Abstract
In the current study, bioethanol has been purified and separated from the culture broth using in situ modified bacterial cellulose (BC) membrane with AMPS. To our knowledge, this is the first report for development of BC composite membrane for bioethanol separation from production media. The characterization of the prepared membrane was investigated for morphology and functional groups via scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, and Raman spectroscopy, in addition to the determination of their water and ethanol uptake. The obtained data proved the formation of cellulose multilayers in addition to the existence of its specific function groups. The Amicon cell pervaporation system containing the prepared BC/AMPS membrane has been used for the separation of the bioethanol from the culture broth using nitrogen gas pressure, and the results revealed that the BC/AMPS composite membrane is more efficient than the neat BC membrane in the separation process of bioethanol. At 50-psi nitrogen pressure, the best separation factor and flux were recorded as 15.43 and 98.94 g/m2.h, respectively, which were accompanied by the elevation of the bioethanol concentration from 1.98 to 3.22 mg/ml before and after separation, respectively. These findings revealed the promising application of BC/AMPS membrane in the field of bioenergy especially the bioethanol separation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Owing to global warming and the shortage of fossil fuels, renewable energy such as hydrogen or bioethanol has been significantly produced in recent years. Among them, ethanol fuels produced from biomass have attracted significant attention because fuel ethanol has the advantages of environmental friendliness and low carbon, and can alleviate the shortage of fossil fuels. Bioethanol as a clean and renewable fuel has gained more attention; however, greater energy inputs make a slow progress in industry. Membrane technology has potential in the bioethanol production process as a highly selective and energy-saving separation process through pervaporation technology to separate ethanol from fermentation with high efficiency and green technology [1, 2]. The majority of bioethanol generated today is made by the fermentation of starchy biomass, such as potato, corn, grains, and seeds, or sugar-based consumable raw materials, typically juice from sugarcane, which is known as the first generation of bioethanol production [3, 4]. However, it was thought that the use of first-generation fuel sources would lead to a fuel or food crisis. Second-generation biofuels were made from non-edible feedstocks, such as agricultural waste, wood residual waste, and energy crops, to solve this problem [5, 6]. In this context, the leftovers from agriculture, forestry, sewage sludge, industrial waste, and municipal garbage are seen as promising sources for the generation of biofuel [7].
The textile and apparel sectors produce a significant volume of bio-waste in a variety of forms and under diverse conditions. The waste is produced during the production of natural fibers, yarn spinning, fabric and apparel manufacturing, and post-consumer processes [8]. Unfortunately, fermentation broths normally contain less than 10 wt% of ethanol since a higher concentration of ethanol would have a suppressive effect on the microorganisms needed for bioethanol fermentation, which would cause the fermentation process to stop [9]. Therefore, the extraction of ethanol from fermentation broths is a crucial step in the conversion of cellulose to ethanol. A batch of fermenting liquid is first distilled as part of a typical separation procedure, which is intermittent. High energy costs associated with distillation translate into high startup costs. Evidently, such intermittent manufacturing methods are inefficient and energy-intensive, which is contrary to the needs of sustainable development and the circular economy [10].
The separation of the liquid mixture achieved by pervaporation (PV) refers to the difference in the dissolution rate. Pervaporation (PV) as a new technology has attracted the attention of researchers for the separation and purification of biofuels. PV’s advantages include highly efficient separation, simple equipment, low cost, low pollution, and low energy consumption. In order to achieve separation, a pervaporation system must be used, together with both feed and vacuum pumps [11]. The Amicon cell’s pervaporation-based operation is inexpensive and energy efficient due to its nitrogen pressure feeding instead of utilizing pumps that consume electricity [12].
Polymeric membranes are frequently used in pervaporation systems for the recovery of ethanol from aqueous environments, with polydimethylsiloxane (PDMS) producing positive outcomes [13]. Up to this point, the possibility of using membranes made of natural polymers, synthetic polymers, biopolymers, and their chemically modified forms (doped, composites, etc.) has been examined [14]. The separation of ethanol uses a variety of membranes, including zeolites [15], microporous silica [16], and polyhedral oligomeric silsesquioxanes (POSS) [17] membranes.
