Abstract
Torrefaction encourages homogeneity and enhances the energy-producing capabilities of biomass. In the current study, bean husk (BH) and sesame stalks (SS) were torrefied for 30 and 60 min at operating temperatures of 200, 225, 250 and 275, and 300 °C with nitrogen purging. Mass yield (MY), higher heating value (HHV), energy yields (EY), and torrefaction severity index (TSI) were examined. The variations of the biochar characteristics, pyrolysis kinetics by applying two models (Coats and Redfern (CR) and Direct Arrhenius (DA)), and crystallinity index (CRI) were depicted. Depending on pyrolysis kinetics, thermodynamic activation parameters were derived to elucidate biomass pyrolysis. The alterations in the torrefied materials’ composition were also analyzed using Fourier transform infrared spectroscopy (FTIR). The calculations revealed that the torrefied SS and BH decreased MY by 32.74, 29.02% and decreased EY 26, 20.97%, increased high heating values by 14.1, 13.52%, increased fixed carbon by 55.1, 39.91% respectively, and had a slight reduction in bulk density (approximately 2%). Generally, 275 °C and 30 min were the optimal conditions for a balanced torrefaction of SS and BH based on the HHV that reached to 20.5, 16.2 MJ/kg and EY that reached to 86.16 and 85.56% respectively. The FTIR, XRD, and the thermogravimetric results showed that the torrefaction treatment altered samples owing to carbohydrate breakdown, a rise in lignin, and a reduction in hemicellulose as the temperature of the torrefaction process increased. The CR methodology yielded greater frequency factor (A) and activation energy (Ea) values than the DA method. The broadest peak width, lowest average Ea, and lnA were seen in sesame stalks that had been torrefied at 300 °C and 30 min that reached to 107.85 (kJ/mol) and 13.57 (min−1). Results indicated an excellent linear relationship with the index of comprehensive pyrolysis (CPI), CRI, atomic H/C ratio, severity index, and EY.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The increasing depletion of fossil fuels has urged researchers to look for renewable alternatives [1]. Biomass is the most viable alternative to fossil fuels because of its plentiful supply and replenishment capacity [2]. Over 180 billion tons of lignocellulosic biomass are produced every year, making it a potentially useful feedstock [3]. According to the raw materials conversion factor, 1 kg of biomass could be transformed into 6 MJ of energy or 0.8 kg of chemicals [3]. In this regard, Egypt implemented effective renewable energy initiatives during the last decade [4]. More than half of the agricultural waste is burned directly in the fields or in inefficient burners. Both strategies result in energy losses and environmental harm [5]. Untreated biomass is rarely employed as an industrial fuel, despite its abundance due to its inherent disadvantages compared to fossil fuels. A few of these drawbacks are poor energy density, hygroscopicity, high-level moisture content, and limited processability [5, 6]. Several pretreatments could be performed to improve the raw material conditions, boost the economic viability, and fulfill the real demand of solid lignocellulosic biofuel [7, 8].
Thermochemical torrefaction is performed in an inert environment at temperatures of 200–300 °C and modest heating rates. [9, 10]. Torrefaction improves the biomass characteristics as hydrophobic nature, milling properties, and energy density. On the other hand, it reduces the atomic oxygen to carbon (O/C) and hydrogen to carbon (H/C) ratios and enhances the biomass characteristics to meet those of low-rank coal, peat, and lignite [12]. Due to their enhanced properties, the torrefied biomasses are employed in combustion, pyrolysis, or gasification [13]. It is easier and more efficient to burn torrefied biomass products than raw biomass as it has higher flame temperatures and better combustibility than those of the raw biomass [14].
Numerous researches have been conducted in this area because of the significant interest in the growing field of torrefaction, which is predicted to become a major technology [10, 11]. Studying the effect torrefaction of sugarcane bagasse on the ultimate, proximate and morphological properties at operating temperatures of 200–300 °C and 15-–60-min retention duration showed that treated sugarcane bagasse had been sufficiently enhanced to be used in gasification process [15]. Zhang et al. [16] studied the effect of torrefaction process of nutshell waste at temperatures of 250–300 °C and 10–30 min. The results revealed that the optimal conditions at 250 °C and a retention duration of 20 min, which resulted in a greater energy density and acceptable indices. Torrefaction temperatures of 225–300 °C and retention period of 20–60 min were examined for empty fruit bunches in a batch reactor [17]. While higher torrefaction temperature and retention duration reduced the mass and energy yields, the liquid and gaseous products increased. The torrefaction temperature, residence duration, and heating rate were studied on a mustard stalk. It was found that torrefied biomass has improved characteristics of co-combustion and gasification than those of the raw biomass [18]. Table 1 contains a discussion of the torrefaction of a variety of biomass along with the experimental conditions and results. To the authors’ knowledge, neither bean husk (BH) nor sesame stalks (SS) have been studied for their potential use in thermochemical processes. Hence, valorization of these biomasses needs to be further investigated.
