Abstract
The aim of the present study was to determine changes in the organic nitrogen fraction in the liquid fraction of the digestate during laboratory tests of mono- and co-digestion. Three agricultural wastes were tested: distillery residue, cattle slurry and corn silage. In the mono-fermentation tests, the initial total nitrogen concentration depended on the feedstock used. As a result of the hydrolysis and ammonification of the organic matter contained in the feedstocks, there was an increase in ammonium nitrogen concentration after 28 days of fermentation. Analysis of organic nitrogen fractions showed that different organic nitrogen fractions predominated in each of the feedstocks. Four co-digestion tests were performed with 20% and 80% inoculum content and the combination of feedstocks used in the mono-fermentation. Proper evaluation of the transformation of nitrogen forms for the tests with 20 and 80% inoculum participation required the calculation of concentrations from the mass balance, taking into account the effect of changes taking place in the inoculum itself (control test). For these tests, the initial concentrations of nitrogen forms are similar to those found in the mono-fermentation tests. A greater increase in ammonium nitrogen concentrations was found for the test with 80% inoculum than in the test with 20% (for the same feedstock). An increase in the removal efficiency of the dissolved organic nitrogen fraction (DON), and an increase for the colloidal (CON) and particulate organic nitrogen (PON) fractions after 28 days of co-digestion were observed. The trend of change was similar for 20% and 80% inoculum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent years have seen economic growth leading to increased rates of energy production. The continued growth in demand is linked to the use of existing fossil fuel resources, which can still be used for the next few decades. The use of fossil fuels is becoming increasingly inadequate (among other things, through the high costs of production and exploration, as well as environmental aspects) [1]. This situation is leading to increased interest in renewable energy sources (RES), which are steadily increasing their share of energy production year on year [2]. The RES sector gaining year on year is biogas plants. Europe was one of the leaders in obtaining energy in the form of biogas from biogas plants in 2021. There are approx. 19,000 biogas plants in Europe, producing about 167 TWk of “green energy” per year in the form of biogas [3]. More than 70% of biogas plants located in the European Union use waste from the agri-food and livestock industry for biogas production (e.g. animal waste such as manure or slurry, agricultural waste (e.g. corn silage) and also energy crops) [3, 4].
As an agricultural country with a strongly developed agricultural sector together with an extensive agri-food industry, Poland also has the potential to develop agricultural biogas production [5,6,7,8]. This makes agricultural biogas plants the fastest growing and most promising branch of the renewable energy production sector, specifically in agricultural areas. In 2021, 134 installations were registered in the register of agricultural biogas producers, allowing the production of approximately 540 million m3 of biogas per year. From year to year, the number of operational biogas plants in Poland is increasing systematically. In just 1 year of biogas production alone, almost 5 million tonnes of raw materials were used, of which more than 87% was waste from the agriculture and food industry [9].
In published research, a variety of tests relating to the fermentation process for biogas production can be found for various substrates treated as mono-substrates (e.g. agricultural, industrial or municipal waste, as well as sewage sludge) [10,11,12,13]. However, not every type of feedstock is suitable for direct mono-fermentation due to imbalances in the proportions of nutrients, a lack of diverse microorganisms and adverse effects on the process. A technological alternative increasingly used for methane fermentation is co-digestion. This consists of the mixing of two or more different feedstocks (e.g. sewage sludge together with agricultural, industrial or municipal waste) in one digester at the same time [14,15,16]. Co-digestion enables the potential of the existing digesters to be utilised, the efficiency of the biogas plant to be increased and therefore the energy effect to be greater than that of the mono-fermentation of the product concerned [11, 17,18,19,20]. Co-digestion provides a way to utilise waste and at the same time increase methane production and has the potential to reduce some of the problems that arise with mono-fermentation. It is also one method of increasing the energy self-sufficiency of wastewater treatment plants [15, 21,22,23]. The strategy is in line with the principles of sustainable development [18, 24]. However, a fundamental element of the co-digestion process is the selection of co-substrates with a composition balanced in relation to the main feedstock of the process; the feedstocks used should provide the required balance of nutrients in the feedstock, improve the C/N ratio and dilute compounds inhibiting or toxic to the fermentation process when using the basic feedstock [18, 21, 25, 26]. Feedstocks with a higher carbon/nitrogen (C/N) ratio, such as straw or corn silage, can be used for co-digestion with substrates with a lower C/N ratio, e.g. pig manure or food waste, to achieve a nutrient balance and avoid inhibition of the methane fermentation process [11, 23, 27, 28]. Research has shown, for example, that the use of animal manure with other, different co-substrates (e.g. corn silage) can increase biogas production between 25% and even 400%, compared with the fermentation of the same feedstock in mono-fermentation [16, 29]. Although the co-digestion process has many advantages, its implementation requires the observance of a specific environmental regime and a number of parameters at an optimum level for the microorganisms carrying out the process [11, 20].
The methane fermentation process transforms only part of the organic components contained in the waste into bioenergy. The resulting by-product of biogas production (digestate) is a mass rich in nutrients, such as nitrogen and phosphorus, and can be used as a “green fertiliser” [30, 31]. However, some test results show that it cannot always be treated as a safe product. The digestate may contain toxic contaminants in the form of heavy metals, organic pollutants (e.g. from pesticides or pharmaceuticals) or pathogenic bacteria that can be introduced into the soil during fertilisation [32,33,34]. The heavy metals that are found in the feedstock are not removed during the fermentation process and, in addition, their concentration increases because of the reduction in feedstock mass. It is therefore necessary to assess the heavy metal content to limit the increase in their concentration in the digestate or in the soil above the regulatory limit values [32, 34, 35]. Pathogens found in the feedstock are not fully removed during mesophilic fermentation. Salmonella bacteria have been found in the digestate, as well as the particularly dangerous bacterium L. monocytogenes. Therefore, the use of digestate containing pathogens may pose a threat to the health of food users, as well as contribute to environmental contamination (e.g. crops, soils, groundwater) [33, 34]. The balance of the benefits of using fertiliser digestate for the soil against its possible toxicity should be analysed despite these risks [34].