Bacterial cellulose is an extracellular polysaccharide biosynthesized by certain bacteria with the same structure and chemical formula as cellulose in nature [(C6H10O5)n]. It is widely produced by strains related to the family Acetobacteraceae, particularly the Komagataeibacter genus, formerly known as Gluconacetobacter, viz. Komagataeibacter hansenii [18, 19] and recently produced from Lactobacillus sp. as Gram-positive bacteria [20]. BC exhibits distinctive properties such as high-water retention capacity, degree of crystallinity, mechanical strength, formability, hydrophilicity, biocompatibility, flexibility, and non-toxicity [21, 22]. These excellent properties and features of BC facilitate its applications in different fields such as biomedical [23], foods [24], cosmetics [25], and industries [26].
Numerous attempts have been made to maximize the impacts of BC-based composites, either by combining BC pellicles with other materials (ex situ) or by BC functionalization during the biosynthesis process (in situ) [27]. Several studies were concerned with the in situ modification of BC for the development of new applications like BC/polyaniline/titanium-dioxide as a bioanode in microbial fuel cells [28], BC/cellulose imidazolium as antimicrobial agent [29], BC/calcium carbonate for gas assistance [30], BC/sodium alginate as a drug delivery system [31], and BC/polypyrrole as an electrical conductive material [32]. BC was also used as a separation system for oil removal from wastewater [33], copper [34], dye, [35] and oil/water separation [36, 37].
Current research generally focuses on the extraction of ethanol from the fermentation broth and to increase the bioethanol concentration that produced through optimum microbial growth. To our knowledge, this work presents for the first time the modification of the BC produced by Lactiplantibacillus plantarum AS.6 (L. plantarum AS.6) strain using AMPS material in order to prepare AMPS-modified BC membrane with more enhanced properties that can be effectively used for the separation of bioethanol from the fermentation broth. Both of the blank and the modified BC membranes were initially characterized by FT-IR spectroscopy, Raman spectroscopy, tensile strength, contact angle, and SEM and were then integrated into properly Amicon pervaporation cell, which can provide high ethanol permeability and sufficient mechanical strength. Furthermore, its low cost also favors its industrial application.
2 Materials and methods
2.1 In situ development of BC/AMPS composite membrane
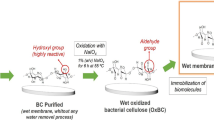
According to our previous work, L. plantarum AS.6 was used as a BC producer strain [38]. For preinoculum preparation, a freshly grown single colony of L. plantarum AS.6 was cultured in Hestrin and Schramm (HS) medium containing glucose 2%, yeast extract 0.5%, peptone 0.5%, disodium hydrogen phosphate 0.27%, citric acid 0.115%, and ethanol 0.5% at pH 5.5 and incubated at 30 °C for 48 h at 200 rpm [39]. AMPS was evaluated for in situ preparation of BC composite membrane according to [29] with little changes as shown in Fig. 1. At the beginning, a 2% concentration of AMPS was added to the broth media containing glucose 1.5%; yeast extract 1.3%; MgSO4 0.1%; KH2PO4 0.4%, and ethanol 0.7% at pH 7.2. A BC control flask was also prepared using the same medium components without the addition of AMPS. The media were autoclaved at 121 °C and 15 psi for 20 min, left to cool at room temperature, and were then inoculated by 11% preinoculum of the bacteria. Both flasks were then incubated at 30 °C for 8 days under static conditions. At the end of the fermentation time, the BC pellicle formed at the air-liquid interface incorporated with AMPS was collected, harvested, and washed several times with distilled water to get rid of the excess medium components. Afterward, each pellicle was then treated by 0.5% NaOH at 90 °C for 30 min to remove any residual bacterial contaminants or other impurities immobilized on the BC membrane, and was then washed by distilled water until neutral pH value [40]. The purified BC and BC/AMPS membranes were dried at 70 °C overnight.
2.2 Bioethanol production
According to our previous work [41], the production of bioethanol occurred through the fermentation of cardboard waste. Briefly, the cardboard waste was treated with 20% HCl at 100 °C for 20 min. For enhancing the liberation of sugars; about 7% of cardboard waste in 50 ml of acetate buffer (pH 5.5) was hydrolyzed with 210 U of cellulase enzyme and then incubated at 50 °C for 72 h under shaking at 150 rpm. For bioethanol production, the hydrolysate was fermented using Saccharomyces cerevisiae at pH 5.5 and 30 °C under static conditions. For the evaluation of bioethanol content, the K2Cr2O7-dependent spectrophotometric method was compared with a standard curve of ethanol [42]. The analytical potassium dichromate method was used to calculate the amount of bioethanol produced with only a few modifications. Each culture was centrifuged at 1000 rpm for 10 min, and then 1 ml of each ferment was combined with 4 ml of distilled water and 1 ml of K2Cr2O7. The tubes were carefully filled with 4 mL conc. H2SO4 while being kept in a cold bath. After a 10-min incubation at room temperature, the absorbance of each sample was assessed by spectrophotometry at 660 nm. To calculate the bioethanol content, the gathered values were compared to an ethanol standard curve.
