Abstract
Crude glycerol (CG) and glycerol pitch (GP) are highly alkaline residues from biodiesel and oleochemical plants, respectively, and have organic content which incurs high disposal cost and poses an environmental threat. Characterization of these residues for composition and properties could provide insight into their quality for proper disposal and can help the biodiesel industry to adopt more sustainable practices, such as reducing waste and improving the efficiency of the production process, hence minimizing the impact of the biodiesel supply chain to the environment. These data also allow the identification and exploration of new ways for their utilization and transformation into highly value-added products. In this study, we evaluated four CG samples (B, C, D, and E) and two GP samples (F and G) obtained from Malaysian palm oil refineries, and the results were compared with pure glycerol (A). Spectroscopic analysis was performed using FTIR, 1H-, and 13C-NMR. All samples had similar density to A (1.26 g/cm3), except for F (1.31 g/cm3), while the density for E and G could not be determined due to their physical states. The pH and viscosity largely varied in the range of 7.26–11.89 and 43–225 cSt, respectively. The glycerol content of CG (B, C, D, and E) was high and consistent (81.7–87.3%) whereas GP F and G had 71.5 and 63.9% glycerol content, respectively. Major contaminants in CG and GP were water and matter organic non-glycerol (MONG), respectively. The water, ash, soap, and salt content were considerably low, which varied from 3.4 to 14.1%, 3.9 to 13.0%, 0.1 to 5.7%, and 4.1 to 9.2% respectively. Thermal analysis of CG and GP exhibited four phases of decomposition attributed to the impurities compared to the single phase in A. All samples had calorific values lower than A (18.1 MJ/kg) between 9.0 and 17.7 MJ/kg. Based on the results, CG and GP have high glycerol content which reveals their potential to be used as feedstock in bioconversion and chemical or thermal treatment while impurities may be removed by pre-treatment if required. As palm oil is one of the main feedstocks for the oleochemical industry, this work underlines the importance of characterization of the residue generated to provide additional data and information on palm-based agricultural industry wastes, minimize the impact of palm oil supply chain on the environment, and explore its potential usage for value-addition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, there has been renewed interest in the transformation of crude glycerol (CG) into value-added products or its direct usage, to address the issue of oversupply of CG. The abundance of CG is driven by one of the most rapidly growing industries in the world, the biodiesel industry, which is a promising alternative energy source due to its renewability and reduction of CO2 emissions compared to fossil fuels. In Malaysia, as of 2021, a total of 18 biodiesel plants based on palm oil feedstock are in operation with an annual processing capacity at 2.33 million tonnes [1].
CG is a by-product of the biodiesel industry with roughly 10 kg of it being produced for every 100 kg of biodiesel (~10%). About 75% of biodiesel is based on vegetable oils, i.e., palm oil (30%), followed by rapeseed oil (25%) and soybean oil (20%). In 2019, the global biodiesel production reached 48 billion liters but contracted in 2020 due to the ongoing COVID-19 crisis. However, in 2021, the renewable energy industry has shown its resilience to the crisis and is expected to grow — though at a much lower pace — supported by higher blending mandates in key markets, development in transportation fleet, and greater demand from consumers [2, 3]. This has further resulted in a decrease in the prices of CG over the years due to its surplus. In 2014, the CG price was US$240/tonne [4] but in 2019, the price declined to US$170/tonne [5].
On top of the availability and low value of CG, there has been extensive interest in this by-product due to the prospect of purification to pure glycerol that has three reactive hydroxyl groups for transformation into highly value-added intermediates, chemicals, and products such as glycerol acetal [6, 7], glycerol ether [8], and glycerol carbonates [9]. Glycerol has found applications in many high value–added industries such as personal care, cosmetics, pharmaceutical, and food industries which require high-purity glycerol as feedstock. However, the high cost of CG refining seems an unappealing option for many biodiesel industry players. This has become a concern as disposing of CG not only incurs additional waste management cost but is also wasteful and causes environmental problems. Therefore, it is essential to explore wiser utilization options such as direct use, direct combustion, or bioconversion to address the growing challenge of surplus CG.
In the cattle industry, glycerol is formulated in the diet of ruminants to prevent ketosis [10]. CG has also been proposed as a potential beneficial energy source for pigs and poultry, but the feedstock source and manufacturing process are the key factors in determining its nutritional values [11]. It was reported that the addition of up to 5% of CG to broiler diets did not have a negative effect on growth performance or carcass yield [12]. However, CG contaminated with methanol can be toxic to poultry. Nevertheless, a study found that methanol content of up to 3.1% in CG was found to give no effect on the performance of broilers [13].
Usage of CG in direct combustion is possible and offers added advantages of energy integration, improved sustainability chain of biodiesel production, and fossil fuel substitution, but this process is hindered by its properties including low energy contents, high viscosity, and high autoignition temperature. The high content of residual inorganic species also makes this process unattractive as this will lead to high particulate emissions [14] and caused furnace contamination by particle coagulation [15]. Another major concern is the possible formation of toxic acrolein. However, Steinmetz and co-workers (2013) showed that the combustion of CG produced a comparable amount of acrolein to those from natural gas combustion. Nonetheless, the combustion of glycerol was found to be viable and the production of acrolein can be avoided in a high-temperature oxy-combustion reactor [16]. The feasibility of the production of renewable energy such as hydrogen from CG has been extensively reviewed and studied [17–20]. It was found that the presence of Na salts of fatty acids (soap) in CG greatly inhibited hydrogen production in the aqueous phase reforming reaction. Furthermore, long-chain fatty acids such as stearic acid in the CG could deactivate the metal catalyst used in the reaction by adsorption on the surface but could be avoided by the addition of activated carbon in the reaction mixture [17]. Integration of waste glycerol into renewable energy production processes is also a practical opportunity [19]. Mixing CG with other fuels was demonstrated to improve fuel properties compared to the individual fuel [21]. Nevertheless, similar to direct combustion, deposits of sediments and sintered products are formed on the heat-exchange surfaces which are dictated by the presence of metals, chlorine, and sulfur. This also led to an increase in the rate of high-temperature corrosion [22]. For this reason, monitoring the content of such elements is important in fuels intended for energy production [23].
In bioconversion, the presence of 5.5% NaCl in CG significantly reduced the conversion into poly(3-hydroxybutyrate) by 10–12% [24]. The impact of contamination present in CG in bioconversion for lipid production had been highlighted by Kumar et al. (2019) [25]. In addition, high soap content (21%) in the CG used as a carbon source had also been reported to inhibit cell growth in the cultivation of Trichosporon oleaginosus for lipid production [26].