Another environmental risk arising from the increase in the number of agricultural biogas plants is the significant increase in the amount of digestate produced, as a result of the large amount of feedstock often brought to the biogas plant from distant areas. The most common solution is to use the produced digest directly to fertilise agricultural fields located close to the biogas plant. This can cause over-fertilisation of these farmlands, resulting in environmental pollution (e.g. with nitrogen and phosphorus compounds) [36,37,38,39]. It is economically unviable to manage the hydrated digestate on sites located further away from the agricultural biogas plant because of the high cost of transport[40]. A solution to this problem could be to separate the digestate into solid and liquid fractions and then manage the dewatered (solid) fraction and the leachate separately [41, 42]. The solid fraction is most often used to produce compost and used as a fertiliser [43, 44]. However, a significant problem may be the management of the liquid fraction (leachate) which accounts for more than 80% of the digestate mass. The leachate contains high concentrations of nitrogen and phosphorus [45, 46], and incorrect management (e.g. overdosing, leading to soil over-fertilisation) can lead to nutrients leaching from the soil or infiltrating into groundwater, thus contaminating nearby rivers and adversely affecting the development of aquatic flora and fauna [39, 47].
A review of the literature indicated that the concentration of total nitrogen in the liquid fraction of the digestate can be between 1000 and 3100 mg N/L, mainly in the form of ammonium nitrogen [48, 49]. At the same time, leachates from digestate dewatering contain high concentrations of phosphorus about 1500 mg P/L [48], with low concentrations of bioavailable organic matter [50]. In the literature, though, the information on organic nitrogen fractions and their contribution to the liquid fraction of the digestate is missing. Therefore, the leachate should be treated before being discharged into the environment. In the case of biogas plants located next to wastewater treatment plants, the leachate is most often discharged into the main line of the treatment plant. Increased nitrogen loading, however, means increased energy consumption for aeration, which reduces or even eliminates the positive energy and environmental effects associated with increased biogas production [51, 52]. The solution to this problem is to treat the leachate in a separate treatment plant focused on nitrogen removal. The use of anammox bacteria is most commonly used for this, in one variant of the deammonification system [51, 53]. Another very interesting solution is the treatment of leachate using microalgae biomass. This method not only ensures efficient removal of nitrogen and phosphorus, but also enables the production of biomass that can be used for biofuel production. Such a solution is therefore in line with the idea of a circular economy and makes it possible to fully exploit the energy potential contained in leachate [54, 55]. Furthermore, for this solution, it would be important to study the fraction of organic nitrogen present in the leachate from the dewatering of the digestate.
The aim of this study was to determine the changes of organic nitrogen fractions in the liquid fraction of digestate in an agricultural biogas plant during the mono- and co-digestion processes. The products of the agriculture and food industry were selected for testing the fermentation processes. Based on 2018 data, these wastes accounted for more than 75% of the feedstocks most commonly used in Polish agricultural biogas plants [9]. The correctness of the fermentation process was verified by determining the biogas production.
2 Materials and methods
2.1 Materials
For research purposes, agricultural waste and waste from the food industry were used as feedstock for the fermentation process:
-
Agricultural lignocellulosic waste in the form of corn silage.
-
Food waste in the form of distillery residue.
-
Animal manure in the form of cattle slurry.
Material collected from a well-operating biogas plant located in central Poland was used as inoculum.
Three mono-fermentation batch tests were performed. The reactor feedstock consisted of 20% inoculum and 80% of the tested substance diluted with water to approx. 4% TS (mass proportions). In addition, a control test was performed with inoculum alone. Four co-digestion batch tests were then performed using the same substrates as for mono-fermentation. In the case of co-digestion, two experiments were additionally performed, differing in the proportion of substrate and inoculum. The tests marked A used 20% inoculum and 80% agricultural substrate feedstock, and the tests marked B used 80% inoculum and 20% agricultural substrate feedstock. The feedstock characteristics for each test are shown in Table 1.
2.2 Research methodology
2.2.1 Fermentation process
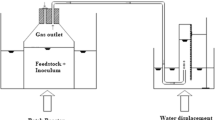
Tests were conducted at laboratory scale on a test station comprising two anaerobic digestion (AD) reactors built with horizontal cylindrical chambers of size a diameter d = 30.5 cm and length l = 60 cm (Fig. 1). The reactors were operated in a batch (non-flow) system. The chamber volume was 44 L (active 30 L). At all times during the test, the reactors were hydrodynamically mixed using external pumps and a temperature was set at 37 °C. The fermentation process was carried out for 28 days. Samples for the analyses were taken at the beginning and end of the tests.
2.2.2 Analyses of biogas
During the test, the volume of biogas produced (with a Ritter MGC1 gas meter (Ritter Apparatebau (Germany)) and its composition (in terms of CH4 and CO2) (with a Geotech GA5000 meter (Geotechnical Instruments (UK)) was checked 3–5 times a week.
2.2.3 Analyses of digestion
Three independent samples of the digestate were each taken at the beginning and end of the methane fermentation process. Dry matter as a fraction of total solids (TS) was determined for the each initial and final sample (triplicate sets of measurements).
The rest of the collected samples were mechanically separated into solid and liquid phases. The mechanical separation was carried out using a Jouan C3i laboratory centrifuge (Thermo Electron Corporation). A centrifugation speed of 4000 rpm and a centrifugation time of 30 min were taken, following the methodology presented in an earlier publication [48].
2.2.4 Analysis of the liquid fraction of the digestate
Analyses of the liquid fraction of the digestate included the forms of total nitrogen (TN), i.e. ammonium nitrogen (NH4-N) and organic nitrogen (ON). Each determination was repeated three times. With regard to organic nitrogen, concentration analyses of the individual fractions were carried out. For this process, the reject water was filtered through Millipore nitrocellulose filters (Billerica, MA) with pore sizes of 0.1, 0.45 and 1.2 μm. For reject water (TNRW) and selected filtrate, determination of total nitrogen (TN0.1 and TN1.2) was also performed using a TOC analyser with TN determination attachment (SHIMADZU Corporation, Japan). The detailed description of Shimadzu method for TN measurement was presented in previous publication [56]. In addition, ammonium nitrogen (NH4-N) concentrations were determined in the filtrate formed by filtration through a 0.45-μm pore size filter using Hach Lange cuvette tests on a DR20000 spectrophotometer. The analytical methodology used by Hach Lange (Germany) and SHIMADZU (Japan) was based on the Standard Methods for Examination of Water and Wastewater [57].
2.2.5 Sequencing of organic nitrogen
Organic nitrogen, due to its physical state, can be divided into particulate organic nitrogen (PON), colloidal organic nitrogen (CON) and dissolved organic nitrogen (DON). On the basis of the results obtained from the analyses of the liquid fraction of the digestate, the concentrations of the individual organic nitrogen fractions were calculated from the following relationships [58]:
2.2.6 Concentrations in the hydrated feedstock
Calculations of the concentrations in the feedstock with the addition of water were carried out based on a mass balance to assess the transformation of nitrogen compounds with respect to the feedstock excluding the influence of the transformation taking place in the inoculum (Eq. 4):
where:
- C:
-
Concentration in diluted substrates at the beginning or the end of individual test
- Ccontrol:
-
Concentration in test C (inoculum alone) at the beginning or the end of test
- CLF:
-
Concentration in the liquid fraction of individual test at the beginning or the end of test
- ηcontrol:
-
Share of inoculum weight in the feedstock
- ηfeedstock:
-
Share of substance weight in the feedstock
- η H2 O:
-
Share of water weight in the feedstock
3 Results and discussion
3.1 Biogas and methane production analysis
Table 2 shows the biogas and methane production potential for mono-fermentation of agricultural feedstocks and for the control test.
