Abstract
Wastewater treatment–derived sludge is a growing concern. Environmental issues, rising sludge production rates, and stringent regulations create the necessity to seek for treatment and valorization alternatives. Sludge is a potential source of high-value materials which can be recovered and transformed into new products such as animal feeds; bioplastics; biofuels, biostimulants; or biobased fertilizers. Considering the current legal constraints hindering the use of certain waste streams, the objective of this work is to show the technical viability for obtaining multiple valuable products from sludge. The emphasis is placed on novel valorization pathways, such as microalgae and purple bacteria cultures growing over sludge. The obtained products are benchmarked against traditional methods for resource recovery such as direct land application and P recovery from ashes. Our results show, besides the nutrient (TKN 7.38, TP 4.41; K 0.47 g 100 g TS-1) and energy content (HHV 22.53 MJ Kg-1 TS), that sludge could be employed to produce a suitable growing medium for microalgae and purple bacteria cultures obtaining, in the latter, remarkable high contents of high-quality proteins (64.50 % dw) for potential valorization as animal feed ingredient. We also obtained nutrient rich microalgae biomass (TKN 7.10, TP 8.10; K 0.40 g 100 g TS-1) which could be used as inputs for biobased fertilizers or biostimulants preliminarily complying with the nutrient requirements in EU 2019/1009. Current global scenario, showing economic and supply risk uncertainties regarding food production inputs, generates the urgent need to find feasible pathways for obtaining recovered products such as the ones presented in this study.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Every year, around 450 km3 of domestic wastewaters is discharged around the world [1]. As a result, and depending on the country, wastewater source, and technology being applied, the global annual sewage sludge generation was estimated to have reached 45 million dry tons by the end of the last decade [2]. Particularly for the EU, the urban sewage sludge production accounts for more than 10 million dry tons [3] which represents 9% of the total organic wastes generated, just behind animal and vegetable wastes, organic fraction of municipal solid waste, and wood wastes [4]. In addition to sewage sludge, there is also sludge generated from agro-industrial wastewaters that is difficult to quantify.
Environmental concerns, rising sludge production rates, and stringent regulations regarding sludge management and disposal creates the necessity to seek for alternatives and feasible solutions for its treatment and further valorization. Indeed, a change of paradigm in sludge management becomes increasingly necessary in the sight of the new European Green Deal, and particularly with the Circular Economy approach. Thus, wastewater treatment plants (WWTPs) play a crucial role within the necessary shift towards resource recovery [5] for being considered as water resource recovery facilities (WRRFs) or wastewater biorefineries (WWBRs) [6]. The recovered materials are aimed to be reintroduced into different production processes to be ultimately transformed into new products [7]. In terms of potential nutrient recovery, it has been estimated that global phosphorus (P) and potassium (K) demands could largely be serviced from waste streams [8] while minimizing geological inputs. Additionally, up to 50% of the global nitrogen (N) market could be supplied using biorecovered products [9] reducing the N load of the highly energy-demanding Haber Bosch process by around 50 Mt per year [10]. Potential value-added products which can be obtained from wastewater-derived sludge include, among others, amino acids and proteins for animal feed; polyhydroxyalkanoates (PHAs); biofuels; and biostimulants [11] along with nutrient-rich materials for biobased fertilizer (BBF) manufacturing.
Particularly addressing to macronutrients (specifically P) in sludges and produce raw materials for BBF manufacturing, the enhanced biological phosphorus removal (EBPR) appears as a very interesting approach. EBPR is generally used to remove pollutants from wastewater rather than to recover them despite this potential application. Briefly, the EBPR process is achieved by submitting wastewaters to alternating anaerobic and aerobic conditions. In this sequence, phosphate-accumulating organisms (PAOs) can take up volatile fatty acids (VFAs) from wastewater under anaerobic conditions and store them as PHAs. Furthermore, in the aerobic stage, using the energy content of the PHAs, PAOs grow and replicate. During this phase, P is uptaken and stored as polyphosphate (poly-P) inside PAO’s cells, resulting in net P removal from wastewater [12]. The ability of PAOs to store P is significantly higher than other heterotrophic organisms (up to 0.15 mg P mg VS-1 vs 0.023 mg P mg VS-1, respectively [13]). Consequently, the sludge produced in an EBPR aerobic phase presents significant higher P concentration than conventional activated sludges. Alternatively, if the sludge is harvested at the end of the anaerobic phase, the resulting EBPR material shows high content of PHAs which creates the opportunity for energy recovery (i.e., biofuels) and obtention of raw materials for bioplastic manufacturing. Moreover, as a source of energy and nutrients, sludge can be used as a suitable growing medium for several microorganisms for valuable biomass production.
One of these emerging technologies involves the obtention of valuable biomass by means of microalgae (MA) cultures growing over different waste streams [14, 15]. This approach shows a great potential since these organisms can efficiently use nutrients present in these streams (N and P removal reported efficiencies of up to 100%) [16]. Moreover, it has been claimed to be a cost-effective and feasible method for bio-fixation of CO2 [17] with high productivity values ranging from 40 to 150 tons ha-1 year -1 (dw) [18, 19]. Furthermore, the harvested biomass can be applied to the soil and act as a slow-release P fertilizer [20, 21] with very good, reported plant production yields [22] or as a biostimulant to enhance plant production [23]. The main advantages of using waste streams for MA cultures is the significant reduction in production expenses due to the avoidance of purchasing a commercial growing medium (which can represent up to 25% of the total production costs) [24]. Among MA species, members of the genus Chlorella were one of the first to be cultured on large scale due to their fast growth rate, resistance to biotic and abiotic stresses, and high nutrient, lipid, protein, and carotenoid content [25, 26]. They gained importance as robust biomass-accumulating strains, allowing for sustainable industrial productions of biomass and high-value products. Coupling with either CO2 abatement technologies or wastewater bioremediation could decrease production cost as well as provide several environmental benefits.
Another valorization approach involves the recovery of microbial proteins by means of purple phototrophic bacteria (PPB) cultures (i.e., Rhodopseudomonas sp. or Rhodobacter sp.). These organisms exhibit high biomass yields (1 g CODbiomass·g CODremoved-1) growing over waste streams and contain up to 60% of crude protein concentrations with additional potentially beneficial compounds such as poly-P, polysaccharides, polyhydroxyalkanoates (PHAs), VFAs, and 5-aminolevulinic acid [27, 28] depending on the metabolic pathway undertaken during the wastewater treatment. The potential obtention of these several value-added products implies great prospects and economic impacts for this technology. Also, PPB biomass is being tested as a raw material in aquaculture feeding, and as a feed ingredient in livestock production chains with promising growth yields and feed conversion ratios [29]. Additionally, [10] reported results on PPB application in agronomic tests showing a significant increase in plant production, which were nearly equal to mineral fertilizers, due to nutrient release and to the biostimulant capacity for soil microorganisms which positively affected plant growth.
Notwithstanding the advantages of recovering resources which are currently being wasted, and the legislation efforts to broaden the materials that can be included for this purpose, there are still normative constraints regarding the use of sludge-derived materials. For any of these products, quality and safety attributes must be entitled according to the specific legal framework. Regarding potential use of the proposed recovered materials as raw materials for BBFs, the European fertilizing product market regulation (Regulation 2019/1009) includes organic and waste-based fertilizers as suitable materials but recognizes their potential content of inorganic and organic pollutants as well as pathogens and identifies the risk of spreading and reintroducing them in the food chain [30, 31]. As such, and although the regulation states that “promising technical progress is being made in the field of recycling of waste, such as sewage sludge,” the use of this material is currently excluded from the Component Material Categories’ compositions. In the same regard, although the Directive 86/278/EEC (and subsequent amendments) encourages the use of sewage sludge in agriculture, it stablishes limits for nuisance components to guarantee their safe use.
Regarding animal feed application of recovered products, Regulation (EU) 767/2009, Directive 2002/32/EC, and amendments ((EU) 2019/1869) sets up the permitted products and threshold values for nuisance compounds contained in animal feeds, seeking to ensure a high level of feed safety and of protection of public health as well as animal welfare. Chapter 1 of this regulation prohibits materials obtained from wastewater treatment process to be used directly as ingredients. However, according to the council directive concerning urban wastewater treatment 91/271/EEC, industrial wastewater is defined as “wastewater which is discharged from the premises” (European Council 1991). Thus, it is prohibited to produce PPB as source of microbial protein on sewage, yet it is permitted to produce microbial protein on process water generated on the factory site [32].
Finally, and in the context of promoting the production of renewable fuels (Directive 2018/2001) towards the mitigation of climate change, the European Commission encourages the development and implementation of technological solutions to produce energy from renewable sources in which PHA rich EBPR sludges could perfectly fit.
Considering the identified legal constraints, the objective of the present work is to show the technical viability for obtaining multiple valuable products from wastewater treatment–derived sludge after an EBPR process at lab scale. The emphasis is placed in novel valorization pathways, such as MA and PPB cultures, for obtaining bioproducts to be used either as biostimulants, BBFs, protein-rich biomass for animal feeds, or biofuels. The results are compared and benchmarked, in terms of costs and market prices, with more traditional methods for resource recovery such as direct application on farmland of sludge, sludge combustion, and field application of the ashes, and also against P recovery from sludge’s ashes. This work’s conclusions provide inputs for a necessary decision-making tool aimed to select the best available alternative for efficient resource recovery and valorization of waste streams.
2 Materials and methods
2.1 EBPR WWTP
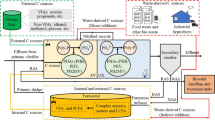
The EBPR sludge for all the tests performed during this work was obtained from the side stream short-cut enhanced nutrient abatement (SCENA) facility installed at the Carbonera WWTP (Treviso, northern Italy). The full-scale (mainstream) plant has a treatment capacity of 40,000 PE and treats around 15,000 m3 day-1 of municipal wastewater without any industrial contribution. The water line is composed by preliminary treatments, primary sedimentation, biological reactor (Schreiber process), secondary sedimentation, disinfection, and final filtration. The sludge line is composed by a static pre-thickener followed by dynamic thickening mixing primary and secondary sludge, equalization tank for thickened sludge, anaerobic digester, and a side stream SCENA-EBPR system for the treatment of sludge reject water (Fig. 1) [33].