The presence of salt in CG was also found to affect chemical reaction catalyzed by the heterogeneous acid catalyst. In the acetalization reaction catalyzed by Amberlyst 15, the conversion was reduced by 25%. In addition, the presence of 15% of water significantly reduced the conversion by nearly 60% whereas zeolite H-beta was found to be less sensitive toward these contaminants [27].
All these examples translated into the importance of the availability of the characteristics and composition information of CG with different sources and manufacturing processes prior to usage. The quality of CG varied depending on the feedstock and process used to produce the biodiesel (Table 1). Typically, it mainly consists of glycerol and impurities including free fatty acid, glyceride, moisture, soap, and ash.
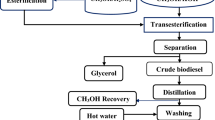
On the other hand, glycerol pitch (GP) is a waste stream generated from the refining of CG that originated from oleochemical and biodiesel production. In the refining process, the undistilled materials will accumulate at the bottom of the vacuum distillation column as residues, which is known as GP. The residues contain a significant amount of useful components including glycerol. GP has been previously studied as a precursor for functionalized carbon [33], and as a binder for roofing tiles [34].
Palm oil, as the most productive and efficient vegetable oil crop, which accounted for 31.6% (76.39 million tonnes) of total global oils and fats produced for 2021 [35] is one of the main feedstocks for the global biodiesel and oleochemical manufacturing industry. It is one of the major contributors to Malaysia’s export revenue, which generated RM108.52 billion in 2021 [1]. Attributed to the surge of biodiesel and oleochemical demand, significant quantities of CG and GP from these industries are also generated which impose challenges in the waste management solutions. An oleochemical plant in Malaysia reported a total GP generation nearly 200 tonnes per month. Currently, glycerol pitch is listed as scheduled waste by the Department of Environment (DOE). Such disposal incurs a significant operation cost between RM1500 and 3500 per tonne. This could reduce the competitiveness of the Malaysian oleochemical industry. Nevertheless, as we have discussed above, these residues could be transformed into value-added products.
Compared to the extensive research on the utilization of these by-products, only a few had reported on their characterization. Although the properties of pure glycerol have been established, limited information is available for CG and GP. To the best of our knowledge, no study dedicated to a comprehensive analysis of palm-based CG and GP obtained from biodiesel and oleochemical route, respectively. The objectives of this study were to obtain the chemical compositional profile, physicochemical, spectroscopy, and thermal characteristics of palm-based CG and GP. This characterization will provide a database for defining these agricultural industry waste for potential transformation into highly value-added products in various applications such as the synthesis of chemicals and polymers, animal feed, and energy.
2 Materials and methods
2.1 Materials
All the samples used in this study were obtained from Malaysia biodiesel or oleochemical industry using palm oil as feedstock: pure glycerol A (Kofa Chemical Works (M) Sdn Bhd, BP/USP grade (99.5%)); all CG and GP samples (B, C, D, E, F, and G) were generously donated by biodiesel and oleochemical manufacturers in Malaysia.
2.2 Physical properties
The density of CG was determined using a DMA 5000 M density meter (Anton Paar, Austria). The homogeneous sample (2 mL) was filled into the measuring cell using a syringe with a Luer tip. The filling was observed through the inspection window to avoid the presence of gas bubbles in the measuring cell. The measurement was repeated three times at 25 °C and reported as the mean value. The viscosity of CG was measured at room temperature according to ASTM D4878–15 using a PMT Tamson NVB Classic (Normalab, France). The selection of different types of capillary viscometer was made according to ASTM D446. Ubbelohde viscometer was purchased from Cannon Instruments (State College, PA). For pH determination, CG (1.00 ± 0.1 g) was diluted in 50 mL of deionized water (DI). The pH of the solution was measured by the SevenEasy pH meter (Mettler Toledo, Switzerland) at room temperature (27 ± 1 °C).
2.3 Glycerol composition
Glycerol content in CG samples was determined according to ASTM Method D6584–17. The analysis was performed by gas chromatography (GC) using an Agilent 7890B GC system equipped with a flame ionization detector (FID) (Agilent Technologies, USA). The GC analysis of the free glycerol content of CG was conducted as follows: weighed CG (40.0–100.0 mg) was acidified by 100 μL 1:1 HCl (v/v) and then dissolved in 10 mL of pyridine in a 15-mL glass test tube (Pyrex, Corning, NJ). Then, an aliquot of the obtained solution and 100 μL of 1,2,4-butanetriol standard solution (0.89 mg/mL) were mixed and derivatized by N-trimethylsilyl-N-methyl trifluoroacetamide (MSTFA) (100 μL) at 38 °C for 15 min. The sample was then filtered and injected at an injection volume of 1 μL into a DB 5HT capillary column (10 m × 0.32 mm × 0.1 μm). Helium was used as the carrier gas at a flow rate of 3 mL/min. The injector and column temperature was ramped from 50 to 110 °C at 5 °C/min with the detector temperature held constant at 380 °C. A calibration curve was constructed by analyzing pure glycerol at different concentration levels. The water content of CG was determined by volumetric Karl Fischer titration using an automatic titration system (Metrohm, Switzerland) with reference to AOCS Official Method Ea 8–58. The measurement was performed in duplicate and reported as an average value. The ash content of CG was determined by burning approximately 1 g of the sample in a furnace at 750 °C for 3 h. The MONG was measured by subtracting the sum of the contents of glycerol, ash, and water based on the standard method (ISO 2464–1973). The soap content was determined with reference to AOCS Recommended Practice Cc 17–95. The soap content was calculated using Eq. (1):
where Vs, volume (mL) of titrant consumed on the titration of the sample; Va, volume (mL) of titrant consumed on the titration of the blank; N, normality, HCl solution; m, mass (g) of CG weighed.
The salt content in glycerol samples was determined according to a reported method [36]. Briefly, 10 mL of glycerin sample was diluted in 250 mL of distilled water. Subsequently, 10 mL of the solution was withdrawn, and 5 drops of the 2 M potassium chromate indicator were added. The solution was titrated with 0.1 M silver nitrate standard (AgNO3) until the color changed from yellow to brown color with precipitation. The analysis was performed in triplicate and reported as average ± standard deviation. The salt content was calculated using Eq. (2):
where Va, volume (mL) of the spent AgNO3 on the titration of the sample; Vb, volume (mL) of the spent AgNO3 on the titration of the blank; [Ag], AgNO3 concentration; V, sample volume (10 mL).