For the control test, biogas and methane production rates were low, reflecting the limited availability of organic matter and the use by microorganisms of feedstocks with lower fermentability. At the same time, the volumes of specific biogas and methane production rates are similar to values obtained in earlier tests for inoculum from a biogas plant located in a sewage wastewater treatment plant (0.075 m3 biogas/kg TS, 0.040 m3 CH4/kg TS) [20].
On the basis of the analysis carried out, corn silage was found to be the best feedstock in relation to unit biogas production. Each TS kilogram of this feedstock produced approx. 0.40 m3 biogas and 0.2 m3 methane. The other two feedstocks were characterised by similar values for biogas (0.26–0.28 m3) and methane (0.16–0.17 m3) production potential from each kilogram of TS. The obtained volumes of biogas and methane production rates for all the feedstocks tested are similar to the values presented in the literature. For cattle slurry, Santa et al. [59] tested biogas production as a function of dry matter content and cattle diet. At 8% dry matter in the input, they obtained between 0.11 and 0.15 m3 biogas/kg TS after 20 days of fermentation. In a review paper by Weiland [60], biogas production from corn silage was reported at 0.23 m3 per ton wet matter. In contrast, a test by Gunes et al. [61] on the fermentation of distillery and brewery wastes yielded 0.18 m3/kg TS. On the basis of a comparison of the biogas and methane production values obtained with those presented in the research literature, it can be concluded that the course of the fermentation process is correct. This provides the composition of the obtained digestate as measured for analyses of the transformation of nitrogen compounds. Table 3 summarises the specific biogas and methane production rates for the co-digestion tests of the analysed feedstocks.
The results show a major difference in biogas production rates between tests A and B. For tests with 20% of inoculum, the unit values for biogas and methane production are about 300% higher for the co-digestion of a feedstock containing distillery residue and corn compared with a feedstock containing slurry and corn. In tests with 80% of inoculum, values about 150% higher were obtained. This may be due to the availability of organic carbon for the microorganisms and thus the effect on microbial consortia and their adaptability. Such a large difference in values for co-digestion, with comparable volumes of unit biogas and methane production for tests with slurry and distillery residue mono-fermentation, indicates a significant positive effect of the feedstock composition prepared in this way on methane bacterial activity. The work of Wojcieszak et al. [62] presents tests on the adaptive effect of corn silage inoculum, which wereobtained from different sites, for methane production. The results obtained indicate that for different compositions of consortia that adapt during the process, different values of methane concentrations in the produced biogas were obtained [62]. The adaptability of the microbial consortia may result in differences in production rates and biogas composition despite using the same inoculation and inoculum.
Figure 2 shows the course of change in the proportion of methane in the biogas produced for the co-digestion tests. The course of the change in the proportion of methane in the biogas shows that for tests with 20% inoculum (tests A1 and A2), a gradual increase in the proportion of methane can be observed, which stabilises at a level of more than 60% by the end of the experimental time. The trend in the concentration of carbon dioxide is different. At the beginning of the test, its share in the biogas is high (more than 80%) and then decreases as the proportion of methane increases. In these experiments, the microorganisms multiply in the fermenter and undergo successive growth phases, which have an impact on the progression of the different methane fermentation phases. Such tests make it possible to trace the dynamics of gaseous product production as the number of microorganisms increases. The results obtained confirm the correct course of growth in size and composition of the biogas. In the case of tests with 80% of inoculum (tests B1 and B2), we observe an immediate (after 2 days) increase in the proportion of methane in the biogas to a value of 60–70% and subsequent stabilisation with slight changes in the methane content of the biogas produced. The trend is similar for the proportion of carbon dioxide, which peaks after 2 days (approximately 40%) and remains at this level until the end of the test. For experiments with 80% of inoculum, we can observe what is the effect of the feedstocks added as the input on the biogas production rate with a high concentration of microorganisms. In this case, the consortium of microorganisms is in a stationary growth phase and, after nutrient depletion, moves to a die-off phase. In this case, a decrease in the amount of biogas produced will be observed, with a stable proportion of methane. This is confirmed by the presented research results.
3.2 Transformation of nitrogen in the liquid fraction
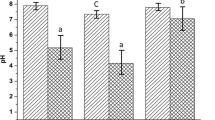
Table 4 shows the concentrations of total nitrogen and ammonium nitrogen in the liquid fraction at the beginning and after 28 days of mono-fermentation, and for the control test. Figure 3, on the contrary, shows the concentrations and the increase or decrease of these values during fermentation, including the influence of inoculum transformations. The concentration of total nitrogen in the liquid fraction depended on the separation of the solid fraction by centrifugation. The initial concentration of total nitrogen in the control test was 829.1 mg N/L, of which ammonium nitrogen accounted for 643 mg NH4-N/L. A higher concentration of ammonium nitrogen was shown for the inoculum from the municipal biogas plant in Swarzewo: 867 mg NH4-N/L [20]. After 28 days of fermentation, an increase of 15.8% in total nitrogen and 26.7% in ammonium nitrogen was found. The increase in these values was due to the decomposition of residual organic matter contained in the inoculum and the transition of nitrogen to the liquid fraction and the ammonification process. In the mono-fermentation tests, initial total nitrogen concentrations depended on the feedstock. Relatively high values were found for feedstocks in the form of cattle slurry (2067.0 mg N/L) and distillery residue (1868.3 mg N/L), and considerably lower values for corn silage (358.0 mg N/L). These differences are due to the characteristics of the batches, including the presence of the dissolved fraction. For all three feedstocks, there was an increase in total nitrogen concentration at the end of the test. Recalculating the concentrations, considering the transformations taking place in the inoculum, the increase in TN concentration for the hydrated feedstocks was relatively small (8–9%), while for the corn silage, it exceeded 20%. This means that for slurry and distillery residue, most of the transformations were related to the nitrogen in the dissolved fraction, while for corn silage, the effect of the decomposition of the feedstock solids was significant (Fig. 3A).