The EBPR plant treats up to 50 m3 day-1 of anaerobic reject water produced in the sludge line of the main WWTP. The plant consists in a concrete sequential batch reactor (SBR) with a total volume of 70 m3 seeded with conventional activated sludge taken from the biological reactor of the main wastewater treatment line. The operating cycle (4 cycles per day) is composed of six different phases: feeding, anaerobic, aerobic, anoxic, settling, and discharge. The carbon source VFA-rich provided during the anaerobic and anoxic phases is produced by a controlled mesophilic (37 °C) acidogenic fermentation of mixed sludge (primary and secondary) operating with a hydraulic retention time (HRT) of 5 days. Before its use as carbon source, the effluent from the acidogenic fermenter follows as solid/liquid separation in a screw-press and is then stored in a 20-m3 storage tank. The solid retention time (SRT) of the SBR is maintained at 15–20 days and the HRT at 2 days. Oxygen is provided during the aerobic conditions by a volumetric blower (Robuschi, Italy) and ten ultrafine bubbles diffusers (INVENT, USA) placed in the bottom of the tank. Four probes (Hach-Lange, Germany) are installed within the reactor for the monitoring of oxidation-reduction potential (ORP) and dissolved oxygen (DO), pH, and conductivity. The blower is controlled by a variable frequency drive to maintain a DO concentration of 2 ± 0.2 mg O2 L-1, resulting in a flowrate between 450 and 500 m3 h-1. The bulk liquid is mixed through a horizontal mixer (Flygt, USA). EBPR sludge was sampled after the aerobic phase and used for the valorization trials without dewatering it.
2.2 Sludge samples
2.2.1 Nutrients
In all sludge samples, raw, dried, and combustion ashes, total Kjeldahl nitrogen (TKN), TP, and K determinations were performed, with the exception of TKN in combustion ashes.
TKN was determined by titration of ammonium ions with 0.1 N HCl, after the digestion of 2.5 g of the solid matrix in 20 ml of concentrated H2SO4 and in the presence of one Kjeldahl catalyst tablet (9 % CuSO2) as indicated by standard methods 4500-Norg B [34].
For P and K determinations, initially 2.5 g of solid samples was digested following the nitric acid-sulfuric acid method in Section 4500-P.B [34]. Furthermore, total P determination was conducted following the colorimetric ascorbic acid method (E) [34]. Separately, K concentration was determined using a Varian AA240FS Flame Atomic Absorption Spectrometer equipped with a K lamp working with 5 mA and measuring at 766.5 nm. The equipment was pre-calibrated between 0.1 and 2 ppm K (KCl stock solution), and samples were diluted accordingly with 2% HNO3 prior to measurement.
Additionally, to determine the different phosphate fractions in the raw sludge, the cold perchloric acid (PCA) for sequential extraction was employed [35]. This method allows to determine individually the (a) chemically bound P fraction; (b) non-orthoP which corresponds to biological origin stored in cells as poly-P; and (c) “interstitial” loosely bound phosphate [36]. The study of the different P fractions in the sludge permits to infer about the bioavailability and mobility of this nutrient.
Total and volatile solids (TS and VS) were determined following the methods in [34].The organic matter content (Corg) was equated to the volatile portion of the burned samples and converted to C using a factor of 1.83 [37].
2.2.2 Heavy metals and emerging contaminants
Metal contents (Cd, Pb, Zn, Cr(VI), Cu, Ni, As) were analyzed in sludge extracts by means of a VARIAN VISTA-MPX, simultaneous inductively coupled plasma-optical emission spectroscopy “ICP-OES.” The instrumental quality control was carried out using Standard Reference Material 2782 (Industrial Sludge; USA). Emerging contaminants were assessed by analyzing polycyclic aromatic hydrocarbons (PAHs) following the method fully described in [38].
2.2.3 Polyhydroxyalkanoates (PHAs)
Raw sludge samples were dried at 60 °C for 24 h for PHA extraction following a modified method from [39] as stated in [40]. Two-milliliter vials were used to mix dried sludge (1.0–1.5 g) with 1 mL chloroform, 0.8 mL methanol-sulfuric solution, and benzoic acid as the internal standard. Sealed vials were placed in a thermostatic bloc at 95 °C for 3.5 h. After cooling, the vial content was transferred into 4-mL vials and mixed with 0.5 mL of NaOH 0.05 M. Methyl-ester monomer of PHA was determined from the organic fraction after separation of the phases.
PHA content of the extracts was determined by gas chromatography (GC) with a flame ionization detector (FID). The GC system (Agilent 7820A) consisted of a FID with an HP-Innowax column (30 m × 0.53 mm × 1 μm) as fully described in [40]. Finally, PHA accumulation was expressed as the total PHA content in grams of polyhydroxybutyrate (P3HB) + polyhydroxyvalerate (P3HV) + polyhydroxy-2-methylvalerate (PH2MV)) per gram of cell dried weight (CDW) [g g−1 CDW].
2.2.4 Calorific power
To assess the potential of the material as a biofuel, dried sludge pellets (0.6–1 g) were electrically ignited using a bomb calorimeter (1341 Plain Jacket Calorimeter with the 1108 Oxygen Combustion Vessel, Parr). Higher heating value (HHV) was determined though the monitoring of heat of combustion according to manufacturers’ instructions, while lower heating value (LHV) can be further corrected from HHV according to the moisture and hydrogen content of original sludges as stated in [41].
2.2.5 Combustion and P extraction
Dry sludge solid samples (5 g) were subjected to a combustion process at 850 °C in a muffle furnace (Carbolite Furnaces, UK) for 5 h until constant weight. The temperature was selected after [42] who reported maximum content of extractable TP in ashes at this temperature. Ash samples were further submitted to an extraction procedure with H2SO4 0.5 M in an S:L ratio of 1:10 (3 g of sample and 30 mL of H2SO4) for 240 min, as stated in [43].
2.3 Preliminary MA and PPB growth tests
In order to test the ability of MA and PPB to grow over EBPR sludge and to define the optimal operational parameters to maximize the biomass production, a preliminary growth test was conducted. Both MA and PPB were grown over a synthetic medium as a positive control (BG 11 (UTEX Culture Collection of Algae, United States) [44] and DSMZ Medium 27 (Leibniz-Institute DSMZ, Germany), respectively, a water extract of dry EBPR sludge and a water extract of EBPR sludge ashes. A tap water negative control was included.
Extracts of both dry sludge and sludge ashes were prepared by putting the solids in contact with Milli-Q water (5 g of solids per L of water) and shaken overnight. Dry sludge was obtained by centrifuging the EBPR sludge and then dried in an oven at 105 °C until constant weight. Sludge ashes were obtained by burning dry sludge in a muffle at 850 °C until constant weight. Main characteristics of all the growing mediums extracts are included in Table 1.
Algal growth tests were conducted with Chlorella sorokiniana strain with an average cell size of ~1.5–2 μm. This strain was originally obtained from the UTEX Culture Collection (University of Texas, Austin, TX) as strain UTEX 1230, and the seed culture was maintained on BG-11 medium in flasks at 25 °C, 70 μmol photons m-2 s-1 at the Laboratory of Biotechnology, University of Verona, Italy.
Preliminary algal growth tests were performed in duplicates in a Multi-Cultivator MC 1000-OD (Photon System Instruments, Czech Republic). The equipment provides independent standard white LED illumination (up to 1000 μmol photons m-2 s-1), thermoregulation, aeration (3% CO2 supply), and optical density monitoring (720 nm with optical path of 27 mm) for 8 independent round glass testing tubes of up to 80 mL of cultivated suspension each. Test started with an initial concentration of 5,000,000 cells mL-1. Data of the optical density at 720 nm was registered at 5-min intervals and was used to estimate the biomass production. The test was run for 3 days, until a steady growth curve was achieved. Daily samples were collected from the tubes, and the actual algal cellular concentration was determined by means of a Countess II cell-counting instrument (Thermo Fisher Scientific Inc., Waltham, MA, USA).
PPB growth tests were conducted with Rhodopseudomonas palustris. The strain was isolated at the Department of Biotechnology, University of Verona, in a lab-scale reactor (5 L) fed with hydrogen as electron donor, light as source of energy, and CO2 as the only carbon source.
Preliminary PPB growth tests were performed in triplicate in sealed glass bottles with 200 mL of working volume leaving an initial headspace of 120 mL. The bottles present a serum stopper which can be surpasses by a syringe for sampling. The mixtures were incubated anaerobically at 37 °C with continuous lightning of ~ 800 μmol photons m-2 s-1 for 5 days until a steady growth curve was achieved. Biomass was sampled twice a day and optical density (OD) at 600 nm with optical path of 10 mm and measured in a visible spectrophotometer. Test started with a 10% (v/v) of fresh PPB inoculum over 70 mL of growing medium [45].
2.4 MA and PPB biomass production tests
Following the results obtained in the preliminary growth tests, the optimal HRT and growing medium for maximizing the biomass production were selected and applied to 1-L photobioreactors. Reactors consisted in open (MA) and closed vessels (PPB) with continuous mechanical stirring, and constant white LED lighting of ~ 800 μmol photons m-2 s-1 (Fig. 2).
HRT was kept at 2 days by daily withdrawing 500 mL of the mixed liquor. Growing medium consisted of dry sludge-water extract (see Table 1). For MA, the initial inoculum was 5,000,000 cells mL-1 which was achieved by mixing 100 mL of concentrated culture with 900 mL of dry sludge extract. PPB initial conditions were achieved by also mixing 100 mL of concentrated culture with 900 mL of dry sludge extract. MA photobioreactor included a CO2 feed delivered by a gas line inserted into the reactor, while PPB reactor was kept in anaerobic conditions by means of stoppers and initial purge with N2. Optical density (720 nm for MA and 600 nm for PPB) was measured 3 times per day in 1-mL mixed liquor samples in a visible spectrophotometer. Harvesting of MA and PPB biomass occurred once per day. After each harvesting event, the volume was recovered by including dry sludge extract to each of the reactors. Total length of the experiment was 4 days. MA and PPB samples were centrifuged, freeze-dried, and kept frozen until further analyses.