2.4 Spectroscopic analysis
1H- and 13C-NMR spectra were recorded in Fourier transform mode on a JEOL JNM-ECZ600R spectrometer (JEOL Ltd, Japan) operating at a nominal frequency of 600 and 150 MHz, respectively. Samples were dissolved in deuterated dimethyl sulfoxide (DMSO-d6) unless otherwise stated. All spectra were processed using Delta NMR v5.0.5.1 software. Infrared spectra were recorded as neat samples using a Perkin-Elmer Spectrum 100 FT-IR Spectrometer (PerkinElmer, USA). All IR data were manipulated using Spectrum v6.3.5 software.
2.5 Thermal analysis
Thermogravimetric analysis (TGA) was performed in a Perkin Elmer STA 6000 (PerkinElmer, USA), under flowing nitrogen with a heating rate of 10 °C/min from 25–800 °C. Differential scanning experiments were performed with a Perkin Elmer DSC 6000 (PerkinElmer, USA). The sample (ca. 3 mg) was placed in a hermetic aluminum pan that was flushed with high-purity nitrogen at a rate of 50 mL/min during the experiment. The sample was cooled to −50 °C, left to equilibrate at this temperature for 3 min, and then underwent an increase in temperature at 5 °C/min up to 20 °C. The calorific values of glycerol samples were determined using a bomb calorimeter, LECO AC600 (LECO, USA) according to ASTM D5868–07. The analysis of the measurement was performed using TruSpeed® software.
2.6 Elemental analysis
The C, H, N, and S analysis was performed using a CHN628 Analyzer (LECO, USA) according to ASTM D5373-93. The oxygen content (O) was determined from the difference between 100% and the total percentage of C, H, N, and S. The amount of trace elements (Na, K, P, Ca, Mg, and Fe) was determined using the ICP Mass Spectrometer ELAN 9000 (PerkinElmer, USA).
3 Results and discussion
3.1 Physicochemical and composition analysis of CG
In this study, one pure glycerol (99.7%), four CG, and two GP samples obtained from local biodiesel and oleochemical plants were used. All the samples have different physical appearances as can be seen in Fig. 1. Four samples (B, C, D, and F) were in liquid form, whereas samples E and G were in solid and paste form, respectively. The color of the CG and GP samples ranged from clear light brown to very dark brown, nearly black whereas pure glycerol A has a clear colorless appearance. For CG samples, sample E appeared in a wax-like form compared to the liquid form for other samples. This distinct physical appearance is attributed by the catalyst used in the biodiesel production. The biodiesel producer where sample E was obtained employed sodium methoxide (NaOCH3) as a catalyst in their biodiesel production process whereas other producers used a potassium-based catalyst. The sodium soap produced in the process is called “hard” soaps as they solidify at room temperature while potassium soaps are “soft” and appear as a liquid at room temperature [37]. Similarly, Hansen et al. have reported one sample was in solid form out of 11 samples of CG collected from seven Australian biodiesel manufacturers. The paper, however, does not provide any explanation of the difference in the physical state [31]. In contrast to the work by Thompson and He [11] who employed NaOCH3 for biodiesel production in their study, the untreated crude glycerol obtained was not in the solid state. A possible explanation for this might be that, unlike other vegetable oils (like rapessed, canola, soybean, and crambe), palm oil is composed of an appreciable amount of palmitic acid (42%) which is in a solid state at room temperature. Additionally, the composition of sample E is slightly different as compared to other CG samples in this study such as the presence of MONG, lower water content, and high soap content. This is discussed further in the later part.
The physicochemical properties of all samples are listed in Table 2. The density of samples B, C, and D were similar to A while F had the highest density of 1.31 g/cm3. Sample D had a slightly lower density with 1.25 g/cm3. The density of samples E and G could not be determined as they were in paste/solid form. Samples B, C, and D had nearly neutral pH between 7 and 8 whereas samples E, F, and G had pH values higher than 10 because of the existence of residual alkalis from the biodiesel and oleochemical production processes. Low pH was obtained for B, C, and D due to the removal of the residual alkalis by some post-treatments in the biodiesel plants [38]. The viscosity of samples B, C, and D were in the range between 43 and 69 cSt, very much lower than A (225 mPa.s). The viscosity of samples E, F, and G was not able to be determined due to their physical appearances.
The composition of CG and GP is commonly defined by eight components, i.e., glycerol, methanol, water, soap, fatty acid methyl esters (FAMEs), glycerides, free fatty acids (FFAs), and ash. The chemical composition is affected by several factors such as the type of catalyst used in the production process, the efficiency of the transesterification or fat-splitting process, the recovery or post-treatment, and the presence of other impurities in the feedstock. MONG consists of the organic components other than glycerol such as soap, alcohol, free fatty acids, mono-, di-, and triglycerides, and methyl esters. As shown in Table 3, all CG samples had glycerol content higher than 80% which varied less significantly from 81.7 to 87.3% whereas GP samples, F and G, had lower glycerol content of 71.5 and 63.9%, respectively. The glycerol content in CG samples was relatively consistent, averaging 84.7%. This result agrees with Liu et al. who reported 80.5% glycerol content derived from a palm-based biodiesel plant [39]. Low glycerol content in F and G was in accordance with a study by Hazimah et al. who reported between 55 and 65% of glycerol content, where 15–25% of diglycerol was present in the GP [40]. Thompson and He reported the glycerol contents of the crude obtained from the transesterification of seven different oil feedstocks varied from 62.5 to 76.6% [11]. Mothes and co-workers also reported high glycerol content of seven CG samples collected from different biodiesel manufacturing plants in Germany, between 77 and 90% [24]. In contrast, a significant difference in glycerol content in CG collected from different biodiesel plants in Australia was obtained from 38.4 to 96.5% [31]. Soy-based biodiesel also had largely varied glycerol content from 22.9 to 63.0% [38]. These results suggested that the composition of CG from biodiesel production may be influenced by a combination of factors such as feedstock, process, and catalyst employed as well as post-treatment. High and consistent glycerol content gave quite similar density for the CG samples (Table 2). This finding is consistent with the study by Hansen et al. CG samples with glycerol content higher than 74% were shown to have a density in the range of 1.25–1.26 g/cm3 [31]. In general, we can conclude that CG samples with glycerol content above 80% may have a density value close to the pure glycerol. The density, however, will also be influenced by other impurities present in the samples as we can see in the GP sample, F.