For the evaluation of organic nitrogen transformations, the concentration of ammonium nitrogen was important (Fig. 3B). Ammonium nitrogen is formed during hydrolysis of proteins and urea contained in the feedstock and is the predominant form of nitrogen in the liquid fraction. An increase in the concentration of ammonium nitrogen indicates that the process is proceeding correctly. The initial concentration of ammonium nitrogen in the tests depended on its concentration in the feedstock initially dissolved with water to obtain a constant TS value of 4%. Considering the proportion of nitrogen contained in the inoculum, the highest concentration of ammonium nitrogen was characterised by slurry (1778.0 mg NH4-N/L). The initial concentration in distillery residue was more than 3.5 times lower (497.4.0 mg NH4-N/L), and by far the lowest for corn silage (96.3 mg NH4-N/L). As a result of hydrolysis and ammonification of the organic matter contained in the feedstocks, there was an increase in ammonium nitrogen concentration after 28 days of fermentation. A particularly high increase was found for the distillery residue (by 271.7%) and this despite the relatively high initial values. By far, the lowest increase was recorded for cow slurry (16.8%), which could be due to the ammonification process in the storage tank from which this feedstock was taken. For corn silage, the increase was 44.7%, which, with a very low initial value, resulted in the lowest concentration for the three feedstocks tested (139.4 mg NH4-N/L). The concentrations of ammonium nitrogen in the mono-fermentation tests are similar to previous research. In the research literature, ammonium nitrogen concentrations vary between 500 and 3100 mgNH4-N/L or 1730 and 2100 mg NH4-N/L [48, 49], depending on the feedstocks used. Ammonium nitrogen accounted for 30 to 75% of total nitrogen at the beginning of the test, and at the end, its percentage had increased to 60–80% of total nitrogen. Similar shares of ammonium nitrogen in relation to total nitrogen can be found in the research literature, e.g. from 16 to 72% for leachates from agricultural biogas plants [49]; from 60 to 90% for pure feedstocks of slurry, distillery residue, corn silage or fruit and vegetable waste [48].
In the research literature, information on nitrogen transformations is mainly restricted to TN and ammonium nitrogen concentrations. However, the results of analyses of the organic nitrogen fraction are limited. In the control test, the initial organic nitrogen fraction contribution was similar. During the 28 days of fermentation, there was more than a 30% decrease in the DON fraction and a 20% decrease in CON. These values are correlated with an increase in ammonium nitrogen concentration, indicating that ammonification mainly affects the DON fraction, which is simultaneously fed from the hydrolysis of CON and PON. Taking into account the limited access to feedstock in this test, it can be concluded that DON after fermentation consists of organic compounds not susceptible to ammonification under digester conditions.
After the start of the mono-fermentation tests, different fractions of organic nitrogen predominated. For slurry, it was the PON fraction (almost 56%);for corn silage, it was the CON fraction (almost 64%); for distillery residue, it was the dissolved DON fraction (almost 80%). After 28 days of fermentation, the percentage distribution of organic nitrogen fractions changed significantly. The greatest change occurred with regard to the DON contained in the distillery residue. A decrease from an initial value of 1325.8 mg N/L to 139.2 mg N/L (concentrations after taking into account the effect of the inoculum) was found. This represents a removal efficiency of 90%. The decrease in DON concentration was due to ammonification, which is associated with a simultaneous significant increase in ammonium nitrogen concentration for this feedstock. Also in the slurry test, a decrease in the proportion of DON was found, but much lower (by 35%). This may support the conclusion from the analysis of ammonium nitrogen concentrations, that there is an intensive process of hydrolysis and ammonification of nitrogen compounds in the storage tank of this feedstock. Only in the test with corn silage was an increase in DON concentration found (by more than 100%), which may be due to the very low initial concentrations of this fraction and the significant proportion of nitrogen compounds not susceptible to ammonification. In the case of slurry and distillery residue, the concentration of CON increased significantly, and in the case of corn silage, PON which became dominant in the liquid fraction of the digestate (Fig. 4A). This increase is a measure of the transformation of nitrogen compounds in the hydrolysis of the solids and individual fractions during the 28-day fermentation. In tests conducted by Tuszynska et al. [63], the proportion of individual organic nitrogen fractions was similar. In the samples containing slurry, as well as in the sample with corn silage, the predominant fractions were the suspended solids and colloidal fractions. Their combined share was almost 80%. In previous tests of leachates from sludge digestion, much lower concentrations of individual organic nitrogen fractions, i.e. 52.0, 72.0 and 61.3 mgN/L, were obtained for DON, CON and PON, respectively [20]. Even lower values for DON (24.6–29.5 mg N/L) and CON (33.7–38.5 mgN/L) concentrations were obtained for digester leachate at two large wastewater treatment plants located in northern Poland [58]. Also lower DON concentrations (28.8 mgN/L, for DON defined as 0.45 µm sieve filtrate) were obtained for mesophilic sludge digestion from the Kelowna WWTP (British Columbia, Canada) [64]. Galvagno et al. [65] show a significant increase in DON concentration (also defined as 0.45 µm sieve filtrate) during sludge methane digestion (from 61.7 to 210 mgN/L) with a significant increase in ammonium nitrogen concentration from 330 to 1186 mg NH4-N/L. In subsequent tests of methane digestion of sewage sludge from two wastewater treatment plants located in Canada, it also showed high final concentrations of DON in the digestate (85.7 and 204 mg N/L) [65]. In comparison, digestion of fruit and vegetable wastes yielded relatively high final concentrations of CON fraction (average 331.0 mgN/L) and DON (average 200.6 mgN/L) [48].
The results of determining the concentrations of nitrogen compounds at the beginning of the co-digestion test and after 28 days of the process are presented in Table 5. The determined values of the initial concentrations of nitrogen forms are similar to the theoretical values determined from the mass balance taking into account the concentrations during the mono-fermentation tests (Figs. 5 and 6). The differences in concentration values are within ± 20%, indicating a similar composition of the inoculum and the agricultural feedstocks used in both test series. In tests A1 and B1, higher initial concentrations of ammonium nitrogen were found compared to tests A2 and B2, which was due to the use of cattle slurry in the inoculum. For the tests with 20% inoculum (A1 compared to A2), 160% higher values were obtained, while for the tests with 80% inoculum (B1 compared to B2), the difference is lower (114%) which is due to the much higher proportion of inoculum. The differences in ammonium nitrogen concentrations calculated for the values obtained from the mass balance, i.e. taking into account the effect of the inoculum (Fig. 5B), are much higher at 192 and 256%, respectively. In contrast, tests A2 and B2 showed significantly higher concentrations of the DON fraction compared to tests A1 and B1, which is related to the properties of the broth used as co-substrate. For tests with 20% inoculum (A1 compared to A2), values 1130% higher were obtained, while for tests with 80% inoculum (B1 compared to B2), the difference is lower (340%), which is also due to the significantly higher proportion of inoculum. The differences in DON concentrations calculated for the values obtained from the mass balance, i.e. taking into account the effect of inoculum (Fig. 6C), are much higher, yet similar for the two tests, at 1440 and 1630%, respectively.
