Abstract
There are several published studies evaluating the possibilities of thermal and non-thermal utilization of pistachio hard shells in many technical sectors; however, there are no relevant data about the possibilities of usage of this homogenous biomass-based by-product as a fuel for automatic household heating appliances for partial or full substitution of standard ENplus A1 pellets, which is the aim of the presented study. The composition and basic properties of both fuels were compared as well as the flue gas composition formed during the 6 different fuel mixture combustion in two real-scale pellet burners. The mass concentration of observed pollutants (CO, total suspended particles, and C3H8) in the flue gas was strongly affected by increasing of pistachio shell mass fraction in the fuel mixture (from 10 to 100%). In comparison to the combustion of ENplus A1 pellets, CO was increased up to 25.9 times, total suspended particles up to 15.3 times, and C3H8 up to 13.7 times. Based on the results of real combustion tests, the equations were listed, describing the increase of the mass concentration of pollutants for the whole spectrum of pellets/pistachio shell ratios applied on the chosen designs of the pellet burners. The Pt-based oxidation honeycomb catalyst, additionally installed at the combustion unit outlets for flue gas purification, showed conversion rates up to 82.2% in the case of CO and up to 33.1% in the case of C3H8. This enables the reaching of the same or lower mass concentrations of mentioned pollutants in the flue gas, formed during the co-combustion of appropriately selected fuel ratios in comparison to ENplus A1 pellet combustion without the catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The worldwide energy situation especially in Europe is a very accented topic nowadays [1]. One of the most important parts of this issue is local household heating. The increasing trend of all kinds of energy pricing, as well as the uncertain future of natural gas boilers, motivates house residents to the installation of different kinds of heat sources such as heat pumps or solid fuel combustion appliances. From the second mentioned ones, automatic pellet boilers and automatic pellet stoves are gaining in popularity especially due to their high comfort of usage, and their sales are constantly increasing [2] as well as the production and consumption of pellets in the EU, which strongly correlates with the utilization of mentioned combustion equipment [3]. This trend can be associated, in many countries, with voluntary grant programs or with legislatively mandated decommissioning of old, non-compliant combustion units [4,5,6]. Newly sold devices for pellet combustion have to comply with the actual, very strict, conditions from the thermal efficiency and the flue gas composition points of view, thus achieving a very environmentally friendly operation [7].
Another discussed topic nowadays is waste management. The combustion of unused solid waste in large waste incineration plants has a huge potential to substitute old conventional fossil fuel (especially coal) plants, widely used in many European countries [8]. However, several European countries still have not yet developed sufficient infrastructure for the thermal utilization of solid waste for the fulfilment of mandatory European aims [9, 10]. Therefore, a significant part of the waste is still landfilled or, in the case of biomass waste, partly composted [11, 12].
Concerning the mentioned ideas, the combustion of thoroughly selected waste biomass in a modern residential combustion unit (especially automatic pellet boilers and stoves) seems to be beneficial for the potential of partial replacement of conventional fuels, such as pellets or coal. While maintaining low values of mass concentrations of pollutants in the flue gas produced during the waste biomass or pellets and waste biomass mixture, the overall amount of the produced waste could be slightly decreased, and part of the energy and the resources will not have to be spent on the production and transportation of substituted pellets. This way of thermal utilization could be very beneficial, especially during the treatment with woody materials which are difficult to compost.
Several studies about alternative biomass-based fuels for combustion in residential solid fuel appliances were published: argan nut shells [13], pellets produced from coconut and cashew nut shells [14], rice husk [15], beech leaves briquettes [16], spent coffee ground pellets [17], straw pellets [18], palm kernel shells [19], date stones [20], brewers’ spent grain [21], olive pits [22], and almond shells [23]. Another possible representative of appropriate waste biomass for home utilization seems to be pistachio hard shells (only hard shells will be considered for this research). Worldwide pistachio production reached more than 1.3 million tons in the year 2021, while Iran and the USA are the biggest producers followed by Turkey, China, and Syria [24]. Shell weight ranges between 35 and 45% of whole nut [25], which represents between 455 and 585 thousand tons of relatively homogenous biomass waste material produced every year. Part of the produced pistachios is sold with the shell to the final customers, and part is sold (usually for the following treatment) peeled off, while the pistachio producer has to deal with the shells as with waste. Compared to the other biomass-based alternative fuels, no additional treatment such as crushing, pelletizing, or drying is necessary before the utilization in the home combustion units. Due to its low moisture content (a huge advantage in comparison to most other types of waste biomass) and relatively high higher heating value (HHV) [25], both similar as in the case of wooden pellet quality A1 [26], it can represent a favourable energy source for different ways of thermal utilization.
Several studies describing different ways of thermal utilization of the pistachio shells were published previously, such as vacuum pyrolysis [27], rapid and slow pyrolysis [28], and gasification [29] mostly in order to characterize solids and liquid residues from the process. The solid residues (ash) from the combustion process were also described as the feedstock for the cement industry in the previous study [30] while the description of the combustion process was put aside. A theoretical study of kinetic parameters during the combustion process including TGA was described before [25]. In the consideration of different volume/surface ratios of pistachio shells and the results of the mentioned TGA, a different course of the combustion process can be expected in comparison to standard wooden pellets [25].
The main advantage of the pistachio hard shell combustion in existing combustion units originally designed for pellets is the presumption of the possibility of the usage of actually existing feeding system without any modification due to similar granulometry of both fuels. Moreover, no electricity needs to be consumed for pelletizing. However, changing the fuel can also have a negative impact. By changing the fuels for the residential combustion units, different times of burnout of each piece of fuel, different ash properties, and, for example, different volume flow of fuel to the burner can occur. Simultaneously, the composition of the flue gas, especially from the pollutants formed during the incomplete combustion process (such as carbon monoxide (CO) and organic gaseous compounds (OGC)) point of view, can be affected negatively by combustion and co-combustion of pistachio shells in standard residential units (without any optimizing of the burner design), as was proved in the previous study with different biomass-based, alternative fuel combustion [31]. The suitability of using a catalyst in a wide range of biomass-burning devices for household heating is undermined by studies proving that local heating can be a significant source of CO [32, 33]. A possible precaution against the increasing mass concentration of pollutants from the incomplete combustion process in the residential combustion units could be oxidation honeycomb catalysts, as was proved in many previous studies based on artificial flue gas testing [34] and also based on real flue gas testing such as the following: Ferrandon et al. [35] described the ability of the precious and non-precious metal–based catalyst on the ignition phase of the wood-fired boiler. The suitability of using a catalyst in the wooden sauna stove was presented by Hukkanen et al. [36]. The impact of platinum- and palladium-based catalysts integrated in firewood stoves on mass concentration of pollutants in the flue gas under real-life operating conditions was described by Reichert et al. [37]. The influence of flue gas parameters on palladium- and palladium-platinum-based catalyst was described by Ryšavý et al. [38]. The platinum- and palladium-based catalytic converter was tested in two different combustion units (wood log stove and automatic pellet stove) in order to determine the emission reduction potential of the device in comparison to the electrostatic precipitator in the study of Vicente et al. [39].