For industrial palm-based biodiesel production, multistage batch reactors are used to push the reaction toward the completion whereby glycerol is withdrawn after each stage of the reaction. The glycerol will go through a few purification steps before being stored in a storage tank as CG [41] which explained the consistent quality of CG obtained from Malaysian biodiesel manufacturers.
The water and ash content in the samples ranged from 3 to 14% and from 4 to 13%, respectively. All CG samples had water content as a major contaminant above 10% leading to their low viscosity except E. High water content in CG originated from washing during biodiesel production to remove the catalyst. Of all samples, E and F had the lowest water content, and interestingly, these two samples were supplied by the same refinery. High water content in CG was shown to affect the viscosity of the sample. As we observed, the sample with high water content has lower viscosity (Table 2). The viscosity of the samples is as the following: C > B > D where the water content of the samples from the lowest is 10.13, 12. 04, and 14.13% for C, B, and D, respectively. For GP samples F and G, the contaminant is primarily comprised of MONG followed by ash and water content. The ash content in CG is mainly from the catalyst employed in the transesterification process [11].
All crude samples had low soap content between 0.3 and 5.76%. Sample E had the highest soap content which may be attributed to its wax-like form. This result is in contrast with very high soap content reported for soybean oil–based CG between 20.5 and 31.4% [38] and other sources: 22.4–29.1% [42]. The soap content in CG is highly related to the character of feedstock oil of biodiesel production. The CG with low soap content was normally generated in the biodiesel plant using oil feedstock with low FFA content. The presence of soap in CG can be attributed to an unfavorable side reaction — saponification — during the biodiesel production process, where the FFA in the oil react with sodium or potassium catalyst to form soap. In base-catalyzed reaction, the feedstock should have an FFA content of less than 3% or an acid value of less than 1 mg KOH/g [43] to reduce soap formation which led to drastic product loss and problems associated with product separation and purification [44]. Two common palm-based feedstocks for industrial biodiesel production are crude palm oil (CPO) and refined-bleached-deodorized palm oil (RBDPO). The former is cheaper but requires pre-treatment to reduce the high initial FFA content — ranging from 3 to 6.5% [45] — whereas the latter has low FFA content but is higher in price. In Malaysia, RBDPO is the predominant feedstock for biodiesel production (94.5%) [46] which explained the low soap content in all palm-based glycerol.
The salt content of all samples ranged from 4.1 to 9.2%. Sample F had the highest salt content followed by D, and B with 9.0 and 7.6%, respectively. The average salt content in CG and GP is 6.7 and 6.9%, respectively. These results are slightly higher than other reported salt content around 4% [36, 47]. Based on this result, only samples E and G are suitable feedstock for bioconversion with less than 5.5% of salt content. The formation of salt — commonly between 5 and 7% — is the result of the neutralization of homogeneous alkaline catalyst in biodiesel production. This is uneconomical in the purification of glycerol via classical ion-exchange techniques as the cost for chemical regeneration of the resins becomes exceedingly high. As an alternative, heterogeneous processes could be considered in the biodiesel production to avoid salt formation. Nevertheless, the purification of CG may still be required to remove impurities existing in the natural raw feedstock [48].
The ultimate and trace metal analysis of the CG and GP samples gave an account of any metal/element present. The carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) contents of glycerol samples ranged from 23.9 to 38.2%, from 5.7 to 9.5%, from 0.13 to 0.24%, and from 0.01 to 0.19%, respectively (Table 4).
Except for sample E, all CG had lower carbon content than pure glycerol A (35.7%). Sample F had the lowest carbon content of 23.9%. Thompson and He reported C content between 24.0 and 26.3% in CG from various oil feedstocks except for CG from waste vegetable oil transesterification which had high carbon content (37.7%) [11]. High carbon content in sample E can be attributed to the presence of certain impurities which have higher C content than glycerol such as soaps, FAME, and glycerides [40]. This is supported by high soap content in E (Table 3).
All palm-based CG had low metal content of less than 1 ppm. Sample G had the highest Na element trace, evidenced by the high ash content in G (Table 3). Other elements (K, P, Ca, Mg, and Fe) were detected below 0.05 ppm.
To better understand the differences in palm-based CG and GP with other reported literatures, we summarized the composition and properties of our samples and compared them with selected references [11, 31, 38]. As can be seen in Table 5, the glycerol contents of two GP samples are in the range of glycerol content in CG reported by Thompson and He [11] whereby all the CG reported by Hu et al. have lower glycerol contents [38]. With glycerol content above 80%, the quality of our CG and GP samples can be considered good and consistent as they are less varied compared to others.
The crude glycerol in the study by Thompson and He was obtained from transesterification of six different first-use oil and one waste vegetable oil (WVO) with methanol in the presence of NaOCH3. Based on the results, all the first-use oil had very consistent glycerol content between 62.5 and 67.8% (despite having varied fatty acid composition) whereas WVO had the highest glycerol content. It was reported that the latter contained 4% of FFA which then formed soap during the reaction. Thus, we can conclude here, little variation in the CG quality can be obtained using the same process while the presence of FFA in the feedstock can significantly affect the biodiesel yield and the quality of the CG. This finding is consistent with our results.
In contrast, Hu and co-workers reported significantly varied compositions for three samples of soy-based CG, one WVO, and one soy bean-WVO mixture-based CG obtained from a biodiesel producer. A sample which contained the highest glycerol content (63%) was found to have no detectable levels of organic fraction due to the post-treatment during the production process and partial refining process to remove most of its organic impurities. Three of the samples had low glycerol content in the range of 23 to 33% and were characterized by a high amount of other organic impurities such as soap, FAME, and methanol [38]. Post-treatment and partial refining in the biodiesel process were shown to affect the quality of CG generated, and this could also be the reason for the non-detectable organic content in CG samples B, C, and D (Table 3).
In another study by Hansen, three out of 11 samples had very high glycerol content between 83.4 and 96.5%. Seven samples had glycerol content between 61.1 and 77% whereas only one sample had very low glycerol content (38.4%). The CG samples were collected from seven biodiesel producers in Australia where the major feedstocks are canola, tallow, and used cooking oil [49]. Large variations in the composition and properties of their samples were reported to be depending on the refining process used [31].