During the 28-day co-digestion of cattle slurry and corn silage, a 21.9% increase in ammonium nitrogen concentration (from 603.8 to 735.8 mg NH4-N/L) and a 23.7% increase in TN concentration (from 784.4 to 970.0 mgN/L) were found with 20% inoculum (A1 test) (values for concentrations calculated from the mass balance, taking into account the effect of inoculum). This increase is similar to the values obtained in the mono-fermentation tests for both inputs used. Significantly higher concentration increases were found for the test with 80% inoculum and the same batch composition (test B1). These were 50.3% for ammonium nitrogen and 74.9% for TN, respectively. At the same time, the final concentrations determined from the mass balance (1570.8 mgN/L and 1006.7 mg NH4-N/L) are close to the literature values for fermentation of cow dung and corn silage (from 1900 to 25,000 mg N/L and from 650 to 840 mg NH4-N/L [66]. The greater increase in ammonium nitrogen concentration for tests with 80% inoculum, compared with tests with 20% inoculum, is due to the limited access of microorganisms to the organic feedstock, resulting in the use of compounds with less susceptibility to anaerobic decomposition. The effect of hydrolysis and ammonification of these organic compounds on the significant increase in ammonium nitrogen and TN concentrations in the liquid fraction of the digestate became apparent when the concentrations were recalculated based on a formula taking into account the effect of inoculum in the mass balance (formula 4). For values determined directly for the liquid fraction of the digestate, the increase is much smaller (13.5% for ammonium nitrogen and 12.1% for TN, respectively) and can be considered negligible.
The influence of methane fermentation microorganisms on the trend in the hydrolysis of macromolecular compounds is also confirmed for the organic nitrogen fraction. An increase in the removal efficiency of the DON fraction was found from about 10% for the test with 20% inoculum (A1) to almost 20% for the test with 80% inoculum, and this despite the expected higher hydrolysis rate of the CON fraction to DON. At the same time, a greater increase in the CON fraction (from 160% for the A1 test to 220% for the B1 test) and PON (from a decrease of 50% for the A1 test to an increase of 150% for the B1 test) was observed after 28 days of co-digestion, confirming the higher degree of defragmentation and hydrolysis of the solids present in the input and their transition to the liquid fraction, an effect that is more pronounced for the broth and corn silage co-digestion tests. Broth is characterised by high initial concentrations of DON, which resulted in high concentrations of this fraction at the start of the A2 (548 mg N/L) and B2 (181 mg N/L) tests (Table 5).
After taking into account the effect of inoculum and converting from mass balance, the initial concentrations in the hydrated feedstock (distillery residue and corn silage) were 617 and 681 mgN/L for test A2 and B2, respectively (Fig. 6C). After 28 days of fermentation, an increase in CON and PON fraction concentrations was found for both tests, with a significant decrease in DON fraction concentrations. Furthermore, for these tests, the effect of the initial proportion of inoculum is evident. With limited access to feedstock (test with 80% inoculum share), the microorganisms hydrolyse compounds that are difficult to biodegrade in methane fermentation. This process results in an increase in the concentration of the PON fraction, a 218% increase compared to a 52% increase for a test with 20% inoculum participation, and CON (132% increase compared to a 55% increase, respectively) (Fig. 6A, B). Similar to the distillery residue mono-fermentation test, a decrease in the concentration of the DON fraction is observed. For similar process conditions (20% inoculum share), the efficiency of the ammonification process of the DON fraction is comparable, 89% in the M2 test and 84% in the A2 test. In contrast, during tests with 80% inoculum participation, the efficiency of the process is clearly higher and exceeds 98%, resulting in a final DON concentration of 34 mg N/L (value after taking into account the effect of inoculum). With limited access to organic compounds in the B2 test, it can be assumed that this is the non-biodegradable fraction of DON in the digesters. A high degree of ammonification of organic nitrogen results in a significant increase in ammonium nitrogen concentration. The final NH4-N concentrations (after mass balance conversions) were 1298 and 1573 mg NH4-N/L for test A2 and B2, respectively (Fig. 5B). Compared to the initial concentrations, this represents an increase of 313% (test A2) and as much as 502% (test B2). Comparison of the increase in ammonium nitrogen concentration with the decrease in DON concentration indicates that the ammonification also included DON formed by hydrolysis of PON and CON. The concentration of this additionally generated DON fraction is 418 and 643 mgN/L, which is 70% or 90% of the remaining DON at the end of the test, for test A2 and B2, respectively.
An open issue, which requires analysis in further tests, is the evaluation of the biodegradability of residual DON under aerobic conditions. The answer to this question will contribute to the possibility of evaluating the migration of DON to water in surface water or groundwater as a result of using the liquid fraction of the digestate for irrigation/fertilisation of agricultural crops. The biodegradability of DON under aerobic conditions is also important for assessing the impact of the final treatment of the liquid fraction of digestate from agricultural biogas plants in the bioreactors of municipal wastewater treatment plants. Previous tests of sludge co-digestion with vegetables and with vegetables and flotate from on-site wastewater treatment plants (for the agri-food industry) showed an increase in CON concentrations (from 34.4 to 98 mgN/L and 101 to 126.6). At the same time, a significant decrease in DON concentrations was found (by 67.4 mgN/L and 82.2 mgN/L, respectively).
4 Conclusions
This study determined changes in the organic nitrogen fraction in the liquid fraction of digestate in laboratory tests of mono- and co-fermentation processes. Tests were carried out on the digestate from the fermentation of three agricultural and food wastes: distillery residue, cattle slurry and corn silage.
An analysis of the volume of biogas and methane production per unit showed that the fermentation process was stable in all tests, with a typical biogas production volume. An analysis of the mono-fermentation results showed that corn silage was the preferred feedstock in biogas production. The resulting specific biogas and methane production rates for all the feedstocks analysed were similar to values presented in the literature. During the co-digestion process, a combination of cattle slurry and corn silage was shown to be the preferred feedstock for biogas and methane production. During tests with 20% inoculum, a steady increase in the proportion of methane was observed until a value of about 60–70% was reached at the end of the 28-day test. This type of test allowed the fermentation trend to be evaluated with increasing microbial counts. At the higher inoculum proportion (80%), an immediate increase in the proportion of methane in the biogas to a value of approx. 60–70% was observed. For most of the test period, this proportion remained stable, with a slight decreasing trend. In tests with 80% inoculum, the effect of the feedstocks added in the input on the fermentation process was analysed at high microbial concentration.
