chireshe F, Collard F-X, Görgens JF (2020) Production of an upgraded bio-oil with minimal water content by catalytic pyrolysis: optimisation and comparison of CaO and MgO performances. J Anal Appl Pyrolysis. 146. https://doi.org/10.1016/j.jaap.2019.104751
Mohan I, Panda AK, Volli V, Kumar S (2022) An insight on upgrading of biomass pyrolysis products and utilization: current status and future prospect of biomass in India. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02833-2
Feng C, Wu H (2018) Synergy on particulate matter emission during the combustion of bio-oil/biochar slurry (bioslurry). Fuel 214:546–553. https://doi.org/10.1016/j.fuel.2017.11.057
Tong Y, McNamara PJ, Mayer BK (2019) Adsorption of organic micropollutants onto biochar: a review of relevant kinetics, mechanisms and equilibrium. Environ Sci: Water Res Technol 5(5):821–838. https://doi.org/10.1039/c8ew00938d
Wei L, Huang Y, Huang L, Li Y, Huang Q, Xu G et al (2020) The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ Res 184:109324. https://doi.org/10.1016/j.envres.2020.109324
Acomb JC, Wu C, Williams PT (2015) Effect of growth temperature and feedstock:catalyst ratio on the production of carbon nanotubes and hydrogen from the pyrolysis of waste plastics. J Anal Appl Pyrol 113:231–238. https://doi.org/10.1016/j.jaap.2015.01.012
Jiang D-H, Satoh T, Tung SH, Kuo C-C (2022) Sustainable alternatives to nondegradable medical plastics. ACS Sustain Chem Eng 10(15):4792–4806. https://doi.org/10.1021/acssuschemeng.2c00160
Uzoejinwa BB, He X, Wang S, El-Fatah Abomohra A, Hu Y, Wang Q (2018) Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: recent progress and future directions elsewhere worldwide. Energy Convers Manage 163:468–492. https://doi.org/10.1016/j.enconman.2018.02.004
Gouws SM, Carrier M, Bunt JR, Neomagus HWJP (2021) Co-pyrolysis of coal and raw/torrefied biomass: a review on chemistry, kinetics and implementation. Renew Sustain Energy Rev. 135. https://doi.org/10.1016/j.rser.2020.110189
Meng M, Meng W, Cheng S, Xing B, Yi G, Zhang C (2022) Effect of pyrolysis temperature on pyrolysis of Camellia oleifera shell. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03317-z
Zhou H, Long Y, Meng A, Li Q, Zhang Y (2015) Interactions of three municipal solid waste components during co-pyrolysis. J Anal Appl Pyrol 111:265–271. https://doi.org/10.1016/j.jaap.2014.08.017
Miskolczi N (2013) Co-pyrolysis of petroleum based waste HDPE, poly-lactic-acid biopolymer and organic waste. J Ind Eng Chem 19(5):1549–1559. https://doi.org/10.1016/j.jiec.2013.01.022
Huang S, Qin J, Chen T, Yi C, Zhang S, Zhou Z et al (2021) Co-pyrolysis of different torrefied Chinese herb residues and low-density polyethylene: kinetic and products distribution. Sci Total Environ 802:149752. https://doi.org/10.1016/j.scitotenv.2021.149752
Park JH, Park YK, Kim YM (2020) Kinetic analysis and catalytic pyrolysis of spent medicinal herb over HZSM-5 and HY. Environ Res. 187:109632. https://doi.org/10.1016/j.envres.2020.109632
Huang S, Su Y, Luo W, He Q, Huang S, Zhou N et al (2021) Kinetic analysis and in-situ no support catalytic pyrolysis product distribution of Chinese herb residue. J Anal Appl Pyrol. 156. https://doi.org/10.1016/j.jaap.2021.105114
Zhang Z, Wang Q, Li L, Xu G (2020) Pyrolysis characteristics, kinetics and evolved volatiles determination of rice-husk-based distiller’s grains. Biomass Bioenergy. 135. https://doi.org/10.1016/j.biombioe.2020.105525
Huang S, Qin J, He Q, Wen Y, Huang S, Li B et al (2021) Torrefied herb residues in nitrogen, air and oxygen atmosphere: thermal decomposition behavior and pyrolytic products characters. Bioresour Technol. 342:125991. https://doi.org/10.1016/j.biortech.2021.125991
Yao D, Li H, Dai Y, Wang C-H (2021) Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem Eng J. 408. https://doi.org/10.1016/j.cej.2020.127268
Wang T, Tang L, Feng Xa, Xu J, Ding L, Chen X (2022) Influence of organic binders on the pyrolysis performance of rice straw pellets. Journal of Analytical and Applied Pyrolysis. 161. https://doi.org/10.1016/j.jaap.2021.105366
Zhang C, Zhang Z, Zhang L, Zhang H, Wang Y, Hu S et al (2020) Pyrolysis of herb waste: effects of extraction pretreatment on characteristics of bio-oil and biochar. Biomass Bioenergy. 143. https://doi.org/10.1016/j.biombioe.2020.105801
Xu D, Yang S, Su Y, Shi L, Zhang S, Xiong Y (2021) Simultaneous production of aromatics-rich bio-oil and carbon nanomaterials from catalytic co-pyrolysis of biomass/plastic wastes and in-line catalytic upgrading of pyrolysis gas. Waste Manag 121:95–104. https://doi.org/10.1016/j.wasman.2020.12.008
Hassan H, Lim JK, Hameed BH (2016) Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour Technol 221:645–655. https://doi.org/10.1016/j.biortech.2016.09.026
Ojha DK, Vinu R (2015) Fast co-pyrolysis of cellulose and polypropylene using Py-GC/MS and Py-FT-IR. RSC Adv 5(82):66861–66870. https://doi.org/10.1039/c5ra10820a
Rahman MH, Bhoi PR, Saha A, Patil V, Adhikari S (2021) Thermo-catalytic co-pyrolysis of biomass and high-density polyethylene for improving the yield and quality of pyrolysis liquid. Energy. 225. https://doi.org/10.1016/j.energy.2021.120231