3.2 Spectroscopic analysis of CG
The spectroscopic analysis was performed using 1H-, 13C-NMR, and FT-IR. One sextet and two multiplet peaks between 3.40–3.36 ppm and 3.34–3.22 ppm, respectively, were observed in the 1H-NMR spectrum of pure glycerol. The two multiplet peaks can be assigned to the protons (Ha and Hb) on terminal carbons (C1 and C3), while the sextet belonged to the proton (Hc) attached to the secondary carbon (C2) (Fig. 2). The protons of the hydroxyl groups on C2 appeared at 4.47 ppm (d, J = 4.8 Hz), and the protons of two hydroxyls on C1 and C3 were observed at 4.39 ppm (t, J = 5.5 Hz). A strong H2O peak was observed at 3.44 ppm in samples B, C, D, and F. Two signals observed at 63.5 and 72.9 ppm for pure glycerol A in 13C-NMR presented the primary carbon (C1 and C3) and secondary carbon (C2), respectively. Similar spectra were obtained for crude samples B, C, D, and E. The signal at 49.1 ppm in sample E justified the presence of methyl carbon in methanol. The protons of this methyl appeared as two overlapping singlet peaks at 3.13 and 3.12 ppm in the 1H-NMR spectrum while the hydroxyl appeared at 4.08–4.05 ppm. These results are consistent with those reported by Gottlieb and co-workers [50]. The presence of fatty matters demonstrated signals between 0.70 and 2.60 ppm for the aliphatic chain. This is evidenced by the high MONG content in samples F and G. The NMR spectra are supplied in Supplementary Information.
In 13C-NMR, the presence of fatty matters such as mono- and diglyceride in samples F and G was justified by the signals in the range from 10 to 32 ppm for the aliphatic chain whereas peaks for the carbons at glycerol backbone appeared between 60 and 83 ppm [28].
As shown in Fig. 3, in FT-IR spectra, strong and medium peaks can be observed in all samples which can be assigned to the bonding in glycerol molecule: 3300 (–OH stretching), 2934 (CH2 anti symmetry stretching), 2879 (CH2 symmetry stretching), and 1413 cm−1 (CH2 bending). Two strong peaks between 1300 and 1000 cm−1 are characteristics of C–O stretch: 1108 (secondary alcohol C–O stretching) and 1030 cm−1 (primary alcohol C–O stretching). Peaks at 993, 851, and 674 cm−1 were from C–C stretching vibration, C–C–O symmetric stretch, and –OH wag of glycerol, respectively.
The impurities in the samples were indicated by three additional peaks between 1800 and 1500 cm−1 (dotted circle in Fig. 3). All samples gave an absorbance at 1652 cm−1 including A which can be attributed to the H–O–H bending of water. This absorbance was more intense for samples B, C, and D due to higher water content. In samples B, E, and G, absorbance at 1560 cm−1 was assigned to COO– stretching, indicative of soap impurities as reported by Bansal et al. in CG [28] and Hazimah and co-workers in the glycerol pitch sample [40]. In sample F, C=O stretching is observed at 1740 cm−1 indicating the presence of carboxylic acid or fatty acid esters [29, 47].
3.3 Thermal analysis of CG
Thermal degradation of pure glycerol, CG, and GP was measured using TGA. The mass loss of pure glycerol A was straightforward and only presented one phase between 150 and 250 °C, accounting for 96% of its mass loss. The decomposition of pure glycerol was nearly complete with only 2% mass residue left upon further heating. However, CG and GP samples had different thermogravimetric behavior owing to the impurities present. Typically, the thermal degradation of CG consists of four stages which can be clearly identified in samples E, F, and G (Fig. 4). The mass losses at 50–105 °C in all CG and GP samples were correlated with methanol and water contents, respectively, which was not observed in sample A. The highest mass loss of glycerol occurred between 150 and 250 °C which corresponded to the main decomposition of glycerol [51], accounting for 53–80% of mass loss. The next mass loss consisted of the thermal degradation of impurities in the form of FAMEs, soap, and free fatty acids, and that of their residues from early degradation of glycerol, extended from 300 to 400 °C. The absence of a drop-in mass was observed in this temperature range for samples B, C, and D, indicative of the low content of the said impurities.
A similar pattern was observed for jatropha-based CG treated with acid, which removed the impurities except for water, methanol, and ash content [28]. A final phase of thermal degradation of the samples was comprised of 490–800 °C, which consisted of coke and ash, in amounts up to 6%. At 800 °C, sample G had the highest remaining mass followed by F with 12% and 8%, respectively, which can be explained by its high MONG and ash content. It emerges that the coke was produced from the pyrolysis of glycerol, and FAMEs may occur as a result of secondary reactions between the products of thermal cracking [52].
The energy content was determined by measuring the calorific values of the samples. All samples had lower energy content that ranged from 9.0 to 17.7 MJ/kg compared to pure glycerol A (18.1 MJ/kg). GP sample, F, had the lowest calorific value of 9.0 MJ/kg, a consequence of its low C and H, and greater O concentration, followed by D (14.8 MJ/kg), B (15.3 MJ/kg), C (15.7 MJ/kg), G (16.2 MJ/kg), and E (17.7 MJ/kg). The low calorific value of D can also be attributed to its high moisture content (14.1%). These values are lower than those reported by Thompson and He between 18.6 and 20.5 MJ/kg [11]. The heat of combustion is half of those of fossil fuels but is comparable with other types of biomass, such as wood, straw, oilseed cake, bark, and sawdust. Except for sample F, others have heating values within the expected range of 15–21 MJ/kg for plant species.
Based on the results obtained, we found that palm-based CG and GP had high glycerol and carbon content, which can potentially be explored as an alternative biomass feedstock for the production of carbonaceous material. The impurities present are not critical for this transformation as suggested in several studies investigating the acid-catalyzed reactions using glycerol-based sulfonated carbon catalysts. The glycerol-based activated carbon can be generally prepared by reacting glycerol and concentrated sulfuric acid at a reaction temperature between 150 and 200°C. Okoye et al. reported a 99% conversion of glycerol in esterification with acetic acid using a sulfonated carbon catalyst prepared from biodiesel waste with 88% combined DAG and TAG selectivity [53]. These results are similar to those reported by Malaika et al. who prepared a catalyst from pure glycerol. As high as 95% conversion of glycerol was obtained with 70% selectivity for DAG and TAG in this study [54]. In another work, Prabhavathi Devi and co-workers have shown that carbon catalyst derived from glycerol pitch can be used in tetrahydropyranylation and dehydropyranylation [55]. These results suggest that the impurities in the glycerol used as a precursor for the partial carbonization and sulphonation did not affect the performance of the carbon catalyst produced. Among different approaches, various thermochemical conversions for energy applications such as steam reforming, gasification, pyrolysis, and torrefaction are also straightforward.