In the mono-fermentation tests, the beginning total nitrogen concentrations depended on the feedstock. The highest nitrogen concentrations were found for the feedstock in the form of cattle slurry (2067.0 mg N/L) and significantly lower for corn silage (358.0 mg N/L). These differences are due to the characteristics of the feedstocks, including the forms of nitrogen in the dissolved fraction. For all three feedstocks, an increase in total nitrogen concentration was recorded at the end of the test, confirming the correct trend of the methane fermentation process. Of significance in terms of evaluating the transformation organic nitrogen were the changes in ammonium nitrogen concentration values. As a result of hydrolysis and ammonification of organic matter contained in the feedstocks, there was an increase in ammonium nitrogen concentration after 28 days of fermentation. The lowest increase was recorded for cow slurry (16.8%) and the highest for distillery residue (44.7%). However, considering the initial ammonium nitrogen concentration in each of the inputs, it was the distillery residue that showed the lowest final ammonium nitrogen concentration of 139.4 mgNH4-N/L. Furthermore, the analysis of organic nitrogen fractions showed that different fractions of organic nitrogen predominated in each of the feedstocks. For slurry, it was the PON fraction (almost 56%); for corn silage, it was the CON fraction (almost 64%); for distillery residue, it was the dissolved DON fraction (almost 80%). After 28 days of fermentation, the percentage distribution of organic nitrogen fractions changed significantly. The greatest change occurred with regard to the DON contained in the distillery residue. An approx. 90% decrease in DON concentration was shown for this feedstock, which, undergoing ammonification, contributed to the highest increase in ammonium nitrogen concentration.
In tests of the co-digestion process, the initial concentrations of nitrogen forms are similar to those found in the mono-fermentation tests. In particular, higher initial values for ammonium nitrogen concentrations were found for the tests using cattle slurry, and higher initial concentrations of the DON fraction were found for the tests using distillery residue. Proper evaluation of the transformation of nitrogen forms for the tests with 20 and 80% inoculum participation required the calculation of concentrations from the mass balance taking into account the effect of changes taking place in the inoculum itself (control test). After 28 days of fermentation, increases in ammonium nitrogen concentrations were found in all tests. Significantly greater increases in concentration were found for tests with 80% inoculum and the same feedstock composition compared to tests with 20% inoculum. On this basis, it can be concluded that with limited access to organic feedstock, microorganisms also use compounds with lower biodegradability in anaerobic conditions.
The results obtained require further verification for other digester feedstock compositions in municipal and agricultural biogas plants. Another important issue for further testing is the biodegradability of the DON contained in the liquid fraction of the digestate under aerobic conditions. This process will take place in the ground environment after application of leachate to agricultural fields and in the activated sludge chambers at the final management of leachate in municipal wastewater treatment plants (e.g. in winter).
References
Iglinski B, Flisikowski K, Pietrzak M, Kiełkowska U, Skrzatek M, Zyadin A, Natarajan K (2021) Renewable energy in the Pomerania Voivodeship—institutional, economic, environmental and physical aspects in light of Eu energy transformation. Energies 14(24):8221. https://doi.org/10.3390/en14248221
Chovancová J, Tej J (2020) Decoupling economic growth from greenhouse gas emissions: the case of the energy sector in V4 countries. Equilibrium Q J Econ Econ Pol 15(2):235–251. https://doi.org/10.24136/eq.2020.011
EBA (2021) European biogas association statistical report 2020. https://www.europeanbiogas.eu/eba-statistical-report-2021/. Accessed 01.09.2022
Scarlat N, Dallemand JF, Fahl F (2018) Biogas: developments and perspectives in Europe. Renew Energ 129:457–472. https://doi.org/10.1016/j.renene.2018.03.006
Hernik J, Noszczyk T, Rutkowska A (2019) Towards a better understanding of the variables that influence rEnergy sources in Eastern Poland. J Clean Prod 241:118075. https://doi.org/10.1016/j.jclepro.2019.118075
Igliński B, Buczkowski R, Iglińska A, Cichosz M, Piechota G, Kujawski W (2012) Agricultural biogas plants in Poland: investment process, economical and environmental aspects, biogas potential. Renew Sustain Energy Rev 16(7):4890–4900. https://doi.org/10.1016/j.rser.2012.04.037
Piwowar A (2019) Development of the agricultural biogas market in Poland – production volume, feedstocks, activities and behaviours of farmers. Zeszyty Naukowe SGGW w Warszawie - Problemy Rolnictwa Światowego 19(1):88–97. https://doi.org/10.22630/PRS.2019.19.1.8
Piwowar A (2020) Agricultural biogas-an important element in the circular and low-carbon development in Poland. Energies 13(7):1733. https://doi.org/10.3390/en13071733
KOWR (2021) Rejestru Wytwórców Biogazu Rolniczego 2021. Krajowy Ośrodek Wsparcia Rolnictwa. https://www.kowr.gov.pl/odnawialne-zrodla-energii/biogaz-rolniczy/wytworcy-biogazu-rolniczego/rejestr-wytworcow-biogazu-rolniczego. Accessed 01.09.2022
Babaee A, Shayegan J, Roshani A (2013) Anaerobic slurry co-digestion of poultry manure and straw: effect of organic loading and temperature. J Environ Health Sci Eng 11(1):15. https://doi.org/10.1186/2052-336X-11-15
Hagos K, Zong J, Li D, Liu C, Lu X (2017) Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew Sustain Energy Rev 76(September):1485–1496. https://doi.org/10.1016/j.rser.2016.11.184
Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L (2011) The anaerobic digestion of solid organic waste. J Waste Manag 31(8):1737–1744. https://doi.org/10.1016/j.wasman.2011.03.021
Salminen E, Rintala J (2002) Anaerobic digestion of organic solid poultry slaughterhouse waste - a review. Bioresour Technol 83(1):13–26. https://doi.org/10.1016/S0960-8524(01)00199-7