The functional catalytic materials on the rare metal basis found their application in a broad range of technical subjects including purification of flue gas formed during the solid fuel combustion as was described above, purification of the flue gas formed during the liquid fuel combustion [40], or for example catalyst synthesis of gases to the liquid fuels [41].
Concerning the increasing production of pistachio hard shells worldwide, their higher utilization in thermo-chemical processes is offered, so this study is aimed at determining possibilities of combustion and co-combustion of pistachio shells with standard wooden pellets in the two real automatic residential combustion units, used for direct household heating (originally designed for standard pellet combustion).
The novelty and originality of the paper lie in the innovative approach to the use of pistachio shells as a fuel for real combustion devices for household heating originally designed for certified pellets, which were additionally equipped with an additive oxidation catalyst. This approach was never described before.
Obtained data will be beneficial for pistachio producers (by the possible opening of the new way for utilization of continuously produced residual material), for manufacturers of the residential combustion units intended for the combustion of pellets (in the case of the intention of extending the spectrum of the allowed fuel to the pistachio shells) as well as for the end users of the pellet stoves and boilers.
2 Materials and methods
All described experiments were carried out in the accredited testing laboratory of boilers in Energy Research Centre, Centre for Energy and Environmental Technologies, VSB–Technical University of Ostrava.
2.1 Fuel
For the combustion tests, pistachio shells in combination with standard pellets labelled as ENplus A1 quality were chosen in different mixture ratios. Pellets were made by one of the front EU manufacturers. According to information from the producer, the used material for pellet production was pure spruce wood shavings without any bark or additional binders.
Pistachio shells were collected as the waste product of Californian pistachio nut consummation by final customers. Due to the manual cracking of the nuts, small fragments occurred only rarely, and most of the pieces were half shells. Before the combustion tests, shells were stored in the laboratory for several months.
Proximate and ultimate analyses were performed for both fuels. The results of the analyses are shown in Table 1. The composition of the chosen pellets is in accordance with the ENplus A1 quality standard. Pistachio shells’ composition slightly differs from the pellets, especially from the carbon and ash mass fraction point of view. Lower carbon mass fraction is consequently related to lower heating value as well as the higher mass fractions of ash. The sulphur and nitrogen contents of the pistachio shells are very low, on the same level as in the case of ENplus A1 pellets, which is different from some other observed alternative biomass-based fuels such as beech leaves (Sr = 0.09%) [16], rice husk (Sr = 0.05%), corn cob (Sr = 0.82%) [42], palm kernel shell (Sr = 0.06%), wheat straw (Sr = 0.22%) [43], or Napier grass (Sr = 0.35%) [44]. The low sulphur and nitrogen contents are important for the low formation of (fuel) nitrogen and sulphur oxides. Presented pistachio shell analysis results are similar to analyses performed as part of previous studies [25, 45]. Small differences in mass fraction of water between obtained results and previous research results may be caused by different conditions during the storage before the combustion tests.
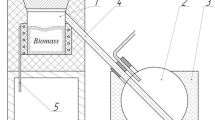
For combustion tests, 6 different fuels were prepared by mixing pellets and pistachio shells. The lower heating values were calculated as a weighted average from the pure material results. Bulk density was measured individually for all fuel mixtures. A basic description of used mixtures is listed in Table 2. Mixtures are visualized in Fig. 1
2.2 Used combustion units
For the combustion tests, two combustion units were used. The first one was a prototype of an automatic pellet stove which still did not pass the certification process (hereinafter referred to as unit 1). Since unit 1 is still a prototype unit, the value of nominal heat input (approximately 6 kW) was determined from previous combustion tests, performed with pellets during the optimizing process of unit 1. The advantage of this unit 1 usage, in comparison to commercially sold pellet stoves, which are usually able to change heat output only by pre-set programs, is the possibility of detailed changing settings of the combustion process (time of fuel batch, time of pause between the fuel batch, flue gas fan performance, etc.). Thanks to this, fuel feeder settings could be changed for each fuel mixture to reach similar heat output between the tests (more suitable for mutual comparison). The disadvantage of the chosen unit 1 is that there are not any labelled values of operating parameters obtained during the certification process, so, the obtained values from the combustion tests serve primarily for fuel comparison and not for classification of unit 1 according to actual legislation.
Unit 1 consists of a fuel tray (for approximately 8 kg of certified ENplus A1 pellets), from where the fuel is lifted by a screw conveyor (21 cm long, 8 cm in diameter) to a dropping point. From the dropping point, the fuel falls to a bowl burner with a rod grate. Combustion air is supplied under the grate and thereupon flows through the grate to the fuel. Flue gas flows from the combustion chamber through the flue gas/air heat exchanger, then through the second draught (inside part of the flue gas duct). At the flue gas outlet, a flue gas fan is placed.
The second used combustion unit was a prototype of a gutter burner installed into the conical combustion chamber with a diameter of 250 mm (hereafter referred to as unit 2) without any heat exchanger. The pellet burner inside unit 2 was designed for nominal heat input of approximately 20 kW. Input parameters of the combustion process such as time of fuel batch, time of pause between the fuel batch, and flue gas fan performance were controlled by the same control unit as in the case of unit 1 (including the above-mentioned advantages and disadvantages).
The whole unit 2 consists of a fuel tray (for approximately 30 kg of certified ENplus A1 pellets), from where the fuel is lifted by a screw conveyor (160 cm long, 8 cm in diameter) to a dropping point. From the dropping point, fuel falls to the bottom part of the burner, from where the fuel was moved by a second screw conveyor to the gutter-shaped grate, where the combustion process took place. Combustion air is supplied under the grate and thereupon flows through the grate to the fuel. Flue gas consequently flows through the combustion chamber right to the flue gas outlet, to the flue gas duct. This disposition enabled the reaching of high flue gas temperature, which is very important for catalyst operation. These combustion units are experimental and were optimized for catalyst testing (high flue gas temperature) for simulation of catalyst installation before the heat exchanger in the boiler or stove.
Chosen units were not originally designed for the combustion of any kind of biomass-based alternative fuel. The prototype units were chosen intentionally to avoid violations of rules listed in user manuals of the commonly sold units by manufacturers (these rules usually do not allow the combustion of different fuel which was not used during the certification process under threat of loss warranty). The reasons for the choice of mentioned two types of units with different types of burners (bowl and gutter) were their high representation in the combustion units in the EU, different combustion process courses (given by different designs and different methods of fuel transportation to the grate), and different nominal heat input and output. Unit 2 was designed intentionally for catalyst testing at high flue gas temperature, which could be reached inside the combustion chamber or inside the heat exchanger in the pellet boiler, or for example in the wood log stove without a water heat exchanger.