2.3 Separation of bioethanol using BC/AMPS membrane
The separation procedure of the bioethanol from the microbial broth was carried out in accordance with [41]. The generated bioethanol/broth mixture was forced through an Amicon ultrafiltration cell that included BC and BC/AMPS membrane as illustrated in Fig. 2. Different nitrogen pressures (20–70 psi) were tested to force the mixture to move from one side to the other side through the cell at different times. The Amicon cell had a 12.56 cm2 surface area, and the experiment was carried out at varying nitrogen pressures for a total of 18 h at 30 °C. The permeate flux and separation factor have both been studied according to the following formula:
where F refers to the permeate fraction, A is the membrane area, and t is the time of test.
where Xi and Yi, respectively, represent the mass fractions of component i in the feed and permeate sides.
2.4 Characterization of BC/AMPS membrane
The chemical bonding and possible interactions within the produced BC and BC/AMPS membranes were investigated using Fourier transform infrared (FT-IR) spectra (Shimadzu FT-IR-8400 S, Japan) and a Raman Scope III (SENTERRA-Bruker, USA) compact bench top FT-Raman microscope at the following conditions: range 4000–400 cm−1 at red leaser 785 nm and 50 mW power and 110 °C. The tensile strength of polymer-based membranes was determined at room temperature using the universal testing machine (Shimadzu UTM, Japan). SEM was also used to analyze the membrane morphologies in cross-sections and top surfaces (JEOL JSM-6360LA, Japan). Water contact angle measurement was performed using the contact angle meter VCA 2500 XE equipped with a CCD camera and analysis software (AST Products, Billerica, MA).
3 Results and discussion
3.1 FT-IR spectrum analysis
FT-IR spectra for BC and BC/AMPS are shown in Fig. 2. The spectrum obtained for BC (Fig. 3A) shows the characteristic bands of cellulose. For instance, β-glycosidic linkages between the glucose units at 901 cm−1 and OH stretching vibration at 3341 cm−1. In addition to C-O symmetric stretching of primary alcohol and C-O-C antisymmetric bridge stretching of the sugar ring at 1053 cm−1and 1158 cm−1, respectively, CH stretching of CH2 and CH3 groups at 2900 cm−1 was also detected [43, 44]. Additionally, the obtained spectra related to BC/AMPS before and after separation (Fig. 3B and C) showed N-H stretching vibration band at 3450 cm−1, which is combined with O-H stretching vibration band. The characteristic absorption bands of AMPS that appeared at 1050 and 1131 cm−1 were ascribed to the asymmetric and symmetric vibration of S=O. The band at 1650 cm−1 resulted from the C=C stretching vibration of the amide group and the band at 1720 cm−1 for the C=O; the N–H band absorbed around 3409 cm−1 [45]. FT-IR spectra of the prepared membrane after the separation process of bioethanol have some changes in band intensities and band positions (shifts) as compared to that of the blend before the separation process. The typical absorbance due to C=O stretching at 1650 cm−1 is shifted to 1660 cm−1 and became intense following the separation process, while a peak of 3341 cm−1 has been shifted to 3360 cm−1 after separation, which indicates a considerable change in the blends after the separation process.
3.2 Raman spectroscopy
The Raman spectra of the BC and modified BC membranes are shown in Fig. 3. In the case of neat BC (Fig. 4A), a band at 2820 cm−1 was observed, which represents the aliphatic C-H stretching vibration. A sharp and steep band observed at 1080 cm−1 is due to the presence of C-O-C stretching vibrations. In the case of the BC/AMPS membrane (Fig. 4B and C), the N-H stretching vibration band is located at 3420 cm−1, which is combined with O-H stretching vibration band. The bands observed at 1700 cm−1, 1632 cm−1, and 1375 cm−1 are attributed to the amide, which exists in the AMPS molecules. The band at 1031 and 1100 cm−1 of the sulfonic group of AMPS was also observed. The bands at 1543 and 618 cm−1 are assigned to C-N- and S-O groups, respectively. After the ethanol/broth separation process, the band of C=O becomes more intense [46, 47].