Kainthola J, Kalamdhad AS, Goud VV (2019) A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem 84(May):81–90. https://doi.org/10.1016/j.procbio.2019.05.023
Mata-Alvarez J, Macé S, Llabrés P (2000) Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour Technol 74(1):3–16. https://doi.org/10.1016/S0960-8524(00)00023-7
Ali Shah F, Mahmood Q, Rashid N, Pervez A, Raja IA, Shah MM (2015) Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew Sustain Energy Rev 42:627–642. https://doi.org/10.1016/j.rser.2014.10.053
Davidsson Å, Lövstedt C, Jansen JI, Gruvberger C, Aspegren H (2008) Co-digestion of grease trap sludge and sewage sludge. J Waste Manag 28(6):986–92. https://doi.org/10.1016/j.wasman.2007.03.024
Masłon A, Czarnota J, Szaja A, Szulzyk-Cieplak J, Łagód G (2020) The enhancement of energy efficiency in a wastewater treatment plant through sustainable biogas use: case study from Poland. Energies 13(22):6056. https://doi.org/10.3390/en13226056
Nag R, Auer A, Markey BK, Whyte P, Nolan S, O’Flaherty V, Russell L, Bolton D, Fenton O, Richards K, Cummins E (2019) Anaerobic digestion of agricultural manure and biomass – critical indicators of risk and knowledge gaps. Sci Total Environ 690:460–79. https://doi.org/10.1016/j.scitotenv.2019.06.512
Wilinska-Lisowska A, Ossowska M, Czerwionka K (2021) The influence of co-fermentation of agri-food waste with primary sludge on biogas production and composition of the liquid fraction of digestate. Energies 14(7):1907. https://doi.org/10.3390/en14071907
Grosser A, Neczaj E (2016) Enhancement of biogas production from sewage sludge by addition of grease trap sludge. Energy Convers Manag 125:301–308. https://doi.org/10.1016/j.enconman.2016.05.089
Jenicek P, Bartacek J, Kutil J, Zabranska J, Dohanyos M (2012) Potentials and limits of anaerobic digestion of sewage sludge: energy self-sufficient municipal wastewater treatment plant? Water Sci Technol 66(6):1277–1281. https://doi.org/10.2166/wst.2012.317
Rabii A, Saad A, Yaser D, Elbeshbishy E (2019) A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 12(6):1160. https://doi.org/10.3390/en12061106
Werle S (2015) Sewage sludge-to-energy management in Eastern Europe: a Polish perspective. Ecol Chem Eng S 22(3):459–469. https://doi.org/10.1515/eces-2015-0027
Cano R, Pérez-Elvira SI, Fdz-Polanco F (2015) Energy feasibility study of sludge pretreatments: a review. Appl Energy 149:176–185. https://doi.org/10.1016/j.apenergy.2015.03.132
Siddique MNI, Wahid ZA (2018) Achievements and perspectives of anaerobic co-digestion: a review. J Clean Prod 194:359–371. https://doi.org/10.1016/j.jclepro.2018.05.155
Panpong K, Srisuwan G (2014) O-Thong S, Kongjan P, Anaerobic co-digestion of canned seafood wastewater with glycerol waste for enhanced biogas production. Energy Procedia 52:328–336. https://doi.org/10.1016/j.egypro.2014.07.084
Sosnowski P, Wieczorek A, Ledakowicz S (2003) Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv Environ Res 7(3):609–616. https://doi.org/10.1016/S1093-0191(02)00049-7
Meiramkulova K, Bayanov A, Ivanova T, Havrland B, Kára J, Hanzlíková I (2018) Effect of different compositions on anaerobic co-digestion of cattle manure and agro-industrial by-products. Agron. Res. 16(1):176–187. https://doi.org/10.15159/ar.18.008
Atelge MR, Krisa D, Kumar G et al (2020) Biogas production from organic waste: recent progress and perspectives. Waste Biomass Valor 11:1019–1040. https://doi.org/10.1007/s12649-018-00546-0
Haiyuan M, Guo Y, Qin Y, Li YY (2018) Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour Technol 269:520–531. https://doi.org/10.1016/j.biortech.2018.08.114
Bonetta S, Bonetta S, Ferretti E, Fezia G, Gilli G, Carraro E (2014) Agricultural reuse of the digestate from anaerobic co-digestion of organic waste: microbiological contamination, metal hazards and fertilizing performance. Water Air Soil Pollut 225:2046. https://doi.org/10.1007/s11270-014-2046-2
Kuusik A, Pachel K, Kuusik A, Loigu E (2017) Possible agricultural use of digestate. Proc Est Acad Sci 66:64–74. https://doi.org/10.3176/proc.2017.1.10
Cucina M, Tacconi C, Ricci A, Pezzolla D, Sordi S, Zadra C, Gigliotti G (2018) Evaluation of benefits and risks associated with the agricultural use of organic wastes of pharmaceutical origin. Sci Total Environ 613–614:773–782. https://doi.org/10.1016/j.scitotenv.2017.09.154
Sager M (2007) Trace and nutrient elements in manure, dung and compost samples in Austria. Soil Biol Biochem 39:1383–1390. https://doi.org/10.1016/j.soilbio.2006.12.015
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour Technol 143:632–641. https://doi.org/10.1016/j.biortech.2013.06.030
Kratzeisen M, Starcevic N, Martinov M, Maurer C, Müller J (2010) Applicability of biogas digestate as solid fuel. Fuel 89(9):2544–2548. https://doi.org/10.1016/j.fuel.2010.02.008
Roy ED (2017) Phosphorus recovery and recycling with ecological engineering: a review. Ecol Eng 98:213–27. https://doi.org/10.1016/j.ecoleng.2016.10.076
Tambone F, Orzi V, D’Imporzano G, Adani F (2017) Solid and liquid fractionation of digestate: mass balance, chemical characterization, and agronomic and environmental value. Bioresour Technol 243:1251–1256. https://doi.org/10.1016/j.biortech.2017.07.130
Rehl T, Müller J (2011) Life cycle assessment of biogas digestate processing technologies. Resour Conserv Recycl 56(1):92–104. https://doi.org/10.1016/j.resconrec.2011.08.007
Herbes C, Roth U, Wulf S, Dahlin J (2020) Economic assessment of different biogas digestate processing technologies: a scenario-based analysis. J Clean Prod 255:120282. https://doi.org/10.1016/j.jclepro.2020.120282
Urbanowska A, Kabsch-Korbutowicz M (2021) The use of flat ceramic membranes for purification of the liquid fraction of the digestate from municipal waste biogas plants. Energies 14(13):3947
Piccoli I, Virga G, Maucieri C, Borin M (2021) Digestate liquid fraction treatment with filters filled with recovery materials. Water 13(1):1–12. https://doi.org/10.3390/w13010021