In existing liquid fuel combustion systems, several drawbacks of crude glycerol for direct usage are high viscosity, high density, low heating values, and high ash content. Despite improved combustion efficiency with the latest technology, the combustion fly ash associated with the ash content remains as the primary emission concern when using crude glycerol directly. Nevertheless, the integration of CG and GP could mitigate this issue and improve the combustion performance of low-rank solid fuels [56]. In addition, all palm-based CG and GP had distinctively low metal content.
High salt and soap content are also the main challenges for direct usage of CG and GP. Despite being detrimental in bioconversion and acetalization reaction by niobium oxyhydroxide catalyst [57], its presence was found to have no influence or even beneficial in some chemical conversions such as in the synthesis of acrolein over a tungsten-doped zirconia catalyst. The soap and residual base catalyst in CG was found to act as a catalyst in the production of monoglyceride, polyglycerol, and polyol. As high as 94.9% yield of polyglycerol was obtained from CG containing 12.5% soap [58]. In the formation of CG-based polyol, impurities such as soap, FFAs, and FAMEs participated in reactions. It was shown that polyurethane derived from CG-based polyol exhibited comparable performance to that of petroleum-based [59, 60,61].
In summary, the usage of palm-based CG and GP can potentially be explored in several reactions such as via thermochemical route, and certain chemical transformation. Pre-treatment of the waste could be performed to remove unwanted impurities from the CG and GP which could tremendously enhance the product obtained.
4 Conclusions
The palm-based CG and GP collected from different biodiesel and oleochemical refineries, respectively, had varied colors and properties. Nevertheless, high and consistent glycerol content of up to 87.3% was observed in all samples with the major contaminant in CG and GP being water and MONG, respectively. The presence of various levels of impurities led to the differences in physical appearance, pH, viscosity, density, and elemental and metal composition as well as the energy content in the CG and GP investigated. The variation of the impurities is related to the contaminants in the feedstock for the biodiesel production, processes involved during the production, and post-treatment of the by-product. The spectroscopy analysis — NMR, IR, and thermal analysis — could be employed as a quick tool to determine the presence of impurities in the CG and GP samples. The calorific values of palm-based CG were lower than pure glycerol but comparable to biomass, between 9.0 and 17.7 MJ/kg. In essence, high and consistent glycerol content in the CG and GP samples indicated their potential use as a precursor for numbers of applications such as in carbon material preparation but may require some pre-treatment for bioconversion which is sensitive to certain impurities present.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Parveez GKA, Kamil NN, Zawawi NZ, Ong-Abdullah M, Rasuddin R, Loh SK, Selvaduray KR, Hoong SS, Idris Z (2022) Oil palm economic performance in Malaysia and R&D progress in 2021. J Oil Palm Res 34:185–218. https://doi.org/10.21894/jopr.2022.0036
IEA (2020) Biodiesel and HVO markets. Renewables 2020. Analysis and forecast to 2025. International Energy Agency, 122–125. https://www.iea.org/reports/renewables-2020 (accessed 9 May 2022)
OECD-FAO (2021) Biofuels. In: OECD-FAO Agricultural Outlook 2021-2030. OECD Publishing, Paris, pp 202–213. https://doi.org/10.1787/19428846-en
Rosaria C, Della PC, Michele R, Mario P (2014) Understanding the glycerol market. Eur J Lipid Sci Technol 116:1432–1439. https://doi.org/10.1002/ejlt.201400229
da Silva Ruy AD, Ferreira ALF, Bresciani AÉ, de Brito Alves RM, Pontes AM (2020) Market prospecting and assessment of the economic potential of glycerol from biodiesel. Biotechnological Applications of Biomass. IntechOpen. https://doi.org/10.5772/intechopen.93965
Armylisas AHN, Hoong SS, Tuan Noor Maznee TI, Yeong SK, Mohammat MF (2021a) Solventless transacetalization of solketal over Amberlyst catalysts into valuable bio-based chemicals. J Chem Technol Biotechnol 96:2667–2674. https://doi.org/10.1002/jctb.6814
Armylisas AHN, Yeong SK, Tuan Noor Maznee TI, Hoong SS (2021b) Effect of bio-based aldehyde structure on the conversion and selectivity towards glycerol acetal over Amberlyst-46 using solvent-free approach. ChemistrySelect 6:256–263. https://doi.org/10.1002/slct.202004215
Sutter M, da Silva E, Duguet N, Raoul Y, Métay E, Lemaire M (2015) Glycerol ether synthesis: a bench test for green chemistry concepts and technologies. Chem Rev 115:8609–8651. https://doi.org/10.1021/cr5004002
Sahani S, Upadhyay SN, Sharma YC (2021) Critical review on production of glycerol carbonate from byproduct glycerol through transesterification. Ind Eng Chem Res 60:67–88. https://doi.org/10.1021/acs.iecr.0c05011
Donkin SS, Koser SL, White HM, Doane PH, Cecava MJ (2009) Feeding value of glycerol as replacement for corn grain in rations fed to lactating dairy cows. J Dairy Sci 92:5111–5119. https://doi.org/10.3168/jds.2009-2201
Thompson JC, He BB (2006) Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agric 22:261–265
Cerrate S, Yan F, Wang Z, Coto C, Sacakli P, Waldroup PW (2006) Evaluation of glycerine from biodiesel production as a feed ingredient for broilers. Int J Poult Sci 5:1001–1007. https://doi.org/10.3923/ijps.2006.1001.1007
Jung B, Batal AB (2011) Nutritional and feeding values of crude glycerin for poultry. 2. Evaluation of feeding crude glycerin to broilers. J Appl Poultry Res 20:514–527. https://doi.org/10.3382/japr.2011-00338