2.3 Catalyst
For the following experiment, a Pt-based honeycomb catalyst was chosen to reduce the concentration of pollutants in the flue gas, especially CO and OGC. The reason for choosing a Pt-based catalyst was the high conversion rates of CO and OGC proved during the previous studies of Ferrandon et al. [35] and Ryšavý et al. [46] and the lower required amount of the active element in the washcoat in comparison to palladium while maintaining a comparable conversion rate. According to the catalyst composition and dimensions, it was designed for installation into the flue gas duct right at the flue gas outlet. A detailed description of the used catalyst is shown in Table 3. The tested catalyst is shown in Fig. 2.
-
The producer did not provide information about the ideal temperature range for reaching the highest conversion rates. Previous tests of the catalyst Cat A showed the possibility of reaching conversion rates of CO around 90% and OGC around 50% with the flue gas (formed during the beech wood log combustion in the stove) temperature at the catalyst inlet ranging between 350 and 380 °C.
-
Catalyst Cat A did not undergo any physical or chemical analysis to characterize its detailed properties, such as, for example, active element layer thickness.
-
The conversion efficiency diagram for Cat A was not provided by the producer.
-
Before the described tests, the catalyst Cat A was used in a real flue gas environment (formed during the wood log combustion process) for approximately 10 h without any extreme flue gas temperatures and without any additives in the fuel, which could cause poisoning of the catalyst. Before the combustion tests with each unit, the catalyst was sintered for 4 h at a temperature 550 °C. According to the mentioned facts, the catalyst was assumed to be in the best possible condition.
-
General properties of the chosen catalyst body material, especially in comparison to the metal body, were described in the previous study [47].
2.4 Testing procedure
Each test started by emptying of fuel tray and screw conveyor. A sufficient amount of fuel mixture was prepared by thorough mixing, and the empty fuel tray was filled by it, as well as the screw conveyor (by 30-min lasting continuous operation of the feeder). At first, the test of the added amount of fuel mixture into the burner by screw conveyor was performed. During the screw conveyor test, all fuel mixture fallen into the burner (at a given control unit setting) in a specified time was weighted. The value of heat input was calculated from this result in consideration of the LHV of each fuel mixture. The cold test lasted at least 2 × 10 min, and the average results were considered. The time of the fuel batch and the time of the pause between each batch were set for each fuel mixture individually to reach approximately the same heat input as during the pure wood pellet combustion.
Before each test, the catalyst was cleaned from settled total suspended particles (further only as TSP) by compressed air to reach the same conditions between the tests.
The ignition process of unit 1 began by filling the bowl burner with 100 g of the fuel mixture along with 10 g of the solid igniter. When unit 1 was turned on, the flue gas fan and the screw conveyor started to work. Simultaneously, the initial fuel dose in the burner was ignited by a propane-butane burner for approximately 40 s. In the case of unit 2, the ignition took place automatically by the ceramic heating element. The flue gas sampling started exactly 15 min after the ignition. Every combustion test lasted 120 min of steady-state operation. Ignition and burnout phases were not considered for their low flue gas temperature, which is connected with lower catalyst activity.
During each test, the flue gas sampling for TSP mass concentration determination took place twice (after 50 and 100 min). Every flue gas sampling lasted 15 min, or less in the case of high mass concentrations of TSP in the flue gas, which led to the rapid increase of pressure loss of the filter for TSP capture. This was a reason for the earlier termination of the sampling.
The heat input to unit 1 was approximately around the nominal heat input level (P = 6 kW), while the heat input of unit 2 was approximately around its minimal heat output level (P = 6 kW).
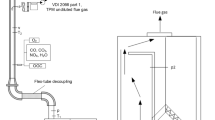
The scheme of the used unit 1 with a marking of the most important parts is shown in Fig. 3.
Scheme of the used combustion units. a Unit 1 on the left side and b unit 2 on the right side with marking of the most important parts: 1, bowl burner; 2, combustion chamber; 3, flue gas-air heat exchanger; 4, inside stove flue gas duct (second draught); 5, flue gas fan (located inside the stove); 6, flue gas outlet from unit; 7, sampling point at the catalyst inlet; 8, location of the catalyst; 9, sampling point at the catalyst outlet; 10, flue gas outlet (to the venturi tube, flue gas ambient air mixer, flue gas fan, and chimney); 11, gutter burner; 12, combustion air fan
2.5 Measuring system and flue gas analyzing
The flue gas was sampled from two places: approximately 50 and 100 cm behind the flue gas outlet. Between these two places, the catalyst Cat A was placed. For the flue gas analysis, the two identical analyzers MRU VARIO luxx were used. The flue gas flowed to the analyzers through the ceramic wool filter, placed within a sampling probe and through a heated hose (heated to 120 °C). Both analyzers were justified before the combustion tests.
The mentioned analyzers can determine the volumetric fraction of CO, CO2, O2, NOx, and C3H8, continually. The OGC was not evaluated during the test, except for propane (C3H8). Analyzers were unable to determine all of the OGC group representatives. According to the last research (not published yet), propane is one of the major components of OGC in the flue gas produced during the household combustion unit operation with a volume fraction of approximately 15%. Propane is representative of the light alkaline hydrocarbon group. In the presence of sunlight, propane reacts with nitrogen oxides to form ground-level ozone. This kind of ozone affects vegetation and the ecosystem and can also cause health problems to humans (for example chest pain, coughing, congestion, or throat irritation) [48, 49].
The pressure loss of the catalyst was monitored during the tests by differential pressure transmitters (Cressto SPD 211 R5UB D type).
The flue gas temperatures were monitored by thermocouples, placed next to the flue gas probes of MRU VARIO luxx. Five thermocouples were used for each place, while their arrangement in the flue gas duct was in accordance with the norm EN 303-5 [50]. A detailed description of the measuring equipment with the measurement principle and accuracy for continuous measurements is in Table 4.
2.6 Data evaluation
All measured values (flue gas composition, flue gas temperature, and pressure drop) were monitored continuously and recorded as 1-min averages, except for the mass concentration of TSP. All volumetric fraction values of gas pollutants obtained by flue gas analyzer MRU VARIO luxx were recalculated to mass concentrations in dry flue gas at STP conditions (P = 101,325 Pa; T = 273.15 K). Consequently, the mass concentration values were recalculated to the reference volume fraction of oxygen φref;[O2] = 13% according to Eq. (1). The reference volume fraction of 13% was chosen for both units, because it is standard for stoves (unit 1), whereas the burner from unit 2 can be also easily installed into the pellet stove. The second reason was enabling easy comparison of the results. The same procedure was applied for the calculation of the mass concentration of TSP from Wöhler SM 500 results. The only values taken into consideration from the Wöhler SM 500 results were the total weight of TSP (measured gravimetrically) on the exposed filter and sucked volume of the flue gas. The average volume fraction of the oxygen in the flue gas during the period of flue gas sampling was obtained by MRU VARIO luxx (due to the higher accuracy of the paramagnetic method of the measurement). From the three mentioned values, the mass concentration of TSP in the flue gas was calculated for each sampling. The results listed below are always the average value from both samplings.