Slepetiene A, Volungevicius J, Jurgutis L, Liaudanskiene I, Amaleviciute-Volunge K, Slepetys J, Ceseviciene J (2020) The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag 102:441–451. https://doi.org/10.1016/j.wasman.2019.11.008
Shi L, Simplicio W, S, Wu G, et al (2018) Nutrient recovery from digestate of anaerobic digestion of livestock manure: a review. Curr Pollution Rep 4:74–83. https://doi.org/10.1007/s40726-018-0082-z
Vaneeckhaute C, Lebuf V, Michels E, Belia E, Vanrolleghem PA, Tack FMG, Meers E (2017) Nutrient recovery from digestate: systematic technology review and product classification. Waste Biomass Valor 8:21–40. https://doi.org/10.1007/s12649-016-9642-x
Svoboda N, Taube F, Kluß C, Wienforth B, Kage H, Ohl S, Hartung E, Herrmann A (2013) Crop production for biogas and water protection-a trade-off? Agric Ecosyst Environ 177:36–47. https://doi.org/10.1016/j.agee.2013.05.024
Tuszynska A, Wilinska-Lisowska A, Czerwionka K (2021) Phosphorus and nitrogen forms in liquid fraction of digestates from agricultural biogas plants. Environ Technol 42(25):3942–3954. https://doi.org/10.1080/09593330.2020.1770339
Akhiar A, Battimelli A, Torrijos M, Carrere H (2017) Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. J Waste Manag 59:118–128. https://doi.org/10.1016/j.wasman.2016.11.005
Ji F, Liu Y, Hao R, Li G, Zhou YG, Dong RJ (2014) Biomass production and nutrients removal by a new microalgae strain Desmodesmus sp. in anaerobic digestion wastewater. Bioresour Technol 161:200–207. https://doi.org/10.1016/j.biortech.2014.03.034
Majtacz J, Grubba D, Czerwionka K (2020) Application of the anammox process for treatment of liquid phase digestate. Water 12(11):2965. https://doi.org/10.3390/w12112965
Zaborowska E, Czerwionka K, Makinia J (2017) Strategies for achieving energy neutrality in biological nutrient removal systems - a case study of the Slupsk WWTP (Northern Poland). Water Sci Technol 75(3):727–740. https://doi.org/10.2166/wst.2016.564
Majtacz J, Grubba D, Kowal P, Czerwionka K (2020) Possibilities of leachate co-treatment originating from biogas production in the deammonification process. J Ecol Eng 21(1):14–19. https://doi.org/10.12911/22998993/113507
Li G, Hua R, Wang N, Yang T, Xu F, Li J, Wu J, Huang Z, Minmin P, Tao L (2022) Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J Clean Prod 355:131768. https://doi.org/10.1016/j.jclepro.2022.131768
Li G, Yuhang H, Tenglun Y, Wenbo X, Minmin P, Shuhao H, Tao L (2022) Enhancing bioenergy production from the raw and defatted microalgal biomass using wastewater as the cultivation medium. Bioengineering 9(11):637. https://doi.org/10.3390/bioengineering9110637
Czerwionka K, Mąkinia J, Pagilla KR, Stensel HD (2012) Characteristics and fate of organic nitrogen in municipal biological nutrient removal wastewater treatment plants. Water Res 46:2057–2066. https://doi.org/10.1016/j.watres.2012.01.020
APHA (2017) Standard methods for the examination of water and wastewater. Federation. Water Environmental American Public Health Association (APHA), Washington, DC, USA. Federation. Washington DC
Czerwionka K, Makinia J (2014) Dissolved and colloidal organic nitrogen removal from wastewater treatment plants effluents and reject waters using physical-chemical processes. Water Sci Technol 70(3):561–568. https://doi.org/10.2166/wst.2014.267
Santana A, Pound B (1980) Paper First Presented at the 5th Annual Meeting of the Dominican Centre for Livestock Research with Sugar Cane, March 1 1980, 2 Technical Cooperation Officer. Overseas Development Administration, London, pp 130–135
Weiland P (2006) Biomass digestion in agriculture: a successful pathway for the energy production and waste treatment in Germany. Eng Life Sci 6(3):302–309. https://doi.org/10.1002/elsc.200620128
Gunes B, Carrié M, Benyounis K, Stokes J, Davis P, Connolly C, Lawler J (2020) Optimisation and modelling of anaerobic digestion of whiskey distillery/brewery wastes after combined chemical and mechanical pre-treatment. Processes 8(4):492. https://doi.org/10.3390/pr8040492
Wojcieszak M, Pyzik A, Poszytek K, Krawczyk PS, Sobczak A, Lipinski L, Roubinek O, Palige J, Sklodowska A, Drewniak L (2017) Adaptation of methanogenic inocula to anaerobic digestion of maize silage”. Front Microbiol 88:1881. https://doi.org/10.3389/fmicb.2017.01881
Dabrowska L, Rosinska A (2012) Change of PCBs and forms of heavy metals in sewage sludge during thermophilic anaerobic digestion. Chemosphere 88:168–173. https://doi.org/10.1016/j.chemosphere.2012.02.073
Abel-Denee M, Abbott T, Eskicioglu C (2018) Using mass struvite precipitation to remove recalcitrant nutrients and micropollutants from anaerobic digestion dewatering centrate. Water Res 132:292–300. https://doi.org/10.1016/j.watres.2018.01.004
Galvagno G, Eskicioglu C, Able-Denee M (2016) Biodegradation and chemical precipitation of dissolved nutrients in anaerobically digested sludge dewatering centrate. Water Res 96:84–93. https://doi.org/10.1016/j.watres.2016.03.036
Seppälä M, Pyykkönen V, Väisänen A, Rintala A (2013) Biomethane production from maize and liquid cow manure - effect of share of maize, post-methanation potential and digestate characteristics. Fuel 107:209–216. https://doi.org/10.1016/j.fuel.2012.12.069
Funding
This study was financially supported by European Regional Development Fund within the framework of Smart Growth Operational Programme 2014–2020 under the project no POIR.04.01.02–00-0022/17.
Author information
Authors and Affiliations
Contributions
Conceptualization: Krzysztof Czerwionka; methodology: Anna Wilinska-Lisowska and Otton Roubinek; formal analysis and investigation: Anna Wilinska-Lisowska and Otton Roubinek; writing—original draft preparation: Anna Wilinska-Lisowska and Otton Roubinek; writing review and editing: Krzysztof Czerwionka and Andrzej Chmielewski; supervision: Krzysztof Czerwionka. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
There are not financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilińska-Lisowska, A., Roubinek, O., Chmielwski, R. et al. Nitrogen transformation during fermentation in agricultural biogas plants. Biomass Conv. Bioref. 13, 16405–16419 (2023). https://doi.org/10.1007/s13399-023-03975-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03975-7