2.5 Biomass yield and nutrient removal
Biomass dry weight produced by MA and PPB was determined in a 50-mL aliquot of culture samples collected at the end of the experiment by centrifugation and freeze-drying the harvested samples. Biomass yield (g L−1) in the different culture mediums was calculated as described by [46] (Eq. 1):
where DW1 and DW0 are biomass dry weight (g L−1) at time tf (final) and t0 (initial), respectively.
Nutrient removal efficiency (RE %) of MA and PPB cultivation was calculated according to Eq. 2
where RE = removal efficiency; Ci = initial concentration, and Cf = final concentration.
2.6 Biomass characterization
Freeze-dried samples were analyzed for NPK, using the same method as for the sludge samples [34].
2.6.1 Total proteins and amino acid profile
Total protein content of MA and PPB cells was measured by the Kjeldahl method via multiplication of total nitrogen by 6.35 for MA [47] and 5.94 for PPB [29].
Amino acid analysis of freeze-dried MA and PPB biomass was carried out after hydrolysis with 6 N HCl at 110 °C for 24 h in a Biotronic LC-5001 Amino Acid Analyser (Germany) according to the method of [48] in accordance with EU Regulation (EC) No 152/2009.
2.7 Statistical analysis
For all the results, statistical differences of the assessed experiments were analyzed using a one-way ANOVA (p < 0.05 confidence level) with post hoc Tukey test after checking the assumptions of normality and equal variance. Assays used to monitor the process have been conducted in triplicates, and the reported values correspond to the mean value and its standard deviation. Minitab 17 (Minitab Inc.) was used to analyze the data and SigmaPlot (Systat Software Inc.) 14.0 was used for data plotting.
3 Results and discussion
3.1 Sludge characterization and multiple valorization alternatives
The quality of the EBPR sludge was addressed with the objective of determining the potential downstream valorization pathways. Table 2 summarizes the global parameters of the raw, dried sludge, and sludge’s ashes in terms of macronutrients (NPK), PHA content, and calorific value (HHV).
In terms of nutrient (NP) content in sludge, our results show interesting valorization opportunities as raw material for biofertilizer manufacturing after a biologic post-treatment resulting in these high nutrient concentrations. As such, they are consistent or even higher than reference concentrations in studies addressing resources recovery. Regarding N, [49] reported 1–2.7 (g 100 g TS-1) in anaerobically digested biowaste and in urban and agro-industrial sludges after a composting process which were then successfully tested in ryegrass pot trials. Moreover, [50] informs 3.87 (g 100 g TS-1) in composted urban sewage sludge aimed for field application as a source of nutrients and soil conditioner which was accepted after a complete decision support framework application. Finally, [51] showed results ranging between 4.9 and 6.7 (g 100 g TS-1) for air-dried, heat-dried, and composted sludge also in line with [52, 53] who concluded that the quality of their obtained products were as efficient as commercially available phosphate fertilizers. Hence, the 7.38 g P 100 g TS-1 found in the dried sludge in our trials shows a very interesting concentration of this nutrient. Regarding P, reference values are around 1–2.3 (g 100 g TS-1) after anaerobic digestion, and several thermal, crystallization, and processes combination of sewage sludge aiming to resource and energy recovery [53,54,55]. Our results, in line with typical P content reported for full-scale EBPR (4–5 g 100 g TS-1) [52, 56, 57], roughly represent two times the P content in conventional activated sludge. As such, considering N and P contents, this nutrient-rich material is a suitable candidate for valorization via BBFs manufacturing for both N and P recoveries.
Available inorganic fertilizers, particularly diammonium phosphate [(NH4)2HPO4], one of the most applied fertilizers, is recognized for its excellent source of P and N for plant nutrition [58]. This mineral fertilizer has a standard grade of 18-46-0 (18% N, 46% P2O5 (~ 20% TP), and 0% K by weight). Our recovered products show approximately half of these nutrient concentrations which shows a great opportunity for nutrient rich recovered materials that would otherwise have been wasted. Moreover, the fertilizing value of any given product is not only related to its NP content, but also organic matter and carbon, microelements, microorganism population, growth-regulating substances, and metabolites which are present in the sludge and can potentially act as a biostimulant enhancing plant growth as demonstrated in several studies [52, 55, 59] and improve the overall health of soils.
Interestingly, the P fractionation analysis showed that around 80% of the TP included in the sludges (raw and dried materials) are mainly poly-P, a polymer of orthophosphate (PO43-). Poly-P serves as a reservoir for inorganic PO43- and an energy source for fueling cellular metabolism [20]. According to the length of the polymer chain, the rate at which the PO43 - is mobilized depends on the activity of phosphorus-solubilizing organisms existing in the soil. Hence, the obtained percentage of poly-p in our material shows an interesting potential to act as a slow/moderate release fertilizer favoring sustained plant growth with reduced PO43- runoff, thus reducing potential environmental impact [60].
In order to address the suitability of the analyzed sludge for fitting in a specific product function category (PFC) within the European regulation (EU) 2019/1009, physicochemical characteristics in terms of nutrient (NPK) and total solids (TS) were established. Regarding nutritional characteristics, considering the produced sludge, the obtained fertilizing product after a stabilization/sanitization process would most likely meet the requirements on nutrient content of PFC 1(A)(I): SOLID ORGANIC FERTILISER and PFC 3(A): ORGANIC SOIL IMPROVER. Moreover, heavy metal content was analyzed against the legal threshold established for those categories and against Spanish law regarding the use of sludge as fertilizer. As it can be observed in Table 3, heavy metal content complies with the EU fertilizer regulation and also with the Spanish regulation which legally authorizes sludge as raw material for fertilizer products, provided it complies with heavy metal maximum concentration values (Spain RD 506/2013 on Fertilizer Products (BOE, 2017).
As it can be observed, it is remarkable to state that metal contents of the studied EBPR sludge (mg Kg-1 dw) were always below the limits established in the analyzed regulations. Therefore, its characteristics make it suitable for its use directly in agricultural field since it complies with the Directive (86/278/EEC), and also with some of the most stringent worldwide regulatory limits for agricultural application of sludge, such as the national regulations from Austria, the Netherlands, Denmark, Canada, or Japan [61, 62]. Regarding other compounds of concern such as PAHs of the sludge, every PAH family component analyzed was below detection limit of 0.010 mg kg-1 TS, except for naphthalene (0.014 ± 0.002) and phenanthrene (0.020 ± 0.003). And the overall PAH content of raw sludge was in the low range of what was found in literature for conventional sludges used to formulate organo-mineral fertilizers [63]. Moreover, our material complies with the EU legislation (EC, 2000 and EU 2019/1009) which propose that the “sum of PAHs,” should not exceed 6 mg kg-1 TS-1 in sludge for land application. In addition, by the application of a downstream biological process such as biodrying or composting exhibiting a thermophilic phase, these compounds along with other micropollutants will be likely degraded resulting in even lower concentrations in the final product as reported in [64].
Finally, regarding safety assessment, EU regulation establishes a limit of Absence of CFU in 25 g for Salmonella spp., and < 1.000 CFU in 1 g of sample for Escherichia coli or Enterococcaceae. In this regard, the proposed methods for drying the material before its field application or use as feedstock in fertilizing products manufacturing (thermal drying or biodrying by the action of thermophilic micro-organisms) will most likely grant the maintenance of the solid matrix at a temperature of at least 70 °C for 1 h (such is the process condition stablished in the EU Regulation 142/2011 for animal by products and End of waste Criteria EUR 23990). Within this high temperature, pathogenic micro-organisms are killed, and the material could be considered hygienically safe.
This work exhibits that wastewater-derived sludges can be considered to be equal to other authorized feedstocks for fertilizing products manufacturing in terms of quality and safety [65,66,67,68,69]. Nevertheless, sewage sludge is currently not considered within the allowed 2019/1009 feedstocks to produce component material categories (CMCs). Consequently, we identify the necessity of deepening the discussion regarding the inclusion of valuable raw materials such as the sludge in this study in future versions of the regulation. Considering the inclusion of this type of materials into the regulation would help boosting the impacts of the current circular economy and zero waste policies.
Another interesting characteristic of the produced EBPR sludge is its high PHA content. It is noteworthy that the EBPR reactor was fed with a short-chain VFA-rich solution produced on-site in a pre-fermenter (See 2.1 EBPR WWTP). As stated by [70], the PHA production is strongly related to the short-chain VFA concentration being high when VFA availability is elevated. As an energy-rich compound, PHA can be derived for biofuel production and to be used as a raw material for bioproducts such as bioplastics or other biopolymers. Our results show a PHA accumulation of 42.21% ± 1.29 (dw) close to some of the highest reported values. For instance, [71] obtained PHA concentration of 57% (dw) treating domestic wastewater, [72] reached 62% (dw) using activated sludge acclimatized in a microaerophilic-aerobic process, and [73] reported about 50% (dw) using activated sludge as a feed for EBPR process.
Recently, [74] obtained 41% (dw) in a side-stream system fed with fermented VFA liquors and remarked that a minimum value of 40% (g PHA g−1 VS) is necessary for an economical down-stream recovery of the PHA polymer. Hence, our produced sludge strikes as being potentially promising for bioplastics production. Moreover, being PHAs an energy-rich material, results also show that EBPR sludge can be derived to biofuel manufacturing since it exhibits a HHV comparable with wood bark, olive husk, and walnut shell [75] after drying it. These previous drying steps will be accomplished by submitting the produced sludge to a biodrying process which enables to reach a moisture content below 40% which is the reported value for an effective and sustained combustion process [76].
All in all, the sludge characteristics open a wide range of potential valorization pathways to be used either as a raw material for direct fertilizing, as an input material for BBFs manufacturing, as biofuels, and alternatively for biopolymer production.