ρB_O2ref — the mass concentration of a pollutant recalculated for reference volume fraction of oxygen (mg·m−3)
ρB_O2m — the mass concentration of a pollutant at measured volume fraction of oxygen (mg·m−3)
φO2m — measured volume fraction of oxygen (%)
φO2ref — reference volume fraction of oxygen (%)
Conversion rates of the catalyst were calculated from the comparison of the mass concentrations of pollutants contained in monitored flue gas at the catalyst inlet and at the catalyst outlet, according to Eq. (2). Conversion rates were determined only for CO and C3H8, which should be affected remarkably by the catalyst.
CR — the conversion rate of a pollutant (%)
ρBO — the mass concentration of a pollutant at the catalyst inlet (mg·m−3)
ρB — the mass concentration of a pollutant at the catalyst outlet (mg·m−3)
Gas hourly space velocity (GHSV) was calculated according to Eq. (3) as the ratio of wet flue gas volume flow at STP conditions (\({\dot{\mathrm{V}}}_{\mathrm{flue}\_\mathrm{gas}}^{\mathrm{wet}}\)) and the effective volume of the catalyst (Veff).
The thermal efficiency of units was determined for each combustion test by the indirect method (from the heat losses) according to Eq. (4) which is in accordance with the related standard EN 14785. [51]
η — thermal efficiency (%)
qA — thermal loss in the flue gas
qB — chemical losses in the flue gas
qr — heat losses due to combustible constituents in the residue passing through the grate
The partial calculations of the individual heat losses are described in detail in the above-mentioned standard EN 14785. [51]
3 Results and discussion
All important results obtained from the combustion tests, according to the methodology mentioned above, are shown in Tables 5 and 6. Six separate combustion tests were performed with each combustion unit, while stable 2-h lasting periods of operation were taken into consideration.
3.1 Evaluation of pistachio shell combustion
The first test only with wooden pellets was established as a reference, and other results of fuel mixtures were compared with it for both units. Considering the reached heat inputs (added chemical energy in the fuel to units), there were only slight differences (up to 0.3 kW in the case of both units) between reference combustion test results and the rest of the results of the tests for both combustion units, which was intentional, and the fuel feeder setting was purposely optimized accordingly before each test.
Despite the lower bulk density of the pellets/pistachio shell mixtures, reaching approximately 43% for pistachio shells in comparison to wood pellets (associated with a longer time of fuel batch and the shorter time between each batch), there were not any problems observed with cumulation of the unburned fuel in the burners (primary optimized and constructed for pellet combustion) which could cause incomplete burnout of the fuel pieces. With increasing mass fraction of pistachio shells in the fuel mixture, the level of the fuel in the bowl burner was increasing, but even during the burning of clean pistachio shells, falling of the unburned fuel off to the ashtray was not observed. In the case of the gutter burner, it was obvious from the ash residues after every combustion test that the combustion process took place only in the first third of the grate during all tests, which is in accordance with the nominal heat input to the burner, for which the burner was designed.
Different fuel shapes of pistachio shells did not cause any operational problems to the fuel feeder. The previous study of thermal utilization of the pistachio shells utilized milled and sieved fuel for maintenance of uniform dimensions between 1 and 2 mm to ensure high bulk density [45]. Pütün et al. [28] also milled and sieved pistachio shells before the thermal process in their study but does not describe the final used granulometry.
The thermal efficiency of units during the pure wood pellet combustion reached 65.9% and 72.5% respectively (determined by an indirect method). Thermal efficiency of unit 1 during other mixture combustion reached similar values ± 2%, while in the case of unit 2, thermal efficiencies reached lower values by 4–8%, what was given by slightly higher flue gas temperature and higher volume fraction of oxygen in the flue gas during the combustion of fuel mixtures and pure pistachio shells. The lowest efficiency 64.7% was reached during the 75/25 mixture combustion, where simultaneously the highest air excess ratio and the second highest flue gas temperature were reached considering the unit 2 results. In the case of recalculation of the results according to the European Commission regulation 2015/1185, related to ecodesign requirements for solid fuel local space heaters, the results would be lower by 6.6%, and the limit value for the seasonal thermal efficiency would not be met during any of the tests [52].
The highest heat loss always was the loss through sensible heat of the products of combustion through the tests, also known as chimney loss. The high value of this loss was caused by a high flue gas temperature (caused by the relatively primitive construction of unit 1 (still a prototype unit) and very primitive construction of unit 2 (only intentionally chosen for reaching high flue gas temperature) and a relatively high-volume fraction of oxygen in the flue gas. In real applications, the burner could be installed in a boiler or stove, with a catalyst installed in front of the part of the heat exchanger, which would significantly increase overall efficiency by a consequence utilization of flue gas heat energy (cooling the flue gas in the heat exchanger). It is also worth mentioning the loss through the incomplete combustion process which was dramatically increasing, especially with the increasing value of the mass concentration of CO in the flue gas (associated with increasing mass fraction of pistachio shells in the fuel mixture) [53].
Flue gas volume flow and GHSV are values strongly connected with basic operating parameters of the combustion unit, such as the volume fraction of oxygen in the flue gas and heat input in the fuel to the combustion unit. Reached values of GHSV (between 31,698 and 35,505 in the case of unit 1 and between 16,672 and 20,712 in the case of unit 2) were similar within the tests for each presented combustion unit. The obtained results are in the same GHSV range as the results presented in the study of Ryšavý et al. [38], where the wood log stove was used as the flue gas source. The determined GHSV corresponds with the heat input and air excess ratio.
The volume fraction of oxygen in the flue gas reached values ranging from 15.2 to 16.1% in the case of unit 1, which is completely in accordance with the previous research by Schmidt et al. [54] (ranging from 15.7 to 17.5%) and of Sippula et al. [55] (ranging from 14.8 to 18.0%) with similarly designed combustion units. In the case of unit 2, the volume fraction of oxygen in the flue gas reached values ranging from 11.7 to 13.0% which is adequate for this kind of burner design during its operation at minimal heat output [53].