Bu Q, Lei H, Ren S, Wang L, Holladay J, Zhang Q et al (2011) Phenol and phenolics from lignocellulosic biomass by catalytic microwave pyrolysis. Bioresour Technol 102(13):7004–7007. https://doi.org/10.1016/j.biortech.2011.04.025
Luo W, Wang T, Zhang S, Zhang D, Dong H, Song M et al (2022) Catalytic co-pyrolysis of herb residue and polypropylene for pyrolysis products upgrading and diversification using nickel-X/biochar and ZSM-5 (X = iron, cobalt, copper). Bioresour Technol. 349:126845. https://doi.org/10.1016/j.biortech.2022.126845
Xu F, Wang B, Yang D, Hao J, Qiao Y, Tian Y (2018) Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: pyrolysis behaviors and kinetic analysis. Energy Convers Manage 171:1106–1115. https://doi.org/10.1016/j.enconman.2018.06.047
Ates F, Miskolczi N, Borsodi N (2013) Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Bioresour Technol 133:443–54. https://doi.org/10.1016/j.biortech.2013.01.112
Zhao Y, Cao H, Yao C, Li R, Wu Y (2020) Synergistic effects on cellulose and lignite co-pyrolysis and co-liquefaction. Bioresour Technol 299:122627. https://doi.org/10.1016/j.biortech.2019.122627
Duangchan A, Samart C (2008) Tertiary recycling of PVC-containing plastic waste by copyrolysis with cattle manure. Waste Manag 28(11):2415–2421. https://doi.org/10.1016/j.wasman.2007.12.010
Faleeva YM, Lavrenov VA, Zaichenko VM (2022) Investigation of plant biomass two-stage pyrolysis based on three major components: cellulose, hemicellulose, and lignin. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03385-1
Burra KG, Gupta AK (2018) Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl Energy 211:230–236. https://doi.org/10.1016/j.apenergy.2017.10.130
Zhao B, Xu X, Xu S, Chen X, Li H, Zeng F (2017) Surface characteristics and potential ecological risk evaluation of heavy metals in the bio-char produced by co-pyrolysis from municipal sewage sludge and hazelnut shell with zinc chloride. Bioresour Technol 243:375–383. https://doi.org/10.1016/j.biortech.2017.06.032
Zhao B, Xu X, Zhang R, Cui M (2021) Remediation of Cu(II) and its adsorption mechanism in aqueous system by novel magnetic biochar derived from co-pyrolysis of sewage sludge and biomass. Environ Sci Pollut Res 28(13):16408–16419. https://doi.org/10.1007/s11356-020-11811-y
Li C, Huang Q, Zhang H, Wang Q, Xue R, Guo G et al (2021) Characterization of biochars produced by co-pyrolysis of Hami melon (cantaloupes) straw mixed with polypropylene and their adsorption properties of cadmium. Int J Environ Res Public Health. 18(21). https://doi.org/10.3390/ijerph182111413
Wang P, Zhang J, Shao Q, Wang G (2018) Physicochemical properties evolution of chars from palm kernel shell pyrolysis. J Therm Anal Calorim 133(3):1271–1280. https://doi.org/10.1007/s10973-018-7185-z
Liu J, Yang X, Liu H, Jia X, Bao Y (2021) Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: co-pyrolysis characteristics and its adsorption capability. Chemosphere. 282:131116. https://doi.org/10.1016/j.chemosphere.2021.131116
Wang H, Wang X, Cui Y, Xue Z, Ba Y (2018) Slow pyrolysis polygeneration of bamboo (Phyllostachys pubescens): product yield prediction and biochar formation mechanism. Bioresour Technol 263:444–449. https://doi.org/10.1016/j.biortech.2018.05.040
Funding
The authors gratefully acknowledge funds from the National Natural Science Foundation of China (Grant 51974123), the Distinguished Youth Foundation of Hunan Province (Grant 2020JJ2018), the Key R&D Projects in Hunan Province (2020WK2016, 2020SK2032, 2021SK2047, and 2022NK2044), the Natural Science Foundation of Hunan Province, China (Grant 2021JJ40261), the Hunan High Level Talent Gathering Project (2019RS1077, 2020RC5007, 2020RC3051), the Scientific Research Fund of Hunan Provincial Education Department (19C0903), the Earmarked fund for China Agriculture Research System (CARS-01–27), Hunan Provincial Key Laboratory of Crop Germplasm Innovation and Resource Utilization Science Foundation (19KFXM12), On 2022 cotton counties, strict control area planting cotton demonstration counties (piece) cotton science and technology innovation services and socialization service demonstration project (Xiang Agricultural Federation [2022] No. 42), thanks are due to Michael henke for assistance with language modification.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Li Li: Conceptualization, Writing—original draft. Zhaoguang Chen: Resources. Zhenhao Guo: Investigation. Hang Dong: Data curation. Yu Xie: Software. Nan Zhou: Writing—review and editing. Zhi Zhou: Supervision. All authors have read and approved the final manuscript before submission.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Chen, Z., Huang, Y. et al. Investigation of gauze and medical bottle co-pyrolysis on the product formation, reactivity, and reaction pathway of char, liquid oil, and gas. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04006-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04006-1