3.2 Sludge valorization by means of MA and PPB cultures
3.2.1 Preliminary growth test (MA and PPB)
The productivity of MA was investigated by following cell number and optical density in batch photobioreactors with 4 growing mediums. Namely, BG-11 standard as a positive control, dry sludge extract, sludge ashes extract, and deionized water as a negative control. Growth curves were followed by measurements of OD at 720 nm (Fig. 3) and showed that the BG-11 medium provided MA with the best suitable growth medium, followed by dry ashes extract, sludge ash extracts, and finally water (negative control). All the curves were fitted with a logistic curve and the slope (first derivative) of the point of maximum grow obtained was used to estimate the productivity of the different conditions. Maximal productivity was highest in the case of BG-11 and dry extract sludge (0.0008 increase of OD per minute for both treatments), 1.6 times higher than sludge ashes, and 8 times higher than water treatment. In addition, maximum productivities were obtained at the different mediums. BG-11 resulted in maximum biomass production followed by dry sludge, sludge ash, and water. The biomass (dw) recovered from each treatment cultures ranged from 1.85 (BG-11), 1.45 (dry sludge), 0.34 (sludge ashes) to 0.091 g L−1 (water). Direct observation of cells counts starting from an inoculum of 5.000.000 cells/mL showed at the end of the experiment ~ 18,000,000 in BG-11, ~ 11,000,000 in dry sludge, ~ 2,000,000 in sludge ash, and ~ 100,000 cells/mL in the water treatment. Biomass production is in line with reference studies of Chlorella sorokiniana growing over several wastewater streams: Up to 0.6 g L-1 growing over cooking cocoon wastewater [77] 0.26 to 0.49 g L-1 over silk wastewater [78], and up to 5.45 g L-1 when growing over swine wastewater [79]
Growth curves (upper panel) and biomass productivity (lower panel) of MA growing over BG-11 standard (positive control), dry sludge (DRY), sludge ashes (ASH) extract, and water (negative control) mediums. In the upper panel, each point reports the mean value and standard deviation of 2 measurements (n=2). Lower panel shows the first derivate of the logistic curves obtained by fitting the different growth curves. Maximum values of the first derivate curves can be used to approximate the highest productivity during the growth over the different mediums and to define the maximum growth period
Likewise, PPB growth tests were performed to define optimal operational parameters over each of the growing mediums (SMDZ-27 standard as a positive control, dry sludge, sludge ashes extract, and water as a negative control). Growth curves were followed by measurements of OD at 600 nm (Fig. 4) and showed that the SMDZ-27 medium provided PPB with the best suitable growth medium, followed by dry ashes extract, sludge ash extracts, and finally water. In this case, the curves fitted a normal curve (Johnson Transformation 95% CI - p value = 0.954) which was used to estimate the productivity of the different conditions. Maximal productivity was highest in the case of SMDZ-27 (0.0008 increase of OD per minute), followed by dry sludge extract (0.0005 increase of OD per minute) almost 2 times higher than sludge ashes and 20 times greater than the tap water treatment. In terms of maximum biomass production, dry sludge treatment showed the highest value, accomplishing final values of 2.74 (dry sludge), 1.98 (SMDZ-27), 0.54 (sludge ashes), to 0.011 g TS L−1 (water). These results are higher than recent reference studies aiming to define the optimal operational strategies to selectively produce PPB for protein obtention, who reports biomass concentrations of 0.81 ± 0.04 g TS L-1 [80].
Growth curves (upper panel) and biomass productivity (lower panel) of PPB growing over SMDZ-27 standard (positive control), dry sludge, sludge ashes extract, and water (negative control) mediums. In the upper panel, each point reports the mean value and standard deviation of 3 measurements (n=3). Lower panel shows the first derivate of the normal curves obtained by fitting the different growth curves. Maximum values of the first derivate curves can be used to approximate the highest productivity during the growth over the different mediums and to define the maximum growth period
These results allow us to consider the dry sludge extract as a suitable medium for both MA and PPB growth, and further biomass production. Results show that this medium enables to obtain similar maximum productivities to commercial standard and cost-intensive mediums and reaching attractive final biomass production for further valorization derived from a waste stream.
From the wastewater treatment point of view, Table 4 shows the COD, TP, and TKN removal efficiencies (RE) of MA and PPB growing over standard mediums (BG-11 and SMDZ-27, respectively) and over dry sludge extract
Regarding MA performance, the final RE shows good performances but are somehow lower than recent reported values (maximum RE: COD ~ 90%; TN ~ 87%; TP ~ 95%) for the same strain growing over several wastewaters (i.e., coffee, dairy, and flour/starch industries) [81,82,83]. The lower N RE obtained in this study could be explained following the results of [84] who showed that N assimilation capacity was significantly enhanced when using a BG-11 medium supplemented with NH4+ as compared with the same medium used in the present study (standard BG-11). By using ammonium, the microalgae cells avoid energy needed for the nitrate/nitrite reduction, as NH4+ is directly incorporated into amino acids [85]. Regarding P RE, our results are in accordance with studies using the same strain for wastewater nutrient removals [83, 86].
On the other hand, remarkably PPB were able to uptake > 93% of the TKN contained in both of the tested growing mediums, showing promising potential N and protein concentration in their biomass for further valorization. These removal efficiencies results are higher than recent reported values [80, 87]. However, for the same culture, TP removals were lower when compared with the same references. It has been previously suggested [88] that higher P accumulation occurs mostly when there is lower infrared (IR) energy available, then PPB biomass accumulate P as luxury poly-P. Our culture received a suboptimal wavelength (white light), which may have contributed to the low P accumulation. [87] reported that high P removals (45–50%) only occurred in events when an increase in suspended solids in the growing medium (wastewater) caused shading and consequently a lower IR radiation. Hence, a chance for enhancing the RE relies in the optimization of the supplied light quality.
In terms of COD removal, a comparison to recent references indicates lower efficiencies [78, 87,88,89,90]. Nonetheless, these studies deeply addressed the operational parameters optimization (i.e., reactor configurations, operation temperature, HRT, SRT, agitation speeds, light quality and intensities, and conditions for bacterial strains). Regarding the latter, the origin of the strain can affect some metabolic yields specially in short-term experiments like the ones included in this research. In our case, the strain of PPB that was isolated from a different source that the one used in our process which could have affected the overall performance. For instance, [89] also experienced unsatisfactory nutrients removal and biomass assimilation while working with Rhodobacter sphaeroides and concluded that optimization of the process in needed regarding different light intensities and agitation speeds for different waste stream treatments. For short-term experiments like the ones presented in this paper, a pre-acclimatation period should be included.
Overall, the fact that the biomass, both MA and PPB, could grow adequately over the waste stream, but failed to comply with the required discharge limits (COD, TKN, TP) (EU Directive 91 /271 /EEC), allow us to consider both MA and PPB treatments over waste streams as a potential side stream unit to be used as a waste valorization step taking advantage of a mainstream performance without jeopardizing the final effluent quality. Another alternative, to reduce the high nutrient levels that may remain after harvesting MA or PPB, is to add more growth cycles without adding wastewater or to reuse the effluent solution in hydroponic crop systems [81]. For example, the co-cultivation of tomatoes with MA has been shown to be advantageous [90].
3.2.2 Scale up for biomass production (MA and PPB)
After defining the optimal experimental conditions in the preliminary trials (i.e., HRT = 2 days), 1 L PBR was set for biomass production and further valorization. Figure 5 shows the evolution of the MA and PPB biomass and the OD for both cultures through the whole experiment. Overall, dry biomass yield of Chlorella sorokiniana resulted in 0.79 ± 0.04 g L-1 d-1, while Rhodopseudomonas palustris yielded 0.75 ± 0.08 g L-1 d-1. Arrows in the figure show the moments in which the mixed liquor was extracted; immediately after this withdrawa, the reactor was refilled with the same waste stream. At the end of the first day of the trial, soluble COD of the mixed liquor showed a reduction of 81% and 38% in MA and PPB treatments, respectively. At that same moment, a biomass of 0.89 g L-1 (MA) and 0.90 g L-1 (PPB) was observed. Roughly, biomass production yielded 0.8 g MA biomass L-1 per g of COD removed, while for PPB, the result was 0.60 g PPB biomass L-1 per g of COD removed.
Regarding biomass production, both MA and PPB showed interesting results and in line with recent reported results. Particularly, for MA [91] reported photoautotrophic stable biomass production between 0.8 and 1.2 g L-1 d-1 in closed reactors operating during a longer period (14 d). Also, although using different species, [92] obtained biomass concentrations of 1.98 ± 0.43, 3.58 ± 0.12, and 2.24 ± 0.34 g L−1 for Chlorella vulgaris mixotrophically cultivated in inorganic media supplemented with cheese whey (CW), hydrolyzed CW, and a mixture of glucose and galactose, respectively. [88] obtained a maximum biomass density of 2 g L−1 (in 6 days) when Chlorella vulgaris grew mixotrophically in urban wastewater with glucose, while [93, 94] reported maximum biomass concentration of 1.24 g L−1 and 1.30 g L−1, respectively. Hence, obtaining around 0.79 g L-1 d-1 in a complex real medium represents a great potential for growing MA over this waste stream for further biomass valorization.
Regarding PPB, production results for synthetic substrates containing VFAs, simple sugars, alcohols, and other organic acids feeds are between 0.5 and 1.0 g COD g COD removed-1 [80, 95,96,97]; thus, our results of 0.75 0.79 g L-1 d-1 can be considered as remarkably good production yields for growth test over real waste streams.
Noteworthy that the used growing medium in this study was produced by extracting soluble compounds from a dried solid with no addition of complementary substrates. Results demonstrate that most likely nutrients and carbon sources were rapidly available for PPB and MA uptake, hence explaining the elevated biomass production. Despite the good results working with 1 L volume, it is necessary to consider that scaling up photobioreactors embodies some intrinsic challenges regarding mixing, light penetration, gas exchange, and harvesting procedures which might hamper the productivity and/or increase the capital and operating costs [98] which must be studied thoroughly.