During the pure wooden pellet combustion in unit 1, the measured mass concentration of CO was 513 mg/m3, which is above the ecodesign limit for the described kind of combustion units (300 mg/m3). By gradual addition of the pistachio shells into the fuel, there was an obvious growth of this value while the highest value (8871 mg/m3) was reached during the clean/pure pistachio shells’ combustion which presents more than 17 times increase of the monitored value. During the clean/pure wooden pellet combustion in unit 2, the measured mass concentration of CO was 175 mg/m3, which is below the mentioned ecodesign limit for the automatic pellet stove. By gradual addition of the pistachio shells into the fuel, there was an obvious growth of this value while the highest value (4528 mg/m3) was reached during the clean/pure pistachio shells’ combustion, which presents more than 25 times increase of the monitored value. Previous studies of different kinds of alternative biomass-based fuels in the pellet boilers proved an increase approximately by 11 times in the case of spent coffee ground pellets [17], 17 times in the case of straw pellets [18], 13 times in the case of date stones [20], and 20 times in the case of almond shells [23] in comparison to standard pellets and alternative fuel results from the CO point of view.
C3H8 mass concentrations in the flue gas reached 7 mg/m3 in the case of unit 1 usage for pure pellet combustion while by gradual addition of the pistachio shells into the fuel, there was an obvious growth to the highest reached value (95 mg/m3) obtained during the pure pistachio shell combustion. In the case of unit 2, mass concentrations of C3H8 were below the detection limit for the following pellets/pistachio shell mixtures: 100/0, 90/10, and 75/25. Increasement between mixtures 50/50 (6 mg/m3) and 0/100 (29 mg/m3) was almost fivefold. Obtained results of mass concentrations of C3H8 are incomparable to mass concentrations of OGC mostly presented in previous studies or to limit listed mentioned in the European Commission regulation 2015/1185 (60 mg/m3).
The obtained values of the mass concentration of TSP during the combustion of the pure pellets, 89 mg/m3 for unit 1 and 104 mg/m3 for unit 2, were more than twice above the mentioned European Commission regulation 2015/1185 limit (40 mg/m3) for both combustion units (measured only at the catalyst outlet). Mass concentration of TSP during unit 1 usage (measured only at the catalyst outlet) was strongly correlated with the mass fraction of the pistachio shells in the fuel mixture, ranging from 3.4 times increase in the case of 90/10 pellets/pistachio shell mixture to 15 times increase (1364 mg/m3) during the combustion of pure pistachio shells. A similar correlation was obvious in the case of unit 2 usage, ranging from 1.3 times increase in the case of 90/10 pellets/pistachio shells mixture to almost 12 times increase (1233 mg/m3) during the combustion of pure pistachio shells. Previous studies of different kinds of alternative biomass-based fuels in the pellet boilers proved an increase approximately by 10 times in the case of spent coffee ground pellets [17], 5 times in the case of straw pellets [18], 7 times in the case of date stones [20], and 10 times in the case of almond shells [23] in comparison to standard pellets and alternative fuel results from the TSP point of view.
Unit 2 was disadvantaged from the mass concentration of the TSP point of view by the absence of any kind of heat exchanger or flue gas flow retarder which usually works as a mechanical separator of the TSP. According to previous studies, the influence of the catalyst on the mass concentration of TSP is lower than 20%, caused especially by the settling of the particles on the catalyst’s surface [37]. Significantly, better results could be reached by using a more sophisticated combustion unit, especially with a particle precipitator [56, 57].
An absolute increment of the mass concentration of CO, C3H8, and TSP in the flue gas at the catalyst inlet as the dependency on the mass fraction of pistachio shells in the fuel mixture was defined by polynomial curves, as is shown in Table 7, Fig. 4 (Appendix) for unit 1, and Fig. 5 (Appendix) for unit 2. The dependency of multiple enlargements (relative increment related to the results obtained during the combustion of the pure pellets considered as reference) of CO, C3H8, and TSP on the mass fraction of pistachio shells in the fuel mixture was also defined by polynomial curves, as is shown in the Table 8, Fig. 6 (Appendix) for unit 1, and Fig. 7 (Appendix) for unit 2. Described curves are very valuable outputs which can be widely used by combustion unit manufacturers for rough prediction of change of mass concentration of CO, C3H8, and TSP in the case of substitution of part of pellets in the fuel by pistachio shells. The burner construction and actual mass fraction of the pollutants in the flue gas and maximally available admixture of the pistachio shell to the fuel mixture can be determined.
From the NOx mass concentration point of view, there was a gradual increase in the case of combustion of fuel mixture with 25% of pistachio shells and more. The fuel mixture with 10% of pistachio shells seems to be unaffected from that point of view. Despite the similar mass fraction of nitrogen in both fuels (N < 0.02%), the NOx increase in the flue gas could be connected with small differences in the fuels below the detection limit.
The second way of the increasement of NOx mass concertation in the flue gas during the tests was the prompt formation due to the locally occurred fuel-rich conditions, where atmospheric nitrogen reacts with the combustion radicals and becomes NOx. This process occurs more significantly during the earliest stage combustion process. [58]
A similar effect of NOx mass concentration increase, during the co-combustion of widely used standard fuel with alternative biomass-based fuel such as switchgrass [53] or beech leaves briquettes [16], was described in previous studies. An increase in NOx formation during the pure alternative biomass-based fuel combustion was also observed during the powders of coconut and cashew nut shell pellets [14]. Reached mass concentration values of NOx during these tests are comparable with the values obtained during the argan nut shell combustion tests [13]. In consideration of the European Commission regulation 2015/1185 limit (200 mg·m−3), all combustion tests were in accordance with the limit.
3.2 Effect of catalyst on produced flue gas
Catalysts, as the secondary measure principle for flue gas purification, should positively affect mass concentrations of pollutants, formed during the incomplete combustion process (CO and C3H8), which were significantly increased by combustion and co-combustion of alternative fuel. As is obvious from the results obtained from previous studies, the catalytic conversion rates of pollutants are connected with many processing parameters of the real flue gas. The most important one is the flue gas temperature at the catalyst inlet (within the active phase of the catalyst, with increasing temperature, the conversion rate is increasing), the second most important parameter is GHSV (with increasing GHSV, the conversion rate of the catalyst is decreasing), and the third most important parameter is the mass concentration of a pollutant at the catalyst inlet (with increasing mass concentration of a pollutant at the catalyst inlet, its conversion rate is decreasing) [59].
According to the combination of mentioned influences, the catalyst conversion rate ranged between 61.3 and 2.5% in terms of CO and between 25.9 and 9.6% in terms of C3H8 in the case of unit 1. These relatively low conversion rates were caused by the combination of flue gas conditions, while the low flue gas temperature had the most significant impact (ranging between 260 and 225 °C). For the combustion tests with lower flue gas temperature and very high mass concentration of the pollutant at the catalyst (mixtures of 75/25 and 0/100 pellets/pistachio shells), only negligible conversion rates were reached. A negative effect of higher GHSV during the 50/50 fuel mixture combustion in comparison to other combustion tests occurred.