A comprehensive thermokinetic investigation on the pyrolysis of biomass samples was also undertaken to help develop and optimize procedures to acquire high-carbon material for energy or other applications [14,15,16,17,18,19,20,21,22]. The determination of pyrolysis kinetic parameters is most often accomplished by thermogravimetric analysis (TGA), which is essential for comprehending the whole thermochemical conversion techniques [19]. Several kinetic approaches are applied to estimate the reaction kinetics of biomass. These approaches can be separated into two main categories: the model-fitting technique and the iso-conversional approach [14, 15]. The model-fitting approach applies several possible reaction mechanisms to the thermogravimetric data, and then uses the one with the best match to get the kinetic parameters. This best fit is produced by assuming the reaction order and then adjusting the rate equation to produce a straight line plot, from which the remaining unknown parameters may be calculated using linear regression. Direct Arrhenius plot (DA), Coats and Redfern's graphical technique (CR) are model fitting methods [16, 17]. Model fitting approaches employ a single TG curve. Consequently, it is simple to employ the model fitting techniques. Several researchers have found that using the CR technique to compute kinetic parameters helps them get closer to the best reaction model for the degradation of biomass [23, 24]. These two approaches were utilized to establish kinetic parameters for Egyptian agricultural and woody biomasses [21] and microwave-treated rice straw [22]. Literature shows that the temperature of pyrolysis, the mathematical approaches used for kinetic studies, and the pyrolysis techniques affect activation energy [16, 17]. Consequently, the literature provides various activation energy values for the same biomass type.
Based on the abovementioned, the current study focuses on investigating the impact of torrefied temperature and duration of residence on the characterization of the produced biochar. Two biomasses are considered, namely sesame stalk and bean husk. The sesame crop is considered one of the most important oil crops in Egypt, as it is well cultivated in light yellow, heavy, and sandy lands that are newly reclaimed. The global production of sesame is about 5,532,000 metric tons per year and has jumped to 6,016,000 metric tons in 2018 with the cultivated area estimated at 11,743,000 ha [25]. In 2022, the annual production of sesame in Egypt was 45.58 thousand tons, and the total marketing loss of the sesame crop in the study sample was about 27.3 kg/ton. According to FAO, bean global harvested area was 33.1 million ha and production was 28.9 million tons in 2019 [26] In 2019, the production volume of beans in Egypt was approximately 132 thousand metric tons [27].
In the study of biomass, the FTIR approach has proven to be beneficial in revealing substantial microstructural changes during torrefaction, illustrating how the FTIR spectra bands are altering [28]. This affects the chemical structure and absorption band assignments in the biomass samples [29]. Furthermore, these alterations involve devolatilization, carbonization, and thermal decomposition of amorphous, low-heat-resistant polymeric chains [30]. Most FTIR spectral changes occur between 1200 and 800 cm−1 owing to hemicelluloses and cellulose decomposition [21].
The crystallinity index (CRI) is investigated by XRD partly because of the prominent diffraction peaks produced by cellulose crystals [31]. The amount that light is scattered and bent as it travels through an aperture or an edge is measured using X-ray diffraction, which can be used to evaluate the level of organization present in the structure of a material [21]. The CRI value decreased as the torrefaction temperature increased, and no crystallinity was identified in the torrefied biomass [32].
A detailed experimental investigation on biochar production optimization from sesame SS and BH wastes is conducted in this framework, and the influence of process factors is studied. The effects of temperatures of 200, 225, 250, and 275 and 300 °C and 30- and 60-min retention duration on the solid products of torrefied samples are investigated. Physicochemical features, elemental analysis, proximate analysis, and calorific values are used to thoroughly investigate the primary physical and chemical aspects of raw and torrefied biomasses. A thermo-kinetic study of thermally treated biomass is also conducted by using two simple kinetics approaches DA and CR to develop and optimize procedures for obtaining high carbon content from torrefied solid products for energy usage. The solid biofuel is evaluated using standard methodologies to determine structural and thermal characteristics. It was also stated how the torrefaction process led to the degradation of biomass (TG/DTG). The thermodynamic characteristics of activation (enthalpy, Gibbs free energy, and entropy) were determined based on Ea and A to elucidate biomass pyrolysis. Hence, the current study opens the door to further research on these two biomasses.
2 Materials and methodology
2.1 Materials
Sesame stalks and bean husks, the agricultural waste products employed in this research, were gathered from large-scale farms in Alexandria, Egypt. The raw biomasses were cleaned with distilled water to eliminate the surface impurities, and then put to dry overnight in a drying oven at 105 °C (Binder ED 53). To create the fine powders needed for this process, the untreated biomasses were first broken into little pieces then, a grain mill, AG Flour Mill, was used to grind them. The fine powders were next subjected to mechanical sieving to achieve particle sizes of less than 400 μm. Powdered materials are stored in sealed containers to avoid moisture absorption before further usage.
2.2 Torrefaction procedure
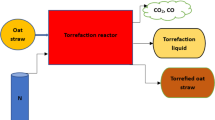
The AMF-25 N 3-kW muffle furnace is used to torrefy the biomass samples. Temperatures are set at 200, 225, 250, 275, and 300 °C to cover the whole spectrum of torrefaction. A total of 30- and 60-min residence times are tested for all prior temperature settings. In a typical experiment, as depicted in Fig. 1, about 75 g ± 3% of the dried SS and BH were put inside a 0.35-L, 80 mm in diameter, and 70-mm high cylindrical stainless steel reactor. This reactor is made specifically to fit inside the furnace in such a way that it can resist extreme temperatures and maintain N2 environment all around the raw biomass. First, a high-purity N2 gas is introduced into the reactor and allowed to circulate there for 15 min to remove any oxygen. The purging port is then locked, and the reactor is put in the muffle furnace and subjected to an additional 20 min of high flow nitrogen purging.
The nitrogen flow rate is lowered to 100 mL per minute while the furnace temperature controller is set to the required temperature. For precise temperature measurements, the reactor contains a port that is used to introduce a K-type thermocouple directly into the sample's core. The duration of the residence time is initiated when the thermocouple reading matches the set temperature that was specified. When the predetermined time has passed, the muffle furnace is turned off, and the N2 supply is increased again. This causes the muffle furnace to cool down quickly, preventing oxidation and secondary reactions before the temperature returns to its normal level [37]. The solid residue from the procedure is then weighed, collected, and sealed for subsequent examination.