Bohon MD, Metzger BA, Linak WP, King CJ, Roberts WL (2011) Glycerol combustion and emissions. Proc Combust Inst 33:2717–2724. https://doi.org/10.1016/j.proci.2010.06.154
Steinmetz SA, Herrington JS, Winterrowd CK, Roberts WL, Wendt JOL, Linak WP (2013) Crude glycerol combustion: Particulate, acrolein, and other volatile organic emissions. Proc Combust Inst 34:2749–2757. https://doi.org/10.1016/j.proci.2012.07.050
Presciutti A, Asdrubali F, Baldinelli G, Rrotili A, Malavasi M, Salvia GD (2018) Energy and exergy analysis of glycerol combustion in an innovative flameless power plant. J Clean Prod 172:3817–3824. https://doi.org/10.1016/j.jclepro.2017.06.022
Boga DA, Liu F, Bruijnincx CAP, Weckhuysen BM (2016) Aqueous-phase reforming of crude glycerol: effect of impurities on hydrogen production. Catal Sci Technol 6:134–143. https://doi.org/10.1039/C4CY01711K
Bobadilla LF, Penkova A, Romero-Sarria F, Centeno MA, Odriozola JA (2014) Influence of the acid-base properties over NiSn/MgO – Al2O3 catalysts in the hydrogen production from glycerol steam reforming. Int J Hydrog Energy 39:5704–5712. https://doi.org/10.1016/j.ijhydene.2014.01.136
He Q, McNutt J, Yang J (2017) Utilization of residual glycerol from biodiesel production for renewable energy generation. Renew Sustain Energy Rev 71:63–76. https://doi.org/10.1016/j.rser.2016.12.110
Remón J, Giménez JR, Valiente A, García L, Arauzo J (2016) Production of gaseous and liquid chemicals by aqueous phase reforming of crude glycerol: influence of operating conditions on the process. Energy Convers Manage 110:90–112. https://doi.org/10.1016/j.enconman.2015.11.070
Feng C, Zhang M, Wu H (2018) Combustion of fuel mixtures containing crude glycerol (CG): important role of interactions between CG and fuel components in particulate matter emission. Ind Eng Chem Res 57:4132–4138. https://doi.org/10.1021/acs.iecr.8b00441
Ściążko M, Zuwała J, Pronobis M (2006) Advantages and drawbacks of co-combustion of biomass in power boilers at the background of operational experiences of first year biomass co-combustion in the industrial scale. Energetics 3:207
Kachel M, Kraszkiewicz A, Subr A, Parafiniuk S, Przywara A, Koszel M, Zając G (2020) Impact of the type of fertilization and the addition of glycerol on the quality of spring rape straw pellets. Energies 13:819. https://doi.org/10.3390/en13040819
Mothes G, Schnorpfeil C, Ackermann J-U (2007) Production of PHB from crude glycerol. Eng Life Sci 7:475–479. https://doi.org/10.1002/elsc.200620210
Kumar LR, Yellapu SK, Tyagi RD, Zhang X (2019) A review on variation in crude glycerol composition, bio-valorisation of crude and purified glycerol as carbon source for lipid production. Biores Technol 293:122155. https://doi.org/10.1016/j.biortech.2019.122155
Chen J, Yan S, Zhang X, Tyagi RD, Surampalli RY, Valéro JR (2018) Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manage 71:164–175. https://doi.org/10.1016/j.wasman.2017.10.044
da Silva CXA, Mota CJA (2011) The influence of impurities on the acid-catalyzed reaction of glycerol with acetone. Biomass Bioenerg 35:3547–3551. https://doi.org/10.1016/j.biombioe.2011.05.004
Bansal N, Dasgupta D, Hazra S, Bhaskar T, Ray A, Ghosh D (2020) Effect of utilization of crude glycerol as substrate on fatty acid composition of an Rhodotorula mucilagenosa IIPL32: assessment of nutritional indices. Biores Technol 309:123330. https://doi.org/10.1016/j.biortech.2020.123330
Chol CG, Dhabhai R, Dalai AK, Reaney M (2018) Purification of crude glycerol derived from biodiesel production process: experimental studies and techno-economic analyses. Fuel Process Technol 178:78–87. https://doi.org/10.1016/j.fuproc.2018.05.023
Hidawati EN, Sakinah AMM (2011) Treatment of glycerin pitch from biodiesel production. Int J Chem Environ Eng 2:309–313
Hansen CF, Hernandez A, Mullan BP, Moore K, Trezona-Murray M, King RH, Pluske JR (2009) A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim Prod Sci 49:154–161. https://doi.org/10.1071/EA08210
Yong KC, Ooi TL, Dzulkefly K, Wan Yunus WMZ, Hazimah AH (2001) Characterization of glycerol residue from a palm kernel oil methyl ester plant. J Oil Palm Res 13:1–6
Devi BLAP, Gangadhar KN, Kumar KLNS, Shanker KS, Prasad RBN, Prasad PSS (2011) Synthesis of sulfonic acid functionalized carbon catalyst from glycerol pitch and its application for tetrahydropyranyl protection/deprotection of alcohols and phenols. J Mol Catal A-Chem 345:96–100. https://doi.org/10.1016/j.molcata.2011.05.025
Teoh WP, Chee SY, Habib NZ, Bashir MJK, Chok VS, Ng CA (2021) Chemical investigation and process optimization of glycerine pitch in the green production of roofing tiles. J Build Eng 43:102869. https://doi.org/10.1016/j.jobe.2021.102869
Oil World (2021) Oil World Monthly 64. ISTA Mielke GmbH, Germany
Demaman Oro CE, Bonato M, Oliveira JV, Tres MV, Mignoni ML, Dallago RM (2019) A new approach for salts removal from crude glycerin coming from industrial biodiesel production unit. J Environ Chem Eng 7:102883. https://doi.org/10.1016/j.jece.2019.102883
Wall J, Van Gerpen J, Thompson J (2011) Soap and glycerin removal from biodiesel using waterless processes. ASABE 54:535–541
Hu S, Luo X, Wan C, Li Y (2012) Characterization of crude glycerol from biodiesel plants. J Agric Food Chem 60:5915–5921. https://doi.org/10.1021/jf3008629
Liu YP, Sun Y, Tan C, Li H, Zheng XJ, Jin KQ, Wang G (2013) Efficient production of dihydroxyacetone from biodiesel-derived crude glycerol by newly isolated Gluconobacter frateurii. Biores Technol 142:384–389. https://doi.org/10.1016/j.biortech.2013.05.055
Hazimah AH, Ooi TL, Salmiah A (2003) Recovery of glycerol and diglycerol from glycerol pitch. J Oil Palm Res 15:1–5
Lam MK, Lee KT (2011) Production of biodiesel using palm oil. In: Pandey A, Larroche C, Ricke SC, Dussap C-G, Gnansounnou E (eds) Biofuels: alternative feedstocks and conversion processes. Academic Press, San Diego, pp 355–359. https://doi.org/10.1016/C2018-0-00957-3