Significantly higher conversion rates were reached during the tests with unit 2, ranging between 82.2 and 78.7% in terms of CO and between 33.1 and 24.8% in terms of C3H8. Higher conversion rates were reached especially due to significantly higher flue gas temperatures (ranging between 352 and 376 °C) than in the case of unit 1 (ranging between 225 and 260 °C). As is obvious, mentioned temperatures were above the transition part of the catalyst characterization, where the mass concentration of the pollutants at the catalyst inlet has less impact on the overall conversion rate [60].
The obtained results of the conversion rates are slightly moved in the direction of higher temperatures with the conversion curve presented in the previous study of Carnö et al. [60] where the Pt-based honeycomb catalysts were tested in the artificial flue gas. In the study of Ryšavý et al. [46], the conversion rate of Pt-based honeycomb catalysts reached 65% for CO at the flue gas temperature of 307 °C, which is also in accordance with the obtained results.
Due to the regular cleaning of the catalyst between each combustion test and their relatively short period, the pressure loss did not rise during the tests due to clogging by TSP (which was one of the main goals for the credible comparison of the fuel mixtures). The catalyst was without any visible layer of TSP after each of the combustion tests.
By installing the catalyst CAT A at unit 1 outlet, which does not meet the European Commission regulation 2015/1185 limit from the mass concentration of CO point of view, compliance with the limits can be easily achieved, even when pistachio shells are mixed into the fuel at pellets/pistachio shell ratio of 92/8 (calculation based on really achieved conversion rate). In the case of catalyst installation right into unit 1 (different catalyst shapes would be necessary), where flue gas temperature is above 350 °C (conversion rate could reach approximately 80%), pellets/pistachio shell ratio could be up to 73/27.
Combustion device 2 already met the European Commission regulation 2015/1185 limit during the combustion of the pure pellets. Due to the high achieved conversion rate within all fuel mixtures, the pellets/pistachio shell ratio can be up to 47/53 while meeting the mentioned limit.
The presented results showed the significant possibility of replacement of pellets in residential automatic combustion units, which can decrease the overall fuel price for the end user and in combination with the Pt-based catalyst; the same or better parameters of flue gas composition from the mass fraction of pollutants formed during the incomplete combustion process point of view can be reached. Locally, the utilization of pistachio shells for the mentioned purpose could improve the energy safety of the region, by decreasing the imported commodities from suppliers from politically unstable regions.
4 Conclusions
The effects of the pistachio shell and pellet ratio as the fuel for household combustion units on mass concertation of gaseous pollutants and TSP were studied in automatic stoves with gutter burner and bowl burner. In total, 6 fuel mixtures were tested. The chemical and physical properties of the certified EN plus A1 pellets were compared with pistachio shells. The most significant differences were observed in energy density, mass fraction of carbon, and mass fraction of ash. The main conclusion which can be drawn from this study is that the pistachio shells can partially substitute wood pellets in residential combustion units from the operational parameter point of view. Concerning the heat output of the combustion unit, there is no problem with partially or fully substituting wood pellets with pistachio shells, but as the research had shown, significant enlargement of observed pollutants in the flue gas can be expected in that case. Mass concentrations of CO, C3H8, and TSP were increased with an increase of the mass fraction of pistachio shells in the fuel mixture and were described by the polynomial curves. According to the type of burner construction and actual mass fraction of the pollutants in the flue gas, the maximally available mass fraction of the pistachio shell in the fuel mixture can be roughly determined for different combustion units.
From the mass concentrations of CO and C3H8 point of view, the gutter burner seemed to be more suitable for the pistachio shells’ co-combustion than the bowl burner. In respect of the increment of mass concentrations of TSP, both burners and combustion units reached very similar values which do not comply with legislation limits.
Installed oxidation honeycomb Pt-based catalyst caused the decrease of the mass concentration of CO and C3H8 in the flue gas. In the case of catalyst installation in the higher temperature areas, for example right inside the combustion unit, closer to the combustion chamber (the combustion unit would have to be designed for that purpose) would mean enabling the burning of a higher mass fraction of pistachios in the fuel mixture. The improvement of residential combustion units for wood pellet combustion by the right catalyst installation might prevent high variability of mass concentrations of pollutants and can enable expanding the spectrum of suitable fuels.
Different construction types of burners in the automatic stoves and boilers for household heating, such as the retort or rotation, could lead to different results and should be investigated separately in the following research as well as the influence of pistachio shell co-combustion on organic compounds in the flue gas.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Canepa F (2022) Euro zone consumers in for a shock as power bills soar. https://www.reuters.com/business/energy/euro-zone-consumers-shock-power-bills-soar-2022-01-18/. Accessed February 18 2022
ExpoBiomasa (2021) The number of pellet stoves and boilers operating in the EU increases by 10% per year. https://www.expobiomasa.com/en/the-number-of-pellet-stoves-and-boilers-operating-in-the-eu-increases-by-10-a-year. Accessed 4th February 2022
Masdemont PR, Stupavský V (2022) Pellet statistics 2021 - detailed description of pellet markets and heating sources. Praha: Czech pellet cluster; p. 79
ERCR (2022) State Environmental Fund of the Czech Republic. https://www.sfzp.cz/en/. Accessed 4th Ferbuary 2022
FME (2022) Blue Angel. https://www.blauer-engel.de/en. Accessed 4th February 2022
ADEME (2022) Flamme VERTE - Le label du chauffage au bois. https://www.flammeverte.org/. Accessed 7th February 2022
EP. Commission Regulation (EU) 2015/1189 of 28 April 2015 implementing Directive 2009/125/EC of the European Parliament and of the Council with regard to ecodesign requirements for solid fuel boilers. In: EP, editor. 2015/1189. Bruxelles: the European Commision; 2015.
Europe C (2021) Europe halfway to closing all its coal plants by 2030. https://caneurope.org/europe-halfway-to-closing-all-its-coal-plants-by-2030/. Accessed 4th February 2022.