The sample notation adopted for this study is SS and BH representing raw sesame stalks and bean husk, respectively. SST,t, and BHT,t represent torrefied sesame stalks and bean husk, while T and t indicate the torrefaction temperature and residence time, respectively. For example, SS200,30 means torrefied SS at 200 °C and 30 min.
2.3 Sample characterization
The proximate and ultimate tests are achieved by utilizing Discovery SDT 650 Simultaneous Thermal Analyzer (ASTM D5142) and Vario Micro Cube Elementar (ASTM D5291), respectively while elemental oxygen is estimated by difference (i.e., O = 100-H-C-S–N). The samples’ higher heating values (HHVs) are a crucial part of figuring out whether or not biomass can be used. (HHVs) are measured by (Barr oxygen bomb calorimeter (Model 1341EE)) based on the steps outlined in the standard UNE-EN 14,918:2011 [38]. Biomass bulk density is significant for storage and transportation. Biomass density affects size, fuel storage, handling, and thermochemical behavior of material [39]. The sample’s weight based on dry basis per unit volume is used to compute the bulk density, according to the process outlined in UNE-EN 15,103. FTIR was carried out in a Bruker Alpha II FTIR to assess the functional groups of raw and torrefied solid products. A sample of approximately 1 mg is examined between 4000 and 400 cm−1 with a step of 2 cm−1. This method may be used to extract pure organic and inorganic components from raw and torrefied SS and BH by detecting the surface functional groups present on their surfaces. To study the thermal breakdown behavior, thermogravimetric (TG) tests are carried out utilizing a TG analyzer Discovery SDT 650 Simultaneous Thermal Analyzer (ASTM D5142) to get the TG and differential TG (DTG) curves. The samples, weighing 5.000 ± 0.500 mg, were added to a TG reactor at a flow rate 100 mL/min of N2 acting as the inert environment. The weight of the sample was purposefully chosen to be somewhat high in order to guarantee that the sample accurately reflects all of the biomass. Dynamic runs were conducted from the room ambient temperature up to 950 °C with heat rate of 20 °C/min. Blank TG runs were performed to compensate for the buoyancy effect. The TG and DTG curves were constantly recorded as a function of time and temperature throughout the experiment. The kinetics, composition, and thermal behavior of a reaction are directly calculated by evaluating the outcomes that shows the onset and offset temperature, which are the temperatures at which the pyrolysis reaction rate accelerates and decelerates, respectively. Shimadzu Xlab 6100 is used for all crystallinity and XRD measurements. The standard group was evaluated at 5.0 to 80.1°, 12°/min, 0.02° sampling pitch, and 40 kV/30 mA X-ray tube voltages. Crystallinity measurements use 1.54060 with the same tube voltage and current. The crystallinity index (CRI) is determined from the subsequent equation [37]:
where the highest peak intensity and lowest possible intensity at 2ɵ = 22°, and at 2ɵ = 16°, respectively.
The fuel ratio (FR) of the biomass was calculated applying the following equation:
Mass yield (MY), enhancement factor (EF), energy yield (EY), torrefaction severity index (TSI), decarbonization (DC), dehydrogenation (DH), and deoxygenation rates (DO) are calculated. The following equations may be used to all torrefied biomasses to track the torrefaction impact and provide a foundation for evaluating the viability and profitability of torrefaction in industrial settings [40]:
The term “mass yield” refers to the proportion of the mass of the torrefied biomass to the mass of the raw biomass. Enhancement factor is the ratio of the improved heating value of torrefied to raw biomass. The energy yield is the ratio of the torrefied solid’s energy to that of the raw biomass. It is an important parameter for analyzing the torrefaction process. As shown in Eq. (7), the torrefaction process results in a loss of, hydrogen, and oxygen from the biomass material [41]. Torrefaction weight loss in biomass samples is measured using the torrefaction severity index (TSI), which is defined as follows.
3 :
For each temperature and time period, WLT,t is the thermally treated biomass weight loss. TSI is calculated at 300 °C and the longest residence time (60 min). The TSI value lies between 0 and 1, where 0 means the torrefaction process has not started and 1 means it has achieved the maximal temperature and time. The pyrolysis reactivity of biomass is quantified using the comprehensive pyrolysis index (CPI, %/(min °C2)) and is determined using the following formula [42]:
where DTGmax is the highest rate of mass loss (%/min), based on the DTG graphs, Tpeak is the associated temperature of the highest rate of mass loss. Tonset is the corresponding temperature of the pyrolysis initiation and Toffset is the corresponding temperature of the pyrolysis ending [42].
3.1 Kinetic parameters determination
The following Equations are given to define the thermochemical degradation of biomass [19]:
where the initial mass is mi, final is mf, and sample mass mt at temperature T, mg.
In terms of reaction kinetics, f (α) is described as:
where n is the reaction’s order. This nth-order reaction is the most prevalent type for biomass, which shows that the rate of the reaction is directly related to the remaining reactants. The Arrhenius equation often uses K in (Eq. 9)) to represent the temperature dependent rate constant as:
where A represents the frequency or pre-exponential factor in min−1, E is the decomposition process activation energy of the apparent in kJ/mol, and R represents the ideal gas constant in kJ/mol. T represents the temperature in Kelvin. Substituting Eqs. (13) and (12) into Eq. (9) yields the following formula for the reaction rate:
The performed TGA runs are non-isothermal so their heating rate is expressed by the following equation:
In non-isothermal TGA tests, the heating rate is changed as a time function:
As a result, Eq. (16) is expressed as:
The kinetic values are obtained from the non-isothermal TGA runs using DA and CR methods [27, 28].