In order to assess the quality of the produced biomass intended for further use as ingredients for biobased fertilizers or animal feed formulations, the MA and PPB biomass obtained in this trial were tested for protein, TKN, K, TP content, and amino acid profile. Table 5 summarizes the MA and PPB biomass characterization and compares them to reference studies.
Regarding our results, the potential valorization as inputs for BBF manufacturing looks promising. In terms of primary nutrients, MA biomass exhibit concentrations of 7.1%, 8.1%, and 0.4% NPK, respectively, and PPB biomass 11.1%, 1.5%, and 2.5% NPK, respectively. In fact, the produced biomass betters some commercially available organic products (biofertilizers and bioactivators) derived from algae biomass exhibiting from 2% onwards of TN. Regarding TP contents, our MA results are in line with reference values for the same genus [103] and species [109]. Likewise, for PPB biomass, TP concentration is in accordance with the only found reference value of TP in PPB biomass [29] growing over synthetic, domestic, pork, and poultry wastewaters. These figures are higher (except for K) than the minimum content required by EU2019/1009 normative by single primary nutrient declaration or by declaring more than one primary nutrient as a sum of % by mass. Moreover, for MA biomass, the obtained concentrations in this study could be enhanced by changing cultivation conditions since changes in operational parameters can favor a specific metabolite (i.e., protein, lipids, carbohydrates) accumulation [110, 111]. Similarly for PPB cultures, carbon and N sources as well as operational parameters can enhance one preferred metabolic pathway leading to specific product [95].
In terms of protein content with the intention to be used as animal feed, MA biomass total protein content resulted a bit lower than some of the reported values. This could be explained by the use of a real growing medium as opposed to synthetic and supplemented mediums included in these references. On the other hand, protein content of the PPB biomass was remarkably high (>65% (dw)) making it possible to use it as substitute for protein source at quite high rates in feeds for carnivorous fish where dietary protein requirement may be between 40 and 55% [29]. Besides protein content, ingredient quality is critical for commercial feed production and is a common problem for formulation [112]. Amino acid profiles for the MA and PPB microalgae biomass were quite similar, PPB exhibiting higher overall content of total amino acids than MA (53.83 ± 1.62 vs 45.23 ± 1.42 g aa g protein-1 respectively). Furthermore, the content of essential amino acids [113] as a measure of protein quality accounted for 53% and of the total amino acid concentration for both MA and PPB biomass.
Quality assessment of the produced biomass and based on the nutritional properties from the amino acid profile allow us to consider these cultures as a good potential source of multipurpose supplement for animal feedstuffs. Figure 6 shows the comparison between our MA and PPB biomass against the amino acid composition within the formulation of experimental diets in [29]. These authors encountered no significant increase in mortality associated with the use of microbial biomass as a feed ingredient compared to commercially available diets and demonstrated that up to 66% replacement of fish meal has no significant adverse effects on fish performance (feed intake, daily gain, feed conversion ratio, growth rate, and mortality) during a 47-day experiment. Thus, our results present strong evidence on the opportunity of obtention of high-quality biomass from waste streams which meets quality requirements for protein production by means of aquaculture.
Amino acid profile for the produced MA and PPB biomass within this study compared with [29] which was successfully tested in fish production trials
3.3 Product benchmarking
Several value-added products have been obtained from sludge at lab scale in this study such as nutrient-rich, PHA-rich, energy-rich sludge, and nutrient- and protein-rich biomass. However, to address the feasibility and the economic potential of these products for reaching full-scale applications, a comparative analysis using commercially available products, which our products intend to replace, must be performed. Hence, a benchmark of alternative products in terms of costs and values is presented hereby. Noteworthy that this preliminary analysis will not consider potential variations of the sludge nutrient composition, transport and spreading costs which are crucial to provide real-time assessment [114]. Also, the saved costs of sludge management and treatment, which WWTP must currently take care of, are not included. Hence, the values presented here could certainly be more attractive when overall economic and environmental saved costs are included.
Regarding the potential use as a BBF, the nutrient fertilizer value of sludge and of the produced MA and PPB biomass depends on the total major plant nutrient (NPK) composition [115] and the economic valuation of these materials which is the total value of available nutrients (TVANS) calculated using Eq. (3) [114]:
where TVAN is the total value available nitrogen per ton (€); TVAP is the total value of phosphorus available per ton (€); and TVAK is the total value of available potassium per ton (€). Net TVANS is calculated by subtracting the production costs or the market price to the TVAN so to get the real value of nutrient normalized by the cost of obtaining it. Net TVANS of our products are compared with net TVANS of commercially available fertilizer products in Table 6.
As it can be observed, with the current production costs and market prices, the TVANS of our products are somewhat less economically profitable and still not convenient as opposed to traditional inorganic alternatives (TVANS 0.18 for raw sludge application vs. 0.58 for average NPK simultaneous application (€ Kg-1)). Also, transport distance and spreading costs need to be thoroughly evaluated to address the economic feasibility of these novel products. Nonetheless, production costs tend to decrease as more research and scaling up of the novel processes are developed. Also, current global scenario reveals problems such as rising food, fuel, and feedstocks prices together with input costs, highlighting the need for EU agriculture and food supply chains to become more resilient and sustainable. As such, biobased fertilizer production strikes as an attractive alternative to avoid economic uncertainties and supply risks. At the same time legal constraints are becoming stricter and EU’s environmental strategy strongly points to zero waste and circular economy strategies. All these combined elements will continue to generate a more favorable scenario for novel fertilizer products derived from waste valorization approaches.
In terms of potential application as ingredients for livestock feeds, the nutritive quality of the product must be checked against commercially available meals (i.e., soybean meal) and the cost can be compared in terms total protein content [123] in a similar way as it was proposed for the fertilizer value (Table 7).
As can be observed, both the protein content and the essential amino acids in MA and PPB biomass are in line with commercially available meals, making our products suitable for this purpose. Moreover, when considering the market prices of available products, it turns out that MA and PPB biomass are more convenient that traditional meals with similar protein content and similar amino acid quality. Hence, if installed as a side stream of a WWTP where a drying facility already exists (i.e., thermal valorization of the sludge), this technology’s production costs would drop dramatically making this alternative even more economically attractive. Moreover, the reduction of the potential sludge-related environmental impacts helps boost the sustainability of the wastewater treatment sector. Nonetheless, it is noteworthy that our results were obtained in lab-scale trials, and the scaling up of this type of processes can make the CAPEX and OPEX to increase leading to a less favorable economic scenarios. Particularly, artificial illumination, centrifugal harvesting/dewatering, and drying are the crucial operational parameter for MA and PPB cultures. These elements will need to be considered when scaling the process.
All in all, the studied sludge and the derived biomass production included in this study meets the physicochemical characteristics to be used as a BBF, as a biofuel, bioplastic precursor, and/or as growing mediums to produce ingredients for animal feed formulations as promising valorization routes.
4 Conclusion
This study provides technical evidence on the feasibility of obtaining high value-added products departing from a waste stream such as EBPR sludge by means of novel valorization pathways. Physicochemical composition of the obtained products meets the quality requirements to be derived for several applications such as BBFs, biofuel, bioplastic precursor, and/or as ingredients for animal feed formulations appearing as promising valorization routes. The results were particularly remarkable in terms of protein content in PPB biomass for further valorization as animal feed ingredient. In our study case, the obtention of dry sludge as a raw material for product manufacturing as well as a growing medium for MA and PPB represents, on one hand an advantage, since such a thermal process warrantee the sanitization of the product (End of waste Criteria EUR 23990). On the other hand, the need for sludge drying makes the process costly and dependent on the existence of a drying facility. Moreover, the fact that the final effluent’s concentrations exceeded some of the discharge limits points out the necessity of optimizing the employed operational parameters while scaling up the process. Notwithstanding, the obtained results highlights the opportunity of developing and applying these technologies as a side stream in wastewater treatment facilities for valorizing the currently produced sludge. This work presents different alternatives for valorizing the inevitable produced sludge with the prospect for obtaining different high value-added products. The various downstream valorization processes presented in this work open the opportunity to derive efforts to the most needed resources for a market-driven decision-making system. Special interest relies on the industrial symbiosis approach since most of the alternatives presented here would be economically enhanced operating as side-stream processes. The recovery of resources from waste streams including domestic and agro-industrial sludges can improve the overall EU sustainability and provide feasible alternatives for inputs with high supply risk and/or elevated costs.