EC (2014) Commission Staff Working Document Executive Summary of the Impact Assessment. In: Commission E, editor. Brussels: European Commission; p. 8
Dziok T, Bury M, Bytnar K, Burmistrz P (2021) Possibility of using alternative fuels in Polish power plants in the context of mercury emissions. Waste Manag 126:578–84. https://doi.org/10.1016/j.wasman.2021.03.053
Linden A, Reichel, A (2020) Bio-waste in Europe : turning challenges into opportunities. European Environment Agency;
Yang Y, Liew RK, Tamothran AM, Foong SY, Yek PNY, Chia PW et al (2021) Gasification of refuse-derived fuel from municipal solid waste for energy production: a review. Environ Chem Lett 19(3):2127–40. https://doi.org/10.1007/s10311-020-01177-5
Rahib Y, Boushaki T, Sarh B, Chaoufi J (2021) Combustion and pollutant emission characteristics of argan nut shell (ANS) biomass. Fuel Processing Technology. 213. https://doi.org/10.1016/j.fuproc.2020.106665
dos Santos GR, de Sousa AM, Lima BKS, Moreira FL, Gondim FL, da Silva GM et al (2021) Combustion of pellets produced from the powders of coconut and cashew nut shells: chemical, thermal and emission analyses. Waste Manag Res. https://doi.org/10.1177/0734242X20983417
Abah EO, Ahamed T, Noguchi R (2021) Catalytic temperature effects on conversion efficiency of pm2.5 and gaseous emissions from rice husk combustion. Energies. 14(19). https://doi.org/10.3390/en14196131
Ryšavý J, Horák J, Kuboňová L, Jaroch M, Hopan F, Krpec K et al (2020) Beech leaves briquettes as fuel for a home combustion unit. WIT Transactions on Ecol Environ 246:75–85. https://doi.org/10.2495/EPM200081
Limousy L, Jeguirim M, Dutournié P, Kraiem N, Lajili M, Said R (2013) Gaseous products and particulate matter emissions of biomass residential boiler fired with spent coffee grounds pellets. Fuel. 107:323–9. https://doi.org/10.1016/j.fuel.2012.10.019
Collura S, Azambre B, Finqueneisel G, Zimny T, Weber JV (2006) Miscanthus × Giganteus straw and pellets as sustainable fuels: combustion and emission tests. Environ Chem Lett 4(2):75–8. https://doi.org/10.1007/s10311-006-0036-3
Pawlak-Kruczek H, Arora A, Mościcki K, Krochmalny K, Sharma S, Niedzwiecki L (2020) A transition of a domestic boiler from coal to biomass – emissions from combustion of raw and torrefied palm kernel shells (PKS). Fuel. 263:116718. https://doi.org/10.1016/j.fuel.2019.116718
Elmay Y, Trouvé G, Jeguirim M, Said R (2013) Energy recovery of date palm residues in a domestic pellet boiler. Fuel Processing Technol 112:12–8. https://doi.org/10.1016/j.fuproc.2013.02.015
Jackowski M, Niedźwiecki Ł, Mościcki K, Arora A, Saeed MA, Krochmalny K, et al (2021) Synergetic co-production of beer colouring agent and solid fuel from brewers’ spent grain in the circular economy perspective. Sustainability (Switzerland). 13(18 C7 - 10480). https://doi.org/10.3390/su131810480
Alves CA, Font O, Moreno N, Vicente ED, Duarte M, Tarelho LAC et al (2019) Mineralogical, chemical and leaching characteristics of ashes from residential biomass combustion. Environ Sci Pollut Res 26(22):22688–703. https://doi.org/10.1007/s11356-019-05231-w
Morán JC, Míguez JL, Porteiro J, Patiño D, Granada E (2015) Low-quality fuels for small-scale combustion boilers: an experimental study. Energy and Fuels. 29(5):3064–81. https://doi.org/10.1021/ef5019252
Statista (2022) Production of pistachios worldwide from 2007/2008 to 2020/2021. https://www.statista.com/statistics/933073/pistachio-global-production/. Accessed 8th February 2022.
da Silva JCG, Alves JLF, Galdino WVdA, Moreira RdFPM, José HJ, de Sena RF et al (2018) Combustion of pistachio shell: physicochemical characterization and evaluation of kinetic parameters. Environ Sci Pollut Res 25(22):21420–9. https://doi.org/10.1007/s11356-017-8945-1
Kocsis Z (2019)Theory and practice of wood pellet production. 1st ed. 2019 ed. Springer;
Lua AC, Yang T (2004) Effects of vacuum pyrolysis conditions on the characteristics of activated carbons derived from pistachio-nut shells. J Colloid Interface Sci 276(2):364–72. https://doi.org/10.1016/j.jcis.2004.03.071
Pütün AE, Özbay N, Varol EA, Uzun BB, Ateş F (2007) Rapid and slow pyrolysis of pistachio shell: effect of pyrolysis conditions on the product yields and characterization of the liquid product. Int J Energ Res 31(5):506–14. https://doi.org/10.1002/er.1263
Alcazar-Ruiz A, Ortiz ML, Dorado F, Sanchez-Silva L (2022) Gasification versus fast pyrolysis bio-oil production: a life cycle assessment. J Cleaner Prod 336:130373. https://doi.org/10.1016/j.jclepro.2022.130373
Tekin İ, Dirikolu İ, Gökçe HS (2021) A regional supplementary cementitious material for the cement industry: pistachio shell ash. J Cleaner Prod 285:124810. https://doi.org/10.1016/j.jclepro.2020.124810
Vicente ED, Figueiredo D, Gonçalves C, Lopes I, Oliveira H, Kováts N et al (2022) In vitro toxicity of particulate matter emissions from residential pellet combustion. J Environ Sci (China). 115:215–26. https://doi.org/10.1016/j.jes.2021.06.008
Nesterovic A, Djatkov D, Viskovic M, Martinov M, Adamovic D (2021) Air pollutants emissions from biomass combustion in the city of Novi Sad. Serbia Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-021-01882-3
Horák J, Hopan F, Kremer J, Kuboňová L, Polcar L, Molchanov O et al (2022) Real measurement of carbon monoxide, total suspended particulate, and thermal efficiency in modern biomass household boilers. Biomass Conversion and Biorefinery. 12(10):4463–72. https://doi.org/10.1007/s13399-022-02657-0
Carnö J, Ferrandon M, Björnbom E, Järås S (1997) Mixed manganese oxide/platinum catalysts for total oxidation of model gas from wood boilers. Applied Catalysis A: General. 155(2):265–81. https://doi.org/10.1016/S0926-860X(97)80129-9
Ferrandon M, Berg M, Björnbom E (1999) Thermal stability of metal-supported catalysts for reduction of cold-start emissions in a wood-fired domestic boiler. Catalysis Today. 53(4):647–59. https://doi.org/10.1016/S0920-5861(99)00152-2
Hukkanen A, Kaivosoja T, Sippula O, Nuutinen K, Jokiniemi J, Tissari J (2012) Reduction of gaseous and particulate emissions from small-scale wood combustion with a catalytic combustor. Atmospheric Environ 50:16–23. https://doi.org/10.1016/j.atmosenv.2012.01.016
Reichert G, Schmidl C, Haslinger W, Stressler H, Sturmlechner R, Schwabl M et al (2018) Impact of oxidizing honeycomb catalysts integrated in firewood stoves on emissions under real-life operating conditions. Fuel Processing Technology. 177:109–18. https://doi.org/10.1016/j.fuproc.2018.04.016
Ryšavý J, Horák J, Hopan F, Kuboňová L, Krpec K, Molchanov O, et al (2022) Influence of flue gas parameters on conversion rates of honeycomb catalysts. Separation and Purification Technology 278. https://doi.org/10.1016/j.seppur.2021.119491