Both approaches are based on the concept of a straight line curve fitting that having fundamental equation:
where a signifies the line’s slope and b represents the point at which the line intersects the y-axis. Before determining the kinetic parameters, the TGA/DTG findings must first be analyzed to find out where the DTG peaks are located. After that, the following computations are carried out only throughout the peak range [27, 28].
3.1.1 Direct Arrhenius plot method
Using this approach, the logarithm is computed for both sides of Eq. (17), and the following equation is produced.
By comparing Eq. (18) and Eq. (19), Y= \(\mathrm{ln}\left\{\frac{1}{{(1-\mathrm{\alpha })}^{\mathrm{n}}}\times \right.\left.\frac{\mathrm{d\alpha }}{\mathrm{dT}}\right\}\) and X = 1/T are used to depict the data. Y vs. X graphs are made for n values from 0.1 to 1.5 with 0.1 increments to identify the optimal reaction order. Straight lines were used to fit all of the plots, and the highest R2 value was selected. The slope represents \(-\frac{\mathrm{E}}{\mathrm{R}} ,\) where E is calculated. Also, the y-axis intercept also equivalent to\(\mathrm{b}=\mathrm{ln}\frac{\mathrm{A}}{\upbeta }\), which yields pre-exponential factor (A).
3.1.2 Integral graphical method
To analyze kinetic data derived from thermogravimetric curves, the graphical approach that was created by Coats and Redfern was put to use. To carry out this study, the following equations are utilized.
Equations (20) and (21) could be simplified as \(\frac{2RT}{E} <<1\), making \(\left(1-\frac{2RT}{E}\right) \simeq 1\) thus both equations could be rewritten as follows:
By comparing Eqs. (22) and (23) with Eq. (18), a relation between \(\mathrm{Y}=\mathrm{ln}\left\{\frac{1-{(1-\alpha )}^{1-n}}{{T}^{2}(1-n)}\right\}\) \(\left(\mathrm{for n}\ne 1\right)\) or Y= \(\mathrm{ln}\left\{\frac{-\mathrm{ln}\left(1-\alpha \right)}{{T}^{2}}\right\}\) \(\left(\mathrm{for n}=1\right)\) and \(\mathrm{X}=\frac{1}{T}\) is obtained.
3.1.3 Thermodynamic parameters
The aforementioned SS and BH torrefaction kinetic analysis can compute activation energy. It does not provide conditions after the torrefaction process equilibrates. Thermodynamic parameters elucidate biomass pyrolysis. Variations in enthalpy (H), Gibbs free energy (G), and entropy (S) are significant for thermodynamic analysis and are calculated by using Eqs. (24), Eq. (25) and Eq. (26), respectively [45].
where T is the pyrolysis temperature (K), KB is the constant of Boltzmann \(\left(1.381 \times {10}^{-23} {m}^{2}\mathrm{ kg}/{s}^{2}K\right)\), h is the constant of Planck \(\left(6.626 \times {10}^{-34} {m}^{2}\mathrm{ kg}/\mathrm{s}\right)\), and Tpeak is the derivative thermogravimetric (DTG) curve’s peak temperature.
4 Results and discussion
4.1 The physical appearance of torrefaction products
The colors of raw and torrefied SS and BH samples under various thermal treatment parameters are shown in Fig. 2. Torrefied biomasses have a distinct color difference from raw samples. Light torrefaction from (200 to 225 °C), moderate torrefaction from (250 to 275 °C), and severe torrefaction occurs at (300 °C) torrefied samples have light brown, dark brown, and black colors, respectively, as seen in Fig. 2. During torrefaction, the cell structure undergoes significant chemical changes, clearly visible in the torrefied biomass samples’ changing color. As seen in Fig. 2, the consistent color of all samples proves that the torrefaction procedure is applied uniformly to the whole sample. There are several possible explanations for this shift in color, including variations in the lignocellulosic content that causes chemical changes and surface changes in the biomass and changes in light absorption, reflection, and diffraction [46]. The color of the samples often changes as the torrefaction temperature increases, indicating that they have undergone a significant amount of conversion. Hydrosols, carbonyls, methoxyls, and phenolic chemicals are responsible for the biomass’s color change after heat conversion. The biomass’s dark color is related to these chemicals’ propensity to absorb visible light [47]. In addition, the color of moderately torrefied biomass might result from the synthesis of phenols and aldehydes under moderate torrefaction conditions (250 °C). This conclusion aligns with research results on improving rice husk’s fuel characteristics [48] and coffee husk and cotton stalk mixed biomass [46].
4.2 Physicochemical properties
4.2.1 Characterization of raw samples
Table 2 lists the proximate, ultimate results, and calorific values of untreated SS and BH. As presented in Table 2, both raw SS and BH have relatively low levels of moisture content, which are desirable in the burning process and approved by the standard less10% [36]. The most significant portion of the proximate analysis is made up of volatile matter, with percentages of 63.57% and 64.18% in SS and BH, respectively. As a result, these biomass wastes are highly reactive and ignitable [36]. The fixed carbon was in the range of 10% for both SS and BH. The ash content is relatively high, reaching 21% for both samples, which will increase after upgrading them using torrefaction, that could be a barrier to their use as fuel [49]. The presence of a high amount of ash could result in a variety of operational issues, including corrosion, slagging, fouling, and blockage of the equipment.
Table 2 shows that the carbon and hydrogen contents of SS are 44.5 and 5.28%, respectively, whereas the corresponding values for BH are 32.77 and 4.77%. Therefore, the data indicate that the higher heating value of SS (18.05 MJ/kg) is more than that of BH (14.98 MJ/kg). There are small levels of nitrogen and sulfur that are ignored and do not raise concerns about the development of NOX or SOX discharged to the environment.