3.3 SEM analysis
SEM micrographs can provide an indication for the membrane surface morphology and homogeneity. As shown in Fig. 5A, the surface of the plain BC membrane presents a smooth surface. After incorporation of AMPS to the BC (Fig. 5B), good compatibility and miscibility between BC and AMPS polymers were exhibited on the surface, indicating that the AMPS was uniformly dispersed and embedded in the BC membrane which is supposed to improve its structure to be effectively used in the bioethanol/broth separation process [48, 49]. Additionally, the cross-sections of the dried BC membranes in Fig. 5C show that the structures are composed of multilayers of thin sheets that showed a lower thickness after the separation process, which is a characteristic feature of the BC membranes.
SEM micrographs of the surface and cross-sections of prepared membranes of BC and BC/AMPS at magnifications 500× and 1000×. A, D Surface SEM images of BC membrane. B, E Surface SEM images of BC membrane before the separation process. C, F Surface SEM images of BC membrane after the separation process at 500 and 1000× magnification, respectively. G Cross-sectional SEM image of BC membrane. H Cross-sectional SEM image of BC membrane before the separation process. I Cross-sectional SEM image of BC membrane after the separation process at 500× magnification
3.4 Mechanical properties
The mechanical properties of BC and BC/AMPS before and after the separation process were analyzed in terms of elongation at break, thickness, and Young’s modulus, as shown in Table 1. The neat BC membrane possessed an elongation at break and Young’s modulus of 0.8% and 6.2 MPa, respectively; meanwhile, the corresponding values for the BC/AMPS membrane were around 2.36% and 20.02 MPa, before the separation process, respectively. After the separation process, the value of elongation at break was 2.03% and the value of Young’s modulus was 18.1 MPa. Accordingly, these results indicate that the incorporation of AMPS into the BC matrices resulted in enhancing the ability of the membrane to absorb more energy, and thus prevent the nanocellulose fibers from breaking. Consequently, when compared with the neat BC, the membranes were demonstrated to elongate more prior to breaking. The enhanced properties of prepared blends indicate that BC/AMPS membrane can be used in the separation process [50].
3.5 Sorption properties of ethanol and water
Water and ethanol absorption of neat BC and BC/AMPS membranes before and after the separation process through the immersion in distilled water and ethanol at room temperature is shown in Table 2. The BC membrane has high water absorption capacity of 90.70%, which might be related to the hydrophilic property of the hydroxyl group in its structure. Water absorption capacity of the BC/AMPS membrane was much lower (35.34%) which reached 21.45% after the separation process. As compared with BC, the BC/AMPS membrane has significantly higher resistance to water and can be used in the ethanol/water separation process. In addition, because of the hydrophilic nature of BC, the BC membrane exhibited a very low ethanol uptake at around 10.77%. The ethanol uptake of BC/AMPS membrane is higher than neat BC membrane (50.60%) which increased to 54.25% after the separation process [51].
3.6 Water contact angle
BC records a value of contact angle 45.33° and 58.12° for BC/AMPS membranes before the separation of the alcohol which is revealing in separation factor of BC and BC/AMPS in which the neat BC membrane has not succeeded to separate the produced bioethanol under the tested nitrogen pressures. While the composite BC/AMPS membrane showed a great result due to the presence of the effective functional group (sulfonic group) in AMPS. The contact angle of BC/AMPS membrane after the separation of the alcohol recorded 67.32° which increased and connected to hydrophobic characteristic.
3.7 Separation of bioethanol
Pervaporation is one of the most popular approaches for the separation of ethanol and water [13, 52]. The current study uses composite of BC/AMPS membranes to mechanically automate the pervaporation process in a similar manner. The neat BC membrane has not succeeded to separate the produced bioethanol under the tested nitrogen pressures, while the composite BC/AMPS membrane showed a great result as presented in Table 3. Moreover, Figs. 6 and 7 show the outcomes of the separated bioethanol under a range of nitrogen pressures using BC/AMPS membrane. According to the obtained results, the higher permeate volume and concentration at 50 psi were 1.41 ml and 3.22 mg/ml, respectively. In addition, the best separation factor and flux were reported as 15.43 and 98.94 g/m2.h, respectively, at 50 psi. These results indicated that the increase in flux is combined with an increase in the separation efficiency [53].