Vicente ED, Duarte MA, Tarelho LAC, Alves CA (2022) Efficiency of emission reduction technologies for residential biomass combustion appliances: electrostatic precipitator and catalyst. Energies.15(11 C7 - 4066). https://doi.org/10.3390/en15114066
Ryšavý J, Horák J, Krpec K, Hopan F, Kuboňová L, Molchanov O (2022) Influence of fuel mixture and catalyst on the ethanol burner flue gas composition. Energy Reports. 8:871–9. https://doi.org/10.1016/j.egyr.2022.10.181
Čespiva J, Skřínský J, Vereš J, Borovec K, Wnukowski M (2020) Solid-recovered fuel to liquid conversion using fixed bed gasification technology and a Fischer-Tropsch synthesis unit – case study. Int J Energy Prod Manag 5(3):212–22. https://doi.org/10.2495/EQ-V5-N3-212-222
Oladeji JT (2010) Fuel characterization of briquettes produced from corncob and rice husk resides
Chang G, Huang Y, Xie J, Yang H, Liu H, Yin X et al (2016) The lignin pyrolysis composition and pyrolysis products of palm kernel shell, wheat straw, and pine sawdust. Energy Conv Manag 124:587–97. https://doi.org/10.1016/j.enconman.2016.07.038
Mohammed IY, Abakr YA, Kazi FK, Yusup S, Alshareef I, Chin SA (2015) Comprehensive characterization of Napier grass as a feedstock for thermochemical conversion. Energies. 8(5):3403–17. https://doi.org/10.3390/en8053403
Açikalin K, Karaca F, Bolat E (2012) Pyrolysis of pistachio shell: effects of pyrolysis conditions and analysis of products. Fuel. 95:169–77. https://doi.org/10.1016/j.fuel.2011.09.037
Ryšavý J, Horák J, Hopan F, Kuboňová L, Krpec K, Kubesa P (2019) Comparison of catalysts in the point of view of pellet stove flue gas purification. Int J Energy Prod Manag 4(2):124–33. https://doi.org/10.2495/EQ-V4-N2-124-133
Wang W, Zhao S, Tang X, Chen C, Yi H (2022) Stainless steel catalyst for air pollution control: structure, properties, and activity. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-21079-z
Ma L, Geng Y, Chen X, Yan N, Li J, Schwank JW (2020) Reaction mechanism of propane oxidation over Co3O4 nanorods as rivals of platinum catalysts. Chemical Eng J 402:125911. https://doi.org/10.1016/j.cej.2020.125911
DNR (2020) Effects of ground level ozone. https://www.iowadnr.gov/Environmental-Protection/Air-Quality/Air-Pollutants/Effects-Ozone. Accessed 15 July 2021
CEN (2021) EN 303:5:2021 Heating boilers - Part 5: Heating boilers for solid fuels, manually and automatically stoked, nominal heat output of up to 500 kW - terminology, requirements, testing and marking. European Committee for Standardization; p. 98.
CEN (2007) EN 14785 Residential space heating appliances fired by wood pellets - requirements and test methods. European Committee for Standardization; p. 68.
EP (2015) Commission Regulation (EU) 2015/1185 of 24 April 2015 implementing Directive 2009/125/EC of the European Parliament and of the Council with regard to ecodesign requirements for solid fuel local space heaters. In: EP, editor. 2015/1185. Bruxelles: the European Commision;
van Loo S, Koppejan J (2008) Handbook of biomass combustion and co-firing, 2nd edn. Earthscan, London
Schmidt G, Trouvé G, Leyssens G, Schönnenbeck C, Genevray P, Cazier F et al (2018) Wood washing: influence on gaseous and particulate emissions during wood combustion in a domestic pellet stove. Fuel Processing Technology. 174:104–17. https://doi.org/10.1016/j.fuproc.2018.02.020
Sippula O, Hytönen K, Tissari J, Raunemaa T, Jokiniemi J (2007) Effect of wood fuel on the emissions from a top-feed pellet stove. Energy and Fuels. 21(2):1151–60. https://doi.org/10.1021/ef060286e
Molchanov O, Krpec K, Horák J (2020) Electrostatic precipitation as a method to control the emissions of particulate matter from small-scale combustion units. Journal of Cleaner Production.246. https://doi.org/10.1016/j.jclepro.2019.119022
Molchanov O, Krpec K, Horák J, Kuboňová L, Hopan F (2020) Comparison of methods for evaluating particle charges in the electrostatic precipitation of fly-ash from small-scale solid fuel combustion. Separation and Purification Technology.248. https://doi.org/10.1016/j.seppur.2020.117057
Zink J (2001) Combustion handbook (industrial combustion). CPR Press, Tulsa, Oklahoma
Kamer P, Vogt D, Thybaut J (2017) Contemporary catalysis: science, technology, and applications
Carnö J, Berg M, Järås S (1996) Catalytic abatement of emissions from small-scale combustion of wood: a comparison of the catalytic effect in model and real flue gases. Fuel. 75(8):959–65. https://doi.org/10.1016/0016-2361(96)00047-6
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported by the doctoral grant competition VSB–Technical University of Ostrava, reg. no. CZ.02.2.69/0.0/0.0/19_073/0016945 within the Operational Programme Research, Development and Education, under project DGS/TEAM/2020-035 “Determination of oxidation catalysts characteristics during the flue gas purification”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jiří Ryšavý. The first draught of the manuscript was written by Jiří Ryšavý, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
It is stated that the manuscript is only submitted in ESPR. The submitted manuscript is original and not forwarded anywhere for proofreads or others. ESPR is authorized to verify plagiarism of the manuscript.
Consent to participate
All the authors have personal consent to perform the completion of the manuscript. No author is forced to perform the work. All the manuscript is mutually cooperated by authors.
Consent to publish
All the authors agreed to submit the manuscript with affiliations of institutes mentioned in the manuscript and also agreed to submit in ESPR.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Pellet burners were able to substitute pellets with pistachio shells without technical issues.
Concentrations of CO, C3H8, and TSP increased with an increase in the pistachio shell share in the fuel.
Pt-based catalyst decreased the concentrations of CO and C3H8 in the flue gas from pistachio shell combustion.
Appendix
Appendix
Dependency of multiple increases of CO and C3H8 mass concentration in flue gas on the fuel mixture. Note: Curve for characterization of C3H8 multiple increases could not be constructed due to lack of reference data caused by very low values below the detection limit during the combustion of the pure pellets
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryšavý, J., Serenčíšová, J., Horák, J. et al. The co-combustion of pellets with pistachio shells in residential units additionally equipped by Pt-based catalyst. Biomass Conv. Bioref. 13, 16511–16527 (2023). https://doi.org/10.1007/s13399-023-03845-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03845-2