An automated fiber analyzer (ankome 2000) determined the two materials’ structural composition. This approach identifies neutral detergent fiber, the detergent-digested residue. Table 2 shows the fiber residues for two biomasses, and cellulose is the most prominent. The lignin content in the SS and BH is higher than the hemicellulose percentages.
Metallic element distribution in biomass fuels affects the temperature of pyrolysis and pyrolysis product composition; hence it is necessary to know their concentrations [50]. Table 2 shows that the presence of significant levels of potassium and calcium results in the creation of eutectic products, which in turn causes the melting point of ash to drop [50]. In addition, the combustion efficiency is reduced when alkalis are mixed with other metals, since deposits form on the combustor’s walls [51]. The two biomass materials include trace amounts of metals including Fe, Cu, and Mn, and their burning in the combustor would release heavy metals into the atmosphere [52].
4.2.2 Characterization of torrefied product
Figure 3 illustrates how torrefaction temperature impacts proximate analysis results. It has been shown that the thermal breakdown of complex polymers at higher torrefaction temperatures results in a growth in FC and the quantity of ash while a reduction in volatile matter [53]. Here, volatile matter removal in SS and BH is attributed to the change in elemental content since it was directly related to the degradation of hemi(cellulose) and lignin. The increment of fixed carbon amount in thermally treated samples results from the carbonization of carbohydrates during torrefaction, which further boosts the carbon concentration [30]. Additionally, a rise in fixed carbon indicates that torrefied solid products have a higher heating value than raw samples.
The fuel ratios of the raw biomass and biochar samples are shown in Fig. 3. Torrefied samples have higher fuel ratios than raw samples, which increase as temperature of torrefaction increases because of the reduction of volatile components of the raw biomass [54]. Industrial coal FR is between 0.5 and 1.0 [55]. The torrefaction of SS could thus be considered to create coal-like fuel. As a result of its high VM, raw biomass burns quickly, resulting in more flame and less unburned char [55]. In comparison to raw SS and BH, torrefied samples have a greater fuel ratio, leading to more efficient burning and fewer emissions. This demonstrates the superiority of torrefaction as a means of enhancing raw biomass’s potential as an energy source [10].
Variations in bulk density between raw and torrefied samples were insignificant (approximately 2%). Generally, torrefaction often causes a slight reduction in bulk density which is undesirable from a storage perspective. This trend could be the result of the removal of volatile matter and moisture, which increased the solid’s porosity. The torrefied biomass needs to undergo a densification procedure to enhance the conditions of transit and storage since it still has a low bulk density.
Figure 4 and Fig. 5 show 3D charts relating the torrefaction conditions and their effect on the elemental parameters for SS and BH, respectively. There are differences between the components in the torrefied samples and the raw materials. The carbon mass fraction increases from 44.45% and 32.77% to 52.67% and 40.96% for SS300,30 and BH300,30, respectively. It also increases to 55.77% and 43.76% for SS300,60 and BH300,60, respectively. As illustrated in Figs. 4b and 5b, the torrefaction process reduces the quantity of oxygen from 48.87% and 61.3% for raw materials to 41.32%, 54.08%, 38.196% and 52.35% for SS300,30, BH300,30, SS300,60, and BH300,60,, respectively, which increases the energy content. Torrefied solid products have better qualities due to oxygen removal in the form of gaseous (H2O, CO2, and CO) and liquid chemicals (e.g., acids, furans, phenols, and ketones) [56]. Owing to the lignocellulosic origin of SS and BH, the hydrogen contents of the torrefied SS and BH were relatively low, ranging from 5.829–4.422% to (4.77–2.38%, respectively. As previously mentioned, under the severe torrefaction conditions, greater quantities of volatile compounds are produced owing to the intense reactions such depolymerization, polymerization, and dehydration [30].
The HHV of the torrefied SS and BH at various operating conditions are shown in Fig. 3d and Fig. 4d, The HHV of raw SS and BH are 18.05 and 14.99 MJ/kg, respectively, which increased gradually with torrefaction intensity owing to carbonization and dehydration at larger torrefaction temperatures and longer residence times. Additionally, thermally processed biomass has more carbon and less oxygen, thus increasing its HHV.
Figure 6 represents the van Krevelen chart for all considered samples. This demonstrates how the fuel properties may be influenced by changes in the elemental composition of the fuel. The ratios of O/C and H/C decline as the degree of torrefaction increases. This occurs because of the removal of volatile substances from the samples, which include a greater proportion of oxygen and hydrogen contents in comparison to carbon contents. The torrefaction conditions of 300 °C and 60 min show a high ability in lowering the O/C and H/C atomic ratios, which is caused by the degradation of hemicelluloses and amorphous cellulose [18, 44]. For co-firing purposes, torrefied SS and BH are very desirable due to their compositional similarities to lignites that have 0.395 for O/C and 0.98 for H/C and peats have 0.428 for O/C and 1.266 for H/C [58].