4 Conclusion
Current research generally focuses on the extraction of ethanol from the fermentation broth and to increase the bioethanol concentration that produced through optimum microbial growth. To our knowledge, this work presents for the first time the modification of the BC produced by L. plantarum AS.6 strain using AMPS material in order to prepare AMPS-modified BC membrane with more enhanced properties that can be effectively used for the separation of bioethanol from the fermentation broth. The composite was confirmed through multiple characterization techniques including SEM, FT-IR spectroscopy, and Raman spectroscopy. The prepared BC/AMPS membrane has been successfully applied for the separation of bioethanol from a culture broth containing glucose units liberated from the hydrolysis of cardboard waste. The efficient application of 50-psi nitrogen pressure succeeded to elevate the bioethanol concentration from 1.98 mg/ml before separation using Amicon cell to 3.22 mg/ml after separation. It could be concluded that, according to the current study, the optimum conditions for the separation of bioethanol from culture broth are the using of Amicon cell integrated with BC/AMPS membrane through the application of nitrogen pressure at 50 psi. Our purpose in future work is to develop a large-scale cost-effective production of high-quality bioethanol by using textile waste cellulosic fibers by using less/energy free technology for the production process.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Szulczyk KR, Ziaei SM, Zhang C (2021) Environmental ramifications and economic viability of bioethanol production in Malaysia. Renew Energy 172:780–788
Rosen Y, Mamane H, Gerchman Y (2021) Immersed ozonation of agro-wastes as an effective pretreatment method in bioethanol production. Renew Energy 174:382–390
Michailos SE, Webb C (2019) Biorefinery approach for ethanol production from bagasse. In: Bioethanol production from food crops. Elsevier, pp 319–342
Melendez JR, Mátyás B, Hena S, Lowy DA, El Salous A (2022) Perspectives in the production of bioethanol: a review of sustainable methods, technologies, and bioprocesses. Renew Sustain Energy Rev 160:112260
Hoang AT, Pandey A, Huang Z, Luque R, Ng KH, Papadopoulos AM, Chen W-H, Rajamohan S, Hadiyanto H, Nguyen XP (2022) Catalyst-based synthesis of 2, 5-dimethylfuran from carbohydrates as a sustainable biofuel production route. ACS Sustain Chem Eng 10(10):3079–3115
Jeyakumar N, Hoang AT, Nižetić S, Balasubramanian D, Kamaraj S, Pandian PL, Sirohi R, Nguyen PQP, Nguyen XP (2022) Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 329:125362
Ghosh R, Bhattacherjee S (2013) A review study on anaerobic digesters with an Insight to biogas production. Int J Eng Sci Invent 2(3):8–17
Khandaker S, Bashar MM, Islam A, Hossain MT, Teo SH, Awual MR (2022) Sustainable energy generation from textile biowaste and its challenges: a comprehensive review. Renew Sustain Energy Rev 157:112051
Peng P, Lan Y, Liang L, Jia K (2021) Membranes for bioethanol production by pervaporation. Biotechnol Biofuels 14(1):1–33
Castro-Muñoz R, Galiano F, Fíla V, Drioli E, Figoli A (2019) Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: current state of the art. Rev Chem Eng 35(5):565–590
Ehsan M, Razzaq H, Razzaque S, Bibi A, Yaqub A (2022) Recent advances in sodium alginate-based membranes for dehydration of aqueous ethanol through pervaporation. J Polym Sci 60(16):2435–2453
Taha RH, Taha TH, Abu-Saied M, Mansy A, Elsherif MA (2022) Successful production of bioethanol from olive waste residues followed by its purification using poly (acrylonitrile-co-methylacrylate)/polymethylmethaacrylate membrane. Biomass Convers Biorefin 1–15. https://doi.org/10.1007/s13399-022-02891-6
Amarante RC, Donaldson AA (2021) Pervaporation separation of ethanol and 2-ethylhexanol mixtures using cellulose acetate propionate and poly (1-vinylpyrrolidone-co-vinyl acetate) blend membranes. Sep Purif Technol 258:117953