Abbreviations
- AA:
-
Amino acids
- BBF:
-
Biobased fertilizer
- COD:
-
Chemical oxygen demand
- CODS:
-
Soluble chemical oxygen demand
- CW:
-
Cheese whey
- DO:
-
Dissolved oxygen
- DW:
-
Dry weight
- EBPR:
-
Enhanced biological phosphorus removal
- EU:
-
European Union
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- HHV:
-
Higher heating value
- HRT:
-
Hydraulic retention time
- IR:
-
Infrared
- K:
-
Potassium
- LCA:
-
Life cycle assessment
- LCC:
-
Life cycle cost
- LHV:
-
Lower heating value
- MA:
-
Microalgae
- N:
-
Nitrogen
- OD:
-
Optical density
- ORP:
-
Oxidation-reduction potential
- P:
-
Phosphorus
- PAHS:
-
Polycyclic aromatic hydrocarbons
- PAO:
-
Phosphate accumulating organisms
- PCA:
-
Perchloric acid
- PE:
-
Person equivalent
- PHA:
-
Polyhydroxyalkanoates
- Poly-P:
-
Polyphosphate
- PPB:
-
Purple phototrophic bacteria
- RE:
-
Removal efficiency
- SBR:
-
Sequential batch reactor
- SCENA:
-
Short-cut enhanced nutrient abatement
- SRT:
-
Solid retention time
- TKN:
-
Total Kjeldahl nitrogen
- TP:
-
Total phosphorus
- TS:
-
Total solids
- TVAK:
-
Total value of available potassium
- TVAN:
-
Total value of available nitrogen
- TVANs:
-
Total value of available nutrients
- TVAP:
-
Total value of available phosphorus
- VFA:
-
Volatile fatty acids
- VS:
-
Volatile solids
- WRRF:
-
Water resource recovery facilities
- WWBR:
-
Wastewater biorefineries
- WWTP:
-
Wastewater treatment plants
References
Mateo-Sagasta J, Raschid-Sally L, Thebo A (2015) In: Drechsel P, Qadir M, Wichelns D (eds) Global wastewater and sludge production, treatment and use BT - wastewater: economic asset in an urbanizing world. Springer, Netherlands, Dordrecht, pp 15–38. https://doi.org/10.1007/978-94-017-9545-6_2
Gao N, Kamran K, Quan C, Williams PT (2020) Thermochemical conversion of sewage sludge: A critical review. Prog Energy Combust Sci 79. https://doi.org/10.1016/j.pecs.2020.100843
Eurostat (2019) Sewage sludge production and disposal [Internet]. Eurostat p. 2019
Alibardi L, Astrup T, Asunis F, Clarke W, de Gioannis G, Dessi P et al (2020) Organic waste biorefineries: looking towards implementation. Waste Manag 114:274–286. https://doi.org/10.1016/j.wasman.2020.07.010
Fernando-foncillas C, Estevez MM, Uellendahl H, Varrone C (2021) Co-management of sewage sludge and other organic wastes: a Scandinavian case study. Energies 14:1–21. https://doi.org/10.3390/en14123411
Holmgren KE, Li H, Verstreete W, Cornal P (2016) State of the art compendium report on resource recovery from water preface. IWA Resource Recovery Cluster, The International Water Association (IWA), London, UK 49
García M, Urrea JL, Collado S, Oulego P, Díaz M (2017) Protein recovery from solubilized sludge by hydrothermal treatments. Waste Manag 67:278–287. https://doi.org/10.1016/j.wasman.2017.05.051
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19:292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Mehta CM, Khunjar WO, Nguyen V, Tait S, Batstone DJ (2015) Technologies to recover nutrients from waste streams: A critical review. Crit Rev Environ Sci Technol 45:385–427. https://doi.org/10.1080/10643389.2013.866621
Zarezadeh S, Moheimani NR, Jenkins SN, Hülsen T, Riahi H, Mickan BS (2019) Microalgae and phototrophic purple bacteria for nutrient recovery from agri-industrial effluents: influences on plant growth, rhizosphere bacteria, and putative carbon- and nitrogen-cycling genes. Front Plant Sci 10:1–13. https://doi.org/10.3389/fpls.2019.01193
Shi S, Xu G, Yu H, Zhang Z (2018) Strategies of valorization of sludge from wastewater treatment. J Chem Technol Biotechnol 93:936–944. https://doi.org/10.1002/jctb.5548
Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL et al (2007) Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res 41:2271–2300. https://doi.org/10.1016/j.watres.2007.02.030
Henze M, van Loosdrecht M, Ekama G, Brdjanovic D (2008) Biological wastewater treatment: principles, modeling and design. IWA publishing. https://doi.org/10.2166/9781780408613
Acién Fernández FG, Gómez-Serrano C, Fernández-Sevilla JM (2018) Recovery of nutrients from wastewaters using microalgae. Front Sustain Food Syst 2:1–13. https://doi.org/10.3389/fsufs.2018.00059
Craggs R, Park J, Heubeck S, Sutherland D (2014) High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. New Zealand J Bot 52:60–73. https://doi.org/10.1080/0028825X.2013.861855
Shahid A, Malik S, Zhu H, Xu J, Nawaz MZ, Nawaz S et al (2020) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci Total Environ 704:135303. https://doi.org/10.1016/j.scitotenv.2019.135303
Cecchin M, Paloschi M, Busnardo G, Cazzaniga S, Cuine S, Li-Beisson Y et al (2021) CO2 supply modulates lipid remodelling, photosynthetic and respiratory activities in Chlorella species. Plant Cell Environ 44:2987–3001. https://doi.org/10.1111/pce.14074
Benemann J, Pedroni PM, Davison J, Beckert H, Bergman P (2003) Technology roadmap for biofixation of CO2 and greenhouse gas abatement with microalgae. Final report to the U.S. department of Energy, National Energy Technology Laboratory. Morgantown-Pittsburgh. https://www.ieaghg.org/docs/01roadmp.pdf. Accessed 13 Feb 2023
Sompech K, Chisti Y, Srinophakun T (2012) Design of raceway ponds for producing microalgae. Biofuels 3:387–397. https://doi.org/10.4155/bfs.12.39
Mukherjee C, Chowdhury R, Ray K (2015) Phosphorus recycling from an unexplored source by polyphosphate accumulating microalgae and cyanobacteria-a step to phosphorus security in agriculture. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01421
Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal L (2016) Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotech Adv 34:550–564. https://doi.org/10.1016/j.biotechadv.2016.01.002
Alvarez AL, Weyers SL, Goemann HM, Peyton BM, Gardner RD (2021) Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res 54:102200. https://doi.org/10.1016/j.algal.2021.102200
Colla G, Rouphael Y (2020) Microalgae: new source of plant biostimulants. Agronomy 10:1–4. https://doi.org/10.3390/agronomy10091240
Fasaei F, Bitter JH, Slegers PM, van Boxtel AJB (2018) Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res 31:347–362. https://doi.org/10.1016/j.algal.2017.11.038
De-Bashan LE, Trejo A, Huss VA, Hernandez JP, Bashan Y (2008) Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Biores Tech 99:4980–4989. https://doi.org/10.1016/j.biortech.2007.09.065
Cazzaniga S, Dall’Osto, L., Szaub, J., Scibilia, L., Ballottari, M., Purton, S. et al (2014) Domestication of the green alga Chlorella sorokiniana: reduction of antenna size improves light-use efficiency in a photobioreactor. Biotech Biofuels 7:1–13. https://doi.org/10.1186/s13068-014-0157-z
Fradinho JC, Oehmen A, Reis MAM (2019) Improving polyhydroxyalkanoates production in phototrophic mixed cultures by optimizing accumulator reactor operating conditions. Int J Biol Macromol 126:1085–1092. https://doi.org/10.1016/j.ijbiomac.2018.12.270
Kim S, Lee Y, Hwang SJ (2013a) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegradation 85:511–516. https://doi.org/10.1016/j.ibiod.2013.05.025
Delamare-Deboutteville J, Batstone DJ, Kawasaki M, Stegman S, Salini M, Tabrett S et al (2019) Mixed culture purple phototrophic bacteria is an effective fi shmeal replacement in aquaculture. Water Res X 4:1–11. https://doi.org/10.1016/j.wroa.2019.100031
Bora RR, Richardson RE, You F (2020) Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: a comprehensive review. BMC Chem Eng1–16. https://doi.org/10.1186/s42480-020-00031-3
Clarke BO, Smith SR (2011) Review of “emerging” organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ Int 37:226–247. https://doi.org/10.1016/j.envint.2010.06.004
Alloul A (2019) Purple bacteria as microbial protein source : technology development, community control, economic optimization and biomass valorization. Doctoral thesis. Faculty of Sciences. Bioscience Engineering, Sustainable Energy, Air and Water Technology (DuEL). UAntwerp University. https://hdl.handle.net/10067/1648200151162165141
Longo S, Frison N, Renzi D, Fatone F, Hospido A (2017) Is SCENA a good approach for side-stream integrated treatment from an environmental and economic point of view? Water Res 125:478–489. https://doi.org/10.1016/j.watres.2017.09.006
American Public Health Association, American Water Works Association, Water Environment Federation (2017) Lipps WC, Braun-Howland EB, Baxter TE, eds. Standard Methods for the Examination of Water and Wastewater. 23th ed. Washington DC: APHA Press
De Haas DW, Wentzel MC, Ekama GA (2000) The use of simultaneous chemical precipitation in modified activated sludge systems exhibiting biological excess phosphate removal. Part 2: Method development for fractionation of phosphate compounds in activated sludge. Water SA 26:453–466
De Haas DW (1989) Chemical fractionation of activated sludge with special reference to enhanced biological phosphate removal. University of Johannesburg, South Africa, p 250
Barrington S, Choinière D, Trigui M, Knight W (2002) Effect of carbon source on compost nitrogen and carbon losses. Bio Tech 83(3):189–194
Suciu NA, Lamastra L, Trevisan M (2015) PAHs content of sewage sludge in Europe and its use as soil fertilizer. Waste Manag 41:119–127. https://doi.org/10.1016/j.wasman.2015.03.018
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly (β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982. https://doi.org/10.1128/aem.54.8.1977-1982.1988
Martínez-Avila O, Llimós J, Ponsá S (2021) Integrated solid-state enzymatic hydrolysis and solid-state fermentation for producing sustainable polyhydroxyalkanoates from low-cost agro-industrial residues. Food Bioprod Process 126:334–344. https://doi.org/10.1016/j.fbp.2021.01.015