The MY, EY, EF, and TSI of torrefied SS and BH are shown in Table 3. During torrefaction, raw biomass loses weight owing to the moisture elimination and removal of light volatile matter, thermal decomposition, and devolatilization of fiber residues. Depending on the results shown in Table 3, the MY and EY of torrefied biomass samples follow a declining pattern with rising torrefaction severity (i.e., temperature and retention durations. The percentage of feedstock mass that was converted into biochar during torrefaction (defined by MY) decreased substantially as the torrefaction temperature is raised. The lowest MY for torrefied SS and BH have been achieved at torrefaction temperature 300 °C and 60 min that reached to 61.11 and 64.986 wt%, respectively. This implies, that approximately 38.89 and 35.014 wt% of the wastes is thermal decomposition from torrefied SS and BH. The least thermally stable materials include hemicelluloses, which can release small molecular gases at relatively low temperature. As SS has higher hemicellulose content than BH, the mass yield of SS clearly decreased at lower torrefaction temperatures. These outcomes are in agreement with the torrefaction of several forms of biomass that has been documented [59].
The energy yield of the biochar is reduced at higher severity because a greater quantity of energy was lost owing to increased SS and BH mass loss [36]. Under (300 °C, 60 min), the most extreme torrefaction condition SS and BH EY was only 71.07 and 73.72%. Choosing the correct torrefaction temperature and time for the torrefaction process of a certain biomass is essential for enhancing the fuel qualities of the products that are obtained from biomass. The enhancement factor (EF) has been frequently utilized as a measure of torrefaction’s efficacy since it represents the change in HHV that occurs throughout the process. The EF values determined for this study are shown in Table 3. Consistent with the literature [53] , the results showed that EF increased with both the temperature and duration of torrefaction, indicating that HHV levels improved throughout the duration of the procedure. The EF of torrefied SS and BH reaches 1.163 and 1.13, respectively, at temperature 300 °C and residence time 60 min. This conclusion is reasonable since carbon densification lead to an increase in products generated at a higher temperature [53]. To be an economically viable solid fuel, the torrefied biomass must have a high calorific value (greater than 20 MJ/kg) and EY (greater than 85%) without losing other physicochemical fuel properties [17] Overall, the condition of 275 °C and 30 min is the appropriate torrefaction temperature and residence time for a reasonable torrefaction of both SS and BH; however, BH possesses 16.2 MJ/kg as HHV.
Elements in the samples are examined for their DC, DH, and DO concentrations to determine how much devolatilization occurred during the torrefaction process. As shown in Table 3, the increasing in DC, DH, and DO levels are influenced mainly by temperature, with insignificant impact from residence duration. Oxygen (DO) and hydrogen (DH) are removed at a rate larger than this of carbon removal (DC) [60]. This also indicates that oxygen is far more affected by torrefaction than carbon. Biomass undergoes dehydration, dehydroxylation, and decarboxylation of its constituents, most notably hemicellulose [46, 47]. Therefore, the biomass loses a significant amount of its oxygen content throughout the torrefaction process [56]. Deoxygenation process is crucial step in producing high-quality biochar. Carbonization of biomass occurs as a result of a reduction in DC and an increase in DH and DO, which lowers the atomic H/C and O/C ratios. From the obtained results of the all analysis, torrefaction as a technique increased the fuel characteristics of SS and BH, creating biomaterials comparable to peat and lignite [56].
4.3 TGA/DTG analysis
Figure 7 displays an example of calculation graph of BHraw for thermal kinetic and pyrolysis parameters together with the second derivative of the thermogravimetric function DDTG. The thermal breakdown of untreated, torrefied SS and BH for all samples begins at around 180 °C. The three pyrolysis zones are the same for all samples: initial, primary, and final decomposition [61]. Water and a minor quantity of volatiles evaporated between 30 and 180 °C during the first zone, causing a mass loss of 3–5%. The second zone (180–600 °C) was responsible for the breakdown of mainly hemicellulose, partly lignin, and mostly cellulose. The third zone (600–1000 °C) was mainly concerned with biochar production due mainly to lignin decomposition. The devolatilization and breakdown of organic materials cause the pyrolysis curves’ progressive shift to the right as torrefaction temperature rises [41, 42]. Due to the difficulty of decomposing carbonaceous compounds, the decreased slop of the TGA profile is due mostly to the removal of volatile substances [62].
The DTG curve is often used to examine how the mass loss rate varies throughout biomass pyrolysis. In the second zone, complex events, such as bond breaking and free radical generation, create oxygen-containing molecules and carbon grounds [62]. Additionally, the hemicellulose’s DTG peak between 200 and 300 °C progressively reduced and nearly disappeared at the temperature of torrefaction process of 300 °C, supporting the large loss of hemicellulose content after torrefaction [37]. Figures 8 and 9 illustrate the trends of TGA, DTG, and parameters of pyrolysis of untreated and 30-min torrefied SS and BH at temperatures between 200 and 300 °C. As the retention time does not affect the results significantly as mentioned before, the thermal analyses are conducted at one residence time of 30 min. With rising the torrefaction temperature from 200 to 300 °C, the pyrolysis trends steadily become smooth and move to the right because of the thermal devolatilization and degradation of organic matter [63]. Also the rise in torrefaction temperature provides a larger quantity of solid residue, which is an appropriate treatment technique for enhancing the solid fuel’s qualities [63].
Table 4 shows that when the torrefaction temperature rose, the initial decomposition temperature Tonset for treated SS gradually increased from 175.32 to 270 °C, and for treated BH from 200 to 265 °C. Tonset is expected to gradually increase as torrefaction affects the thermal stability of cellulose [37]. During torrefaction, the samples’ lignin concentration increased as hemicellulose and cellulose decomposed continuously, increasing their thermal stability and Tonset gradually. The degradation of the primary structure of cellulose is represented by the greatest weight loss rate (DTGmax). As the pretreatment temperature raised, the hydrogen bonds in the cellulose’s ordered structure broken and thus the structure became more complicated [37]. The temperature range of decomposition of the main structure became wider, which led to decrease in DTGmax. As the torrefaction temperature rose, the lignin concentration in the SS and BH continued to rise. Its benzene ring unit was further polymerized, increasing aromaticity and the amount of residue [43,44,45]. The tail portion of the DTG curve, which is thought to be the result of lignin breakdown, characterizes the third stage at temperatures of 400–750 °C [42]. Torrefaction also significantly increases the final decomposition temperature Toffset and decreases the CPI, as illustrated in Table 4 [42], suggesting that the torrefaction process might reduce the activation energy that will be discussed in the kinetic section.