Mali M, Walekar L, Mhamane D, Mali G, Pawar S, Patil V, Parbat H, Gokavi G (2022) Fabrication of ternary polyvinyl alcohol/tetraethyl orthosilicate/silicotungstic acid hybrid membranes for pervaporation dehydration of alcohol. Colloids Surf, A Physicochem Eng Asp 652:129741
Wenten I, Dharmawijaya P, Aryanti P, Mukti R (2017) LTA zeolite membranes: current progress and challenges in pervaporation. RSC Adv 7(47):29520–29539
Nagasawa H, Tsuru T (2017) Silica membrane application for pervaporation process, Current Trends and Future Developments on (Bio-) Membranes. Elsevier, pp 217–241
Chen X, Hung WS, Liu G, Lee KR, Jin W (2020) PDMS mixed-matrix membranes with molecular fillers via reactive incorporation and their application for bio-butanol recovery from aqueous solution. J Polym Sci 58(18):2634–2643
Güzel M, Akpınar Ö (2020) Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. Int J Biol Macromol 162:1597–1604
Saleh AK, Farrag A, Soliman N, Ibrahim M, El-Shinnawy N, Abdel-Fattah YR (2019) Evaluation of culture requirments for cellulose production by Egyptian local isolate alongside reference strain Gluconacetobacter hansenii ATCC 23769. Pak J Biotechnol 16(2):69–80
Khan H, Kadam A, Dutt D (2020) Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr Polym 229:115513
Rajwade J, Paknikar K, Kumbhar J (2015) Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol 99(6):2491–2511
Vasconcelos NF, Feitosa JPA, da Gama FMP, Morais JPS, Andrade FK, de Souza MDSM, de Freitas Rosa M (2017) Bacterial cellulose nanocrystals produced under different hydrolysis conditions: properties and morphological features. Carbohydr Polym 155:425–431
Moniri M, Boroumand Moghaddam A, Azizi S, Abdul Rahim R, Bin Ariff A, Zuhainis Saad W, Navaderi M, Mohamad R (2017) Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 7(9):257
Haghighi H, Gullo M, La China S, Pfeifer F, Siesler HW, Licciardello F, Pulvirenti A (2021) Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll 113:106454
Martins D, Rocha C, Dourado F, Gama M (2021) Bacterial cellulose-carboxymethyl cellulose (BC: CMC) dry formulation as stabilizer and texturizing agent for surfactant-free cosmetic formulations. Colloids Surf A: Physicochem Eng Asp 617:126380
Lin D, Liu Z, Shen R, Chen S, Yang X (2020) Bacterial cellulose in food industry: current research and future prospects. Int J Biol Macromol 158:1007–1019
Eichhorn SJ, Dufresne A, Aranguren M, Marcovich N, Capadona J, Rowan SJ, Weder C, Thielemans W, Roman M, Renneckar S (2010) Current international research into cellulose nanofibres and nanocomposites. J Mater Sci 45(1):1–33
Truong DH, Dam MS, Bujna E, Rezessy-Szabo J, Farkas C, Vi VNH, Csernus O, Nguyen VD, Gathergood N, Friedrich L (2021) In situ fabrication of electrically conducting bacterial cellulose-polyaniline-titanium-dioxide composites with the immobilization of Shewanella xiamenensis and its application as bioanode in microbial fuel cell. Fuel 285:119259
El-Gendi H, Salama A, El-Fakharany EM, Saleh AK (2023) Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr Polym 302:120383
Sun B, Lin J, Wang T, Liu M, Yang L, Ma B, Chaudhary JP, Chen C, Sun D (2021) Gas assisted in situ biomimetic mineralization of bacterial cellulose/calcium carbonate bio composites by bacterial. Int J Biol Macromol 182:1690–1696
Ji L, Zhang F, Zhu L, Jiang J (2021) An in-situ fabrication of bamboo bacterial cellulose/sodium alginate nanocomposite hydrogels as carrier materials for controlled protein drug delivery. Int J Biol Macromol 170:459–468
Müller D, Rambo C, Recouvreux D, Porto L, Barra G (2011) Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synth Met 161(1-2):106–111
Galdino CJS Jr, Maia AD, Meira HM, Souza TC, Amorim JD, Almeida FC, Costa AF, Sarubbo LA (2020) Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem 91:288–296
Urbina L, Guaresti O, Requies J, Gabilondo N, Eceiza A, Corcuera MA, Retegi A (2018) Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr Polym 193:362–372
Saleh AK, El-Gendi H, Ray JB, Taha TH (2021) A low-cost effective media from starch kitchen waste for bacterial cellulose production and its application as simultaneous absorbance for methylene blue dye removal. Biomass Convers Biorefin 1–13. https://doi.org/10.1007/s13399-021-01973-1