González D, Guerra N, Colón J, Gabriel D, Ponsá S, Sánchez A (2019a) Filling in sewage sludge biodrying gaps: Greenhouse gases, volatile organic compounds and odour emissions. Bioresour Technol 291:121857. https://doi.org/10.1016/j.biortech.2019.121857
Li R, Teng W, Li Y, Wang W, Cui R, Yang T (2017) Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. J Clean Prod 140:964–970. https://doi.org/10.1016/j.jclepro.2016.06.177
Donatello S, Tong D, Cheeseman CR (2010) Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manag 30:1634–1642
Allen MM, Stanier RY (1968) Growth and division of some unicellular blue-green algae. Strain 51:199–202
Ehrenreich A, Widdel F (1994) Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol 60:4517–4526. https://doi.org/10.1128/aem.60.12.4517-4526.1994
Zhang Y, Su H, Zhong Y, Zhang C, Shen Z, Sang W et al (2012) The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Res 46:5509–5516. https://doi.org/10.1016/j.watres.2012.07.025
Safi C, Charton M, Pignolet O, Silvestre F, Vaca-Garcia C, Pontalier PY (2013) Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J Appl Phycol 25:523–529. https://doi.org/10.1007/s10811-012-9886-1
Kurbanoglu E, Algur O (2002) Single-cell protein production from ram horn hydrolysate by bacteria. Bioresour Technol 85:125–129. https://doi.org/10.1016/S0960-8524(02)00094-9
Grigatti M, Cavani L, di Biase G, Ciavatta C (2019) Current and residual phosphorous availability from compost in a ryegrass pot test. Sci Total Environ 677:250–262. https://doi.org/10.1016/j.scitotenv.2019.04.349
Laura F, Tamara A, Müller A, Hiroshan H, Christina D, Serena C (2020) Selecting sustainable sewage sludge reuse options through a systematic assessment framework: methodology and case study in Latin America. J Clean Prod 242. https://doi.org/10.1016/j.jclepro.2019.118389
Matsuoka K, Moritsuka N, Masunaga T, Matsui K, Wakatsuki T (2006) Effect of heating treatments on nitrogen mineralization from sewage sludge. Soil Sci Plant Nutr 52:519–527. https://doi.org/10.1111/j.1747-0765.2006.00061.x
Erdincler A, Seyhan LD (2006) Agricultural use of municipal wastewater sludges: phosphorus availability of biological excess phosphorus removal sludges. Water Sci Technol 54:131–138. https://doi.org/10.2166/wst.2006.555
Tyagi VK, Lo SL (2013) Sludge: a waste or renewable source for energy and resources recovery? Renew Sustain Energ Rev 25:708–728. https://doi.org/10.1016/j.rser.2013.05.029
Tarayre C, De Clercq L, Charlier R, Michels E, Meers E, Camargo-Valero M et al (2016) New perspectives for the design of sustainable bioprocesses for phosphorus recovery from waste. Bioresour Technol 206:264–274. https://doi.org/10.1016/j.biortech.2016.01.091
Cydzik-Kwiatkowska A, Nosek D (2020) Biological release of phosphorus is more efficient from activated than from aerobic granular sludge. Sci Rep 10:1–7. https://doi.org/10.1038/s41598-020-67896-5
Levantesi C, Serafim LS, Crocetti GR, Lemos PC, Rossetti S, Blackall LL et al (2002) Analysis of the microbial community structure and function of a laboratory scale enhanced biological phosphorus removal reactor. Environ Microbiol 4:559–569. https://doi.org/10.1046/j.1462-2920.2002.00339.x
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D et al (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182. https://doi.org/10.1128/AEM.66.3.1175-1182.2000
Theregowda RB, González-Mejía AM, Ma X, Garland J (2019) Nutrient recovery from municipal wastewater for sustainable food production systems: an alternative to traditional fertilizers. Environ Eng Sci 36:833–842. https://doi.org/10.1089/ees.2019.0053
Zhang D, Luo W, Yuan J, Li G (2018) Co-biodrying of sewage sludge and organic fraction of municipal solid waste: role of mixing proportions. Waste Manag 77:333–340. https://doi.org/10.1016/j.wasman.2018.04.016
Sanz-Luque E, Bhaya D, Grossman AR (2020) Polyphosphate: a multifunctional metabolite in cyanobacteria and algae. Front Plant Sci 11:1–21. https://doi.org/10.3389/fpls.2020.00938
Colón J, Alarcón M, Gerard Healy M, Namli A, Sanin FD, Tayà C et al (2017) Producing sludge for agricultural applications. In: Lema JM, Suarez S (eds) Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment. IWA Publishing, London, pp 296–322. https://doi.org/10.2166/9781780407876
Collivignarelli MC, Abbà A, Miino MC, Torretta V (2019) What advanced treatments can be used to minimize the production of sewage sludge in WWTPs? App Sci (Switzerland) 9. https://doi.org/10.3390/app9132650
Kominko H, Gorazda K, Wzorek Z (2022) Effect of sewage sludge-based fertilizers on biomass growth and heavy metal accumulation in plants. J Environ Manag 305:114417. https://doi.org/10.1016/j.jenvman.2021.114417
Pilnáček V, Innemanová P, Šereš M, Michalíková K, Stránská WL et al (2019) Micropollutant biodegradation and the hygienization potential of biodrying as a pretreatment method prior to the application of sewage sludge in agriculture. Ecol Eng 212–219. https://doi.org/10.1016/j.ecoleng.2018.11.025
Puyuelo B, Arizmendiarrieta JS, Irigoyen I, Plana R (2019) Quality assessment of composts officially registered as organic fertilisers in Spain. Span J Agric Res 17:1–13. https://doi.org/10.5424/sjar/2019171-13853
Wang P, Changa CM, Watson ME, Dick WA, Chen Y, Hoitink HAJ (2004) Maturity indices for composted dairy and pig manures. Soil Biol Biochem 36:767–776. https://doi.org/10.1016/j.soilbio.2003.12.012
Saha JK, Panwar N, Singh MV (2010) An assessment of municipal solid waste compost quality produced in different cities of India in the perspective of developing quality control indices. Waste Manag 30:192–201. https://doi.org/10.1016/j.wasman.2009.09.041
Martínez M, Ortega R, Janssens M, Angulo J, Fincheira P (2016) Selection of maturity indices for compost derived from grape pomace. J Soil Sci Plant Nutr 16:262–267. https://doi.org/10.4067/S0718-95162016005000021
Soliva M and Zalo M (2008) Compostaje de residuos municipales
Pijuan M, Casas C, Baeza JA (2009) Polyhydroxyalkanoate synthesis using different carbon sources by two enhanced biological phosphorus removal microbial communities. Process Biochem 44:97–105. https://doi.org/10.1016/j.procbio.2008.09.017
Takabatake H, Satoh H, Mino T, Matsuo T (2000) Recovery of biodegradable plastics from activated sludge process. Water Sci Technol 42:351–356. https://doi.org/10.2166/wst.2000.0402
Satoh H, Ramey WD, Koch FA, Oldham WK, Mino T, Matsuo T (1996) Anaerobic substrate uptake by the enhanced biological phosphorus removal activated sludge treating real sewage. Water Sci Technol 34:9–16. https://doi.org/10.1016/0273-1223(96)00489-1
Rodgers M, Wu G (2010) Production of polyhydroxybutyrate by activated sludge performing enhanced biological phosphorus removal. Bioresour Technol 101:1049–1053. https://doi.org/10.1016/j.biortech.2009.08.107
Werker A, Bengtsson S, Korving L, Hjort M, Anterrieu S, Alexandersson T et al (2018) Consistent production of high quality PHA using activated sludge harvested from full scale municipal wastewater treatment - PHARIO. Water Sci Technol 78:2256–2269. https://doi.org/10.2166/wst.2018.502
Acar S, Ayanoglu A (2012) Determination of higher heating values (HHVs) of biomass fuels. Energy Educ Sci Tech Part A: Energy Sci Res 28:749–758
González D, Colón J, Gabriel D, Sánchez A (2019b) The effect of the composting time on the gaseous emissions and the compost stability in a full-scale sewage sludge composting plant. Sci Total Environ 654:311–323. https://doi.org/10.1016/j.scitotenv.2018.11.081
Li D, Amoah PK, Chen B, Xue C, Hu X, Gao K et al (2019) Feasibility of growing Chlorella sorokiniana on cooking cocoon wastewater for biomass production and nutrient removal. Appl Biochem Biotechnol 188:663–676. https://doi.org/10.1007/s12010-018-02942-7
Deng X, Li D, Xue C, Chen B, Dong J, Tetteh PA et al (2020) Cultivation of Chlorella sorokiniana using wastewaters from different processing units of the silk industry for enhancing biomass production and nutrient removal. J Chem Technol Biotechnol 95:264–273. https://doi.org/10.1002/jctb.6230
Chen CY, Kuo EW, Nagarajan D, Ho SH, Di Dong C, Lee DJ et al (2020) Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour Technol 302:122814. https://doi.org/10.1016/j.biortech.2020.122814
Alloul A, Cerruti M, Adamczyk D, Weissbrodt D, Vlaeminck S (2020) Control tools to selectively produce purple bacteria for microbial protein in raceway reactors. Environ Sci Technol 55(12):8278–8286. https://doi.org/10.1101/2020.01.20.912980
Melo JM, Telles TS, Ribeiro MR, de Carvalho Junior O, Andrade DS (2022) Chlorella sorokiniana as bioremediator of wastewater: nutrient removal, biomass production, and potential profit. Bioresour Technol 17:100933. https://doi.org/10.1016/j.biteb.2021.100933
Asadi P, Rad HA, Qaderi F (2019) Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ. Sci Pollut Res 26:29473–29489. https://doi.org/10.1007/s11356-019-06051-8
Moges ME, Heistad A, Heidorn T (2020) Nutrient recovery from anaerobically treated blackwater and improving its effluent quality through microalgae biomass production. Water (Switzerland) 12. https://doi.org/10.3390/w12020592
Psachoulia P, Schortsianiti SN, Lortou U, Gkelis S, Chatzidoukas C, Samaras P (2022) Assessment of nutrients recovery capacity and biomass growth of four microalgae species in anaerobic digestion effluent. Water (Switzerland) 14. https://doi.org/10.3390/w14020221
Lachmann SC, Mettler-Altmann T, Wacker A, Spijkerman E (2019) Nitrate or ammonium: influences of nitrogen source on the physiology of a green alga. Ecol Evol 1070–1082. https://doi.org/10.1002/ece3.4790
Fernandes TV, Suárez-Muñoz M, Trebuch LM, Verbraak PJ and Van de Waal DB (2017) Toward an ecologically optimized N:P Recovery from wastewater by microalgae. Front Microbiol 8:1–6. https://doi.org/10.3389/fmicb.2017.01742