Valerio O, Horvath T, Pond C, Misra M, Mohanty A (2015) Improved utilization of crude glycerol from biodiesel industries: synthesis and characterization of sustainable biobased polyester. Ind Crop Prod 78:141–147. https://doi.org/10.1016/j.indcrop.2015.10.019
Zhang Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: economic assessment and sensitivity analysis. Biores Technol 90:229–240. https://doi.org/10.1016/S0960-8524(03)00150-0
Bouaid A, Vácquez R, Martinez M, Aracil J (2016) Effect of free fatty acids contents on biodiesel quality. Fuel 174:54–62. https://doi.org/10.1016/j.fuel.2016.01.018
Che Man YB, Moh MH, Van De Voort FR (1999) Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using Fourier transform infrared spectroscopy. J Am Oil Chem Soc 76:485–490. https://doi.org/10.1007/s11746-999-0029-z
Yung CL, Subramaniam V, Yusoff S (2021) Life cycle assessment for the production of palm biodiesel. J Oil Palm Res 33:140–150. https://doi.org/10.21894/jopr.2020.0080
Gottlieb HE, Graczyk-Millbrandt G, Inglis GGA, Nudelman A, Perez D, Qian Y, Shuster LE, Sneddon HF, Upton RJ (2016) Development of GSK’s NMR guides – a tool to encourage the use of more sustainable solvents. Green Chem 18:3867–3878. https://doi.org/10.1039/C6GC00446F
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol-a byproduct of biodiesel production. Biotechnol. Biofuels 5:13. https://doi.org/10.1186/1754-6834-5-13
USDA (2021) Australia: biofuels annual. https://www.fas.usda.gov/data/australia-biofuels-annual-2 (accessed on 16th December 2022)
Dhabhai R, Ahmadifeijani E, Dalai AK, Reaney M (2016) Purification of crude glycerol using a sequential physico-chemical treatment, membrane filtration and activated charcoal treatment. Sep Purif Technol 168:101–106. https://doi.org/10.1016/j.seppur.2016.05.030
Dou B, Dupont V, Williams PT, Chen H, Ding Y (2009) Thermogravimetric kinetics of crude glycerol. Biores Technol 100:2613–2620. https://doi.org/10.1016/j.biortech.2008.11.037
Ranjbar M, Pusch G (1991) Pyrolysis and combustion kinetics of crude oils, asphaltenes and resin in relation to thermal recovery processes. J Anal Appl Pyrolysis 20:185–196. https://doi.org/10.1016/0165-2370(91)80072-G
Okoye PU, Abdullah AZ, Hameed BH (2017) Synthesis of oxygenated fuel additivies via glycerol esterification with acetic acid over bio-derived catalyst. Fuel 209:538–544. https://doi.org/10.1016/j.fuel.2017.08.024
Malaika A, Ptaszynska K, Kozlowski M (2021) Conversion of renewable feedstock to bio-carbons dedicated for the production of green fuel additives from glycerol. Fuel 209:538–544. https://doi.org/10.1016/j.fuel.2020.119609
Prabhavathi Devi BLAP, Gangadhar KN, Siva Kumar KLN, Shiva Shanker K, Prasad RBN, Sai Prasad PS (2011) Synthesis of sulfonic acid functionalized carbon catalyst from glycerol pitch and its application for tetrahydrpyranyl protection/deprotection of alcohols and phenols. J Mol Catal A Chem 345:96–100. https://doi.org/10.1016/j.molcata.2011.05.025
Zhang J, Wang Y, Muldoon VL, Deng S (2022) Crude glycerol and glycerol as fuels and fuel additives in combustion applications. Renew Sustain Energy Rev 159:112206. https://doi.org/10.1016/j.rser.2022.112206
Souza TE, Portilho MF, Souza PMTG, Souza PP, Oliveira LCA (2014) Modified niobium oxyhydroxide catalyst: an acetalization reaction to produce bio-additives for sustainable use of waste glycerol. ChemCatChem 6:2961–2969. https://doi.org/10.1002/cctc.201402322
Din NSMNM, Idris Z, Kian YS (2013) Preparation of polyglycerol from palm biodiesel crude glycerine. J Oil Palm Res 25:289–297
Li C, Luo X, Li T, Tong X, Li Y (2014) Polyurethane foams based on crude glycerol-derived polyols: one pot preparation of biopolyols with branched fatty acid ester chains and its effects on foam formation and properties. Polym 55:6529–6538. https://doi.org/10.1016/j.polymer.2014.10.043
Hu S, Wan C, Li Y (2015) Production of polyols and waterborne polyurethane dispersions from biodiesel-derived crude glycerol. J Appl Polym Sci 132:41425. https://doi.org/10.1002/app.41425
Schwengber CA, Alves HJ, Schaffner RA, da Silva FA, Sequinel R, Bach VR, Ferracin RJ (2016) Overview of glycerol reforming for hydrogen production. Renew Sustain Energy Rev 58:259–266. https://doi.org/10.1016/j.rser.2015.12.279
Acknowledgements
The authors would like to thank the Director-General and Deputy Director-General (R&D) of the Malaysian Palm Oil Board (MPOB) for the permission to publish this article and for the support in this project, and the MPOB for the funding. We would also like to express our gratitude to biodiesel and oleochemical manufacturers in Malaysia who generously supplied the CG and GP for this study. We would also like to acknowledge contributions by Bahriah Bilal (QEA Unit, AOTD), Ahmad Lutfi Md. Yusof (SPD Unit, AOTD), Azman Rafiei (PED Unit, AOTD), and Engineering and Processing Division (MPOB) for the assistance provided on the analysis performed.
Funding
This work was supported by the Malaysian Palm Oil Board (MPOB).
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Abu Hassan Noor Armylisas, Seng Soi Hoong, Tuan Ismail Tuan Noor Maznee; methodology: Abu Hassan Noor Armylisas; formal analysis and investigation: Abu Hassan Noor Armylisas; writing — original draft preparation: Abu Hassan Noor Armylisas; writing — review and editing: Abu Hassan Noor Armylisas, Seng Soi Hoong, Tuan Ismail Tuan Noor Maznee; supervision: Seng Soi Hoong, Tuan Ismail Tuan Noor Maznee.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Armylisas, A.H.N., Hoong, S.S. & Tuan Ismail, T. Characterization of crude glycerol and glycerol pitch from palm-based residual biomass. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04003-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04003-4