Wang FP, Zhao XJ, Wahid F, Zhao XQ, Qin XT, Bai H, Xie YY, Zhong C, Jia SR (2021) Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr Polym 253:117220
Hou Y, Duan C, Zhu G, Luo H, Liang S, Jin Y, Zhao N, Xu J (2019) Functional bacterial cellulose membranes with 3D porous architectures: conventional drying, tunable wettability and water/oil separation. J Membr Sci 591:117312
Saleh AK, El-Gendi H, Soliman NA, El-Zawawy WK, Abdel-Fattah YR (2022) Bioprocess development for bacterial cellulose biosynthesis by novel Lactiplantibacillus plantarum isolate along with characterization and antimicrobial assessment of fabricated membrane. Sci Rep 12(1):–2181
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58(2):345
Hsieh J-T, Wang M-J, Lai J-T, Liu H-S (2016) A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J Taiwan Inst Chem Eng 63:46–51
Taha RH, Taha TH, Abu-Saied M, Mansy A, Elsherif MA (2022) Maximization of the bioethanol concentration produced through the cardboard waste fermentation by using ethylenediamine-modifying poly (acrylonitrile-co-methyl acrylate) membrane. Biomass Convers Biorefin 1–19. https://doi.org/10.1007/s13399-022-02303-9
Srivastava AK, Agrawal P, Rahiman A (2014) Pretreatment and production of bioethanol from different lignocellulosic biomass. Int J 2(4):888–896
Chen G, Wu G, Alriksson B, Chen L, Wang W, Jönsson LJ, Hong FF (2018) Scale-up of production of bacterial nanocellulose using submerged cultivation. J Chem Technol Biotechnol 93(12):3418–3427
Molina-Ramírez C, Castro M, Osorio M, Torres-Taborda M, Gómez B, Zuluaga R, Gómez C, Gañán P, Rojas OJ, Castro C (2017) Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials 10(6):639
Mesbah F, El Gayar D, Farag H, Tamer TM, Omer AM, Mohy-Eldin MS, Khalifa RE (2021) Development of highly ionic conductive cellulose acetate-g-poly (2-acrylamido-2-methylpropane sulfonic acid-co-methyl methacrylate) graft copolymer membranes. J Saudi Chem Soc 25(9):101318
Potivara K, Phisalaphong M (2019) Development and characterization of bacterial cellulose reinforced with natural rubber. Materials 12(14):2323
Huang X, Tian F, Chen G, Wang F, Weng R, Xi B (2021) Preparation and characterization of regenerated cellulose membrane blended with ZrO2 nanoparticles. Membranes 12(1):42
Kim J, Cai Z, Lee HS, Choi GS, Lee DH, Jo C (2011) Preparation and characterization of a bacterial cellulose/chitosan composite for potential biomedical application. J Polym Res 18(4):739–744
Santos MV, Barud HS, Alencar MA, Nalin M, Toma SH, Araki K, Benedetti AV, Maciel IO, Fragneaud B, Legnani C (2021) Self-supported smart bacterial nanocellulose–phosphotungstic acid nanocomposites for photochromic applications. Front Mater 8:668835
Yim SM, Song JE, Kim HR (2017) Production and characterization of bacterial cellulose fabrics by nitrogen sources of tea and carbon sources of sugar. Process Biochem 59:26–36
Barud HO, Barud HDS, Cavicchioli M, do Amaral TS, de Oliveira Junior OB, Santos DM, Petersen ALDOA, Celes F, Borges VM, de Oliveira CI, de Oliveira PF (2015) Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr Polym 128:41–51
Zhao D, Li M, Jia M, Zhou S, Zhao Y, Peng W, Xing W (2022) Asymmetric poly (vinyl alcohol)/Schiff base network framework hybrid pervaporation membranes for ethanol dehydration. Eur Polym J 162:110924
Kamtsikakis A, McBride S, Zoppe JO, Weder C (2021) Cellulose nanofiber nanocomposite pervaporation membranes for ethanol recovery. ACS Appl Nano Mater 4(1):568–579
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors conceived the work and performed the experiment and preparation section. All authors carried out the writing, analyses, discussion, and revisions of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansy, A.E., El Desouky, E.A., Saleh, A.K. et al. Separation of bioethanol using in situ composite membrane of bacterial cellulose/poly (2-acrylamido-2-methylpropane sulfonic acid) (AMPS) and their characterization. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03983-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03983-7