Nairn C, Rodríguez I, Segura Y, Molina R, González-Benítez N, Molina MC et al (2020) Alkalinity, and not the oxidation state of the organic substrate, is the key factor in domesticwastewater treatment by mixed cultures of purple phototrophic bacteria. Resources 9:1–15. https://doi.org/10.3390/RESOURCES9070088
Liang CM, Hung CH, Hsu SC, Yeh IC (2010) Purple nonsulfur bacteria diversity in activated sludge and its potential phosphorus-accumulating ability under different cultivation conditions. Appl Microbiol Biotechnol 86:709–719. https://doi.org/10.1007/s00253-009-2348-2
Azad S. Al, Chin FS and Lal MTBM (2019) Efficacy of purple non sulphur bacterium <i>Rhodobacter sphaeroides</i> Strain UMSFW1 in the utilization of palm oil mill effluent. J Geosci Environ Prot 7:1–12. https://doi.org/10.4236/gep.2019.710001
Barone V, Puglisi I, Fragalà F, Lo Piero AR, Giuffrida F, Baglieri A (2019) Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J Appl Phycol 31:465–470. https://doi.org/10.1007/s10811-018-1518-y
Fatima N, Kumar V, Rawat BS, Jaiswal KK (2020) Enhancing algal biomass production and nutrients removal from municipal wastewater via a novel mini photocavity bioreactor. Biointerface Res Appl Chem 10:4714–4720. https://doi.org/10.33263/BRIAC101.714720
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66. https://doi.org/10.1016/j.biortech.2012.05.055
Ruiz J, Álvarez P, Arbib Z, Garrido C, Barragán J, Perales JA (2011) Effect of nitrogen and phosphorus concentration on their removal kinetic in treated urban wastewater by Chlorella vulgaris. Int J Phytoremediation 13:884–896. https://doi.org/10.1080/15226514.2011.573823
Mennaa FZ, Arbib Z, Perales JA (2015) Urban wastewater treatment by seven species of microalgae and analgal bloom: biomass production, N and P removal kinetics andharvestability. Water Res 83:42–51. https://doi.org/10.1016/j.watres.2015.06.007
Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W et al (2020a) Purple phototrophic bacteria for resource recovery: challenges and opportunities. Biotechnol Adv 43. https://doi.org/10.1016/j.biotechadv.2020.107567
Hülsen T, Barry EM, Lu Y, Puyol D, Keller J, Batstone DJ (2016) Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor. Water Res 100:486–495. https://doi.org/10.1016/j.watres.2016.04.061
Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W et al (2020b) Purple phototrophic bacteria for resource recovery: challenges and opportunities. Biotech. Adv. 43:107567. https://doi.org/10.1016/j.biotechadv.2020.107567
Benedetti M, Vecchi V, Barera S, Dall’Osto, L. (2018) Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb Cell Factories 17:1–18. https://doi.org/10.1186/s12934-018-1019-3
Rasouli Z, Valverde Pérez B, D’Este M, de Francisci D, Angelidaki I (2018) Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem Eng J 134. https://doi.org/10.1016/j.bej.2018.03.010
Chia SR, Chew KW, Show PL, Sivakumar M, Ling TC, Tao Y (2019) Isolation of protein from Chlorella sorokiniana CY1 using liquid biphasic flotation assisted with sonication through sugaring-out effect. J Oceanol Limnol 37:898–908. https://doi.org/10.1007/s00343-019-8246-2
Kobayashi N, Noel EA, Barnes A, Watson A, Rosenberg JN, Erickson G et al (2013a) Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol 150:377–386. https://doi.org/10.1016/j.biortech.2013.10.032
Xie F, Zhang F, Zhou K, Zhao Q, Sun H, Wang S et al (2020) Breeding of high protein Chlorella sorokiniana using protoplast fusion. Bioresour Technol 313:123624. https://doi.org/10.1016/j.biortech.2020.123624
Carrasquilla-Batista A, Chacón-Rodríguez A, Murillo-Vega F, Núñez-Montero K, Gómez-Espinoza O, Guerrero-Barrantes M (2017) Characterization of biomass pellets from Chlorella vulgaris microalgal production using industrial wastewater. Energy and Sustainability in Small Developing Economies, ES2DE 2017 - Proceedings. https://doi.org/10.1109/ES2DE.2017.8015352
Kim HS, Park GG, Rhee YH (1999) Biosynthesis of Polyhydroxyalkanoates and 5-Aminolevulinic Acid by Rhodopseudomonas sp. KCTC 1439. The Korean Zoological Society Conference, 2432–2432
Kim S, Lee Y, Hwang SJ (2013b) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegradation 85:511–516. https://doi.org/10.1016/j.ibiod.2013.05.025
Getha K, Vikineswary S, Chong VC (1998) Isolation and growth of the phototrophic bacterium Rhodopseudomonas palustris strain B1 in sago-starch-processing wastewater. World J Microbiol Biotechnol 14:505–511. https://doi.org/10.1023/A:1008855125634
Kornochalert N, Kantachote D, Chaiprapat S, Techkarnjanruk S (2014) Use of Rhodopseudomonas palustris P1 stimulated growth by fermented pineapple extract to treat latex rubber sheet wastewater to obtain single cell protein. Annals Micro 64. https://doi.org/10.1007/s13213-013-0739-1
Honda R, Fukushi K, Yamamoto K (2006) Optimization of wastewater feeding for single-cell protein production in an anaerobic wastewater treatment process utilizing purple non-sulfur bacteria in mixed culture condition. J Biotechnol 125:565–573. https://doi.org/10.1016/j.jbiotec.2006.03.022
Kobayashi N, Noel EA, Barnes A, Watson A, Rosenberg JN, Erickson G et al (2013b) Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol 150:377–386. https://doi.org/10.1016/j.biortech.2013.10.032
Bibi F, Yasmin H, Jamal A, Al-Harbi MS, Ahmad M, Zafar M, Ahmad B, Samra BN, Ahmed AF, Ali MI (2021) Deciphering role of technical bioprocess parameters for bioethanol production using microalgae. Saudi J Biol Sci 28:7595–7606. https://doi.org/10.1016/j.sjbs.2021.10.011
Paliwal C, Mitra M, Bhayani K, Bharadwaj SVV, Ghosh T, Dubey S et al (2017) Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Tangendjaja B (2015) Quality control of feed ingredients for aquaculture. In: Feed and feeding practices in aquaculture. Woodhead Publishing, pp 141–169. https://doi.org/10.1016/B978-0-08-100506-4.00006-4
FAO/WHO (1973) Energy and protein requirements : report of a Joint FAO/WHO ad hoc expert committee [meeting held in Rome from 22 March to 2 April 1971]. World Health Organization, Geneva PP - Geneva
Tesfamariam EH, Ogbazghi ZM, Annandale JG, Gebrehiwot Y (2020) Cost–benefit analysis of municipal sludge as a low-grade nutrient source: a case study from south africa. Sustainability (Switzerland) 12:1–13. https://doi.org/10.3390/su12239950
Grobelak A, Placek A, Grosser A, Singh BR, Almås ÅR, Napora A et al (2017) Effects of single sewage sludge application on soil phytoremediation. J Clean Prod 155:189–197. https://doi.org/10.1016/j.jclepro.2016.10.005
Kacprzak M, Neczaj E, Fijałkowski K, Grobelak A, Grosser A, Worwag M et al (2017) Sewage sludge disposal strategies for sustainable development. Environ Res 156:39–46. https://doi.org/10.1016/j.envres.2017.03.010
Valdovinos-García EM, Barajas-Fernández J, Olán-Acosta MD, Petriz-Prieto MA, Guzmán-López A, Bravo-Sánchez MG (2020) Techno-Economic Study of CO 2 Capture of a Thermoelectric Plant Using Microalgae ( Chlorella). Energies 13:1–19
Rafa N, Ahmed SF, Badruddin IA, Mofijur M, Kamangar S (2021) Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front Energy Res 9:1–11. https://doi.org/10.3389/fenrg.2021.749968
Araújo R, Vázquez Calderón F, Sánchez López J, Azevedo IC, Bruhn A, Fluch S et al (2021) Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front Mar Sci 7:1–24. https://doi.org/10.3389/fmars.2020.626389
Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W et al (2020c) Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotech Adv 43:107567. https://doi.org/10.1016/j.biotechadv.2020.107567
Sakarika M, Spanoghe J, Sui Y, Wambacq E, Grunert O, Haesaert G et al (2020) Purple non-sulphur bacteria and plant production: benefits for fertilization, stress resistance and the environment. Microb Biotechnol 13:1336–1365. https://doi.org/10.1111/1751-7915.13474
World Bank (2015) "Commodity-Price-Data" World Development Indicators. The World Bank Group. https://databank.worldbank.org/databases/commodity-price-data. Accessed 13 Feb 2023
Araújo RRS, Fagundes MMA, Viana AMF, Paulino AHS, Silva ME, Santos EM (2022) Protein quality evaluation in vivo of cricket flour (Gryllus assimilis) reared in Brazil. J Insects Food Feed 8:409–416. https://doi.org/10.3920/JIFF2021.0096
Bernard J (2011) Feed Ingredients: Feed Concentrates: Oilseed and Oilseed Meals. In: Encyclopedia of Dairy Sciences, Second edn. Academic Press, pp 349–355. https://doi.org/10.1016/B978-0-12-374407-4.00165-5
Acknowledgments
This work is part of the REFLOW project with funding from the European Union’s Horizon 2020 program under the Marie Skłodowska-Curie grant agreement no. 814258.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work is part of the REFLOW project with funding from the European Union’s Horizon 2020 program under the Marie Skłodowska-Curie grant agreement no. 814258.
Author information
Authors and Affiliations
Contributions
Pablo M. Binder: Conceptualization, methodology, investigation, writing—original draft. Nicola Frison: Writing (review and editing), supervision. Nagore Guerra Gorostegi: Writing (review and editing). Ipan Hidayat: Methodology, visualization. Lidia Paredes: Writing (review and editing). Laia Llenas: Supervision. Enric Blázquez: Writing—review and editing. Mabel Mora: Supervision. Matteo. Ballottari: Resources, writing—review and editing. Stefano. Cazzaniga: Writing—review and editing. Francesco. Fatone: Writing—review and editing. Sergio. Ponsá Salas: Writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Binder, P.M., Frison, N., Guerra-Gorostegi, N. et al. Innovative multiple resource recovery pathways from EBPR wastewater treatment–derived sludge. Biomass Conv. Bioref. 13, 16421–16440 (2023). https://doi.org/10.1007/s13399-023-03849-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03849-y