4.4 Kinetics parameters
Direct and Coats and Redfern are the two techniques used to undertake the thermal kinetic study of untreated and torrefied solid products in this work. Figure 10 shows a sample of Y versus 1/T as shown in Eq. (17) for BH raw using the Coats and Redfern method with linear fit at the reaction order n, which it has the maximum R2. Figure 11 illustrates the findings of the kinetic values derived from the DA and CR methods for SS and BH. Due to the various methodologies’ assumed assumptions and approximations, there is a variance in the values of activation energy between them [65]. A molecular breakdown of materials requires a minimum amount of energy, which is reflected in Ea estimations. Therefore, larger Ea makes it more challenging for a kinetic reaction to begin. A fuel’s reactivity is also determined using activation energy [65]. The values obtained for activation energy, as well as a pre-exponential component using the Coats and Redfern procedure, are higher than the values obtained using the direct Arrhenius method, as shown in Fig. 11, which agrees with the results found in the literature [46, 47]. The main pyrolysis zones were zones II and III according to the starting and ending of the peaks that appear in DTG curves for all samples [47, 48]. This is consistent with the kinetic data acquired by several other studies [48, 49]. All (R2) values were higher than 0.9, indicating the activation energy’s dependability and accuracy. Coats and Redfern results have higher R2 values when compared with those of direct method, and zone III of all samples have higher R2 when compared with zone II [21].
The Ea and A values of SS samples, and BH samples were higher in zone II than III; for example, the Ea for SS as raw was 218.269 kJ/mol for zone II but was 199.021 kJ/mol for zone III, and it agrees with the work of other researchers [43,44,45,46]. At temperatures below 300 °C, the inert cellulose is first transformed into active cellulose before being thermally broken down into tar, gas, and char. As opposed to the activated cellulose, this phase had a lower Ea value requirement [68]. Since SS200 and BH200 were exposed directly to (hemi)cellulose activity in zone II, its Ea for zones II and III increased as the thermal resistance increased. With SS300 and BH300, the Ea in zone II gradually decreased by 37%, and in zone III decreased by 50.35%, increasing torrefaction severity. Torrefied biomass samples had lower values because of their greater reactivity and surface area because of the more bonds broken by thermal treatment at higher torrefaction temperatures [69]. This might be related to the fact that between 200 and 300 °C, (hemi)cellulose underwent increasing activation and decomposition and that most of the cellulose was activated due to the severe torrefaction [70]. SS300 and BH300 are appropriate for thermochemical conversion due to their lower activation energies. Compared to the results obtained for bean husk in all conditions of torrefaction, SS typically have lower pre-exponential factor and activation energy. One possible explanation for this is because the raw samples’ chemical and physical structures are different from one another.
As shown in Table 5, the reaction order offered the best possible match for the SS samples ranges from 1.1 to 1.8 for DA technique and 0.8 to 1.4 for CR method,. The order of reaction that presented the best fit for the bean husk samples ranges from 0.9 to 1.2 for DA method and from 0.9 to 2 for CR method. To optimize biomass pyrolysis, it is vital to thoroughly understand the chemistry reaction, which is described using pre-exponential factors (A-values). Devolatilization of raw-SS and BH exhibited the highest ln (A) value (59.987 and 63.984 min−1), respectively for zone II and (34.266 and 53.172 min−1) for zone III as compared to torrefied samples. Due to non-lignocellulosic impurities in the sample, raw samples have a lower carbon content than finished samples, which causes this difference [69]. Additionally, non-lignocellulosic impurities increased the effective frequency of collision of molecules with lower Ea values. Pre-exponential factor values vary, indicating that biomass species have varied compositions.
4.5 Thermodynamic parameters
It was also noted that the interactions between the components got stronger as the torrification process developed further. Implementing bioenergy potential relies heavily on the enthalpy of biomass torrification. The enthalpy of biomass pyrolysis is a crucial thermal property for designing the thermal reactor and estimating the yield of the pretreatment process. [71]. Table 6 summarizes that approximately 203.023 kJ/mol and 191.51 kJ/mol; 247.866 kJ/mol and 216.64 kJ/mol of thermal energy is needed for raw (sesame stalks and bean husk), respectively using CR and DA method, while 112.45 kJ/mol and 91.74 kJ/mol; 156.029 kJ/ mol and 101.05 kJ/mol of heat energy is required for torrified (sesame stalks and bean husk) at 300 °C, respectively by utilizing CR and DA techniques. The fact that the enthalpy of the SS/BH and TSS/TBH biomass ratios is positive demonstrates that the torrification process is endothermic [45].
Gibbs free energy (ΔG) increases when complexes develop. ΔG is the amount of internal energy remaining after a reaction is complete, is a useful state function for making these determinations. Table 6 shows the findings of change in ΔG for raw and torrefied SS and BH. According to the findings, 118.62 and 192.89 kJ/mol; 141.52 kJ/mol and 110.34 kJ/mol of energy is required for raw (SS and BH) using CR and DA method. While 235.18 kJ/ mol and 167.45 kJ/mol; 218.953 kJ/mol and 168.492 kJ/mol of energy are needed for torrified (SS and BH) biomass at 300 °C respectively by utilizing CR and DA technique. The average data of △G for raw and torrified biomass showed that the reactions of decomposition occurred spontaneously for both. Both △G and △H are positive, suggesting that the processes are not spontaneous and indeed need the application of thermal heat. Torrified biomass (TSS and TBH) have high levels of usable internal energy, suggesting they could be seriously evaluated as a source of energy generation. [71].
Entropy quantifies a system’s disorder. Low entropy suggests the biomass has aged and reached thermodynamic equilibrium. High activation entropy means the material is not in equilibrium. Due to its great reactivity, the procedure requires short operating durations. Torrified biomass (TSS and TBH) exhibits larger entropy change values than raw biomass (SS and BH), indicating substantial structural disorder. TSS/TBH are more active, disorganized, and have a greater degree of freedom than SS/BH. TSS/TBH biomass’s increasing disorder indicates decreased thermal stability. Higher Entropy means the substance is further from thermodynamic equilibrium. Table 6 shows negative entropy values for raw and torrified biomass, showing they are stable, structured, and near to thermodynamic equilibrium. The current investigation exhibits substantial thermal and kinetic characteristics of original and torrefied (SS and BH) biomass [66]. Also, literature findings are compared. The amount of Ea, ΔS, Δh, and ΔG in the present research is large, indicating that the chosen biomass might be used for green energy generation.
4.6 FTIR analysis of biomass samples
Figure 12 illustrates the FTIR results to analyze the surface functional group variation in TSS and TBH at various torrefaction temperatures. There are noticeable changes in the FTIR spectra that resulted from the thermal treatment. According to the stated results of earlier investigations [45, 52], a distinctive adsorption band was found. Generally, the transmittance of specific adsorption bands reduced with increasing torrefaction temperature, and the majority of FTIR spectrum changes occur between 1200 and 800 cm−1 owing to polysaccharides properties, especially hemicelluloses. According to the primary polymer components of the biomass (hemicelluloses, celluloses, and lignins), Table 7 provides an in-depth explanation of the FTIR peaks for torrefied samples.
The initial peak indicates the rupture of hydrogen bonds between the O–H links and crystalline cellulose at 3291.75 cm−1 and 3331.78 cm−1 for TSS and TBH, respectively. Dehydroxylation happens during the devolatilization of hemicellulose and cellulose, based on the reduction in transmittance at higher torrefaction temperatures. The same trend was observed in Fig. 12 but this decline was not more obvious in Fig. 12b. This is due to the difference in hemicellulose concentration that exists between the two materials [73]. As the more quantity of hemicellulose, the more obvious of decline. The band seen at 2923.07 cm−1 and 2919.15 cm−1 for (TSS) and (TBH), respectively, was brought about by the enlarging vibration of the molecular C-H bond, which was caused mostly by the atomic aliphatic -CH2- [74]. Decreased transmittance suggested that cellulose and hemicellulose’s aliphatic sections were thermally decomposing, and CH2 was the component that had the highest probability of being created as a consequence of the molecular C-H bond breaking [75]. The expanding vibration of the molecular bond C = O in carbonyl and carboxyl classes was associated with the bands at 1706.58 and 1727.68 cm−1 for (TSS) and (TBH), respectively. Due to the decarbonylation and decarboxylation process, the signal of this band became less as the torrefaction severity increased [76]. These bands, span from 1690 to 1500 cm−1, were generated by benzene’s C–C stretching vibrations, mainly due to lignin’s structural units of phenylpropane. Lignin heat breakdown was evident by the reduction in this band [37]. Nitriles and alkynes, found in heterocyclic compounds, alkenes, and other unsaturated compounds, are responsible for the bands of medium intensity seen at 1515.64 cm−1 and 1508.93 cm−1 for (TSS) and (TBH), respectively [37].
The expanding vibrations of molecular bonds C–O and C–H, primarily found in the aliphatic either from the atomic C–H bond deformation bonding in cellulose or carbohydrates, mostly contributed to the distinctive adsorption bands in the fingerprint range of 1475–800 cm−1 [76]. During the torrefaction process, carbohydrate breakdown was evident by the slight reduction in these signals. Figure 12 shows that the biomass functional groups grow simpler when the torrefaction temperature rises. According to the findings, lignin was the most thermally resistant component [76]. This finding agrees with the elemental analysis mentioned in the preceding section and is attributable to the deoxidization impact of torrefaction, as different composition of biomass affect the FTIR [52,53,54,55,56]. It also favored the coordinated and effective usage of biomass to generate clean energy.
4.7 XRD analysis
Crystallinity could be a proxy for cellulose concentration since it is the only crystalline component of biomass (hemicellulose and lignin are both amorphous) [31]. The XRD is a significant technique to analyze the crystallinity of biomass samples because of the diffraction peaks from crystals of cellulose [31]. After torrefaction, the SS and BH XRD patterns are shown in Fig. 13. It is possible to identify the usual crystal structure of cellulose using two diffraction patterns, one at 2θ = 16 and the other at 2θ = 22 [77]. As the operating temperature raised from 200 to 300 °C, the CRI of SS and BH progressively decreased from 31.80 to 19.34% and from 36.93 to 22.57%, respectively. This indicates that the crystal structure of cellulose was obviously destroyed at severe torrefaction. Decomposition of the crystalline regions of samples was found to be weak by the minor change in CRI at minimal operating temperatures (200–250 °C) [36].
The trends of the CRI versus (CPI) and H/C ratio for SS and BH are displayed in Fig. 14a and b. It is found that that a linear rise in CRI and the atomic H/C ratio follows closely after an increase in CPI. Specifically, the (R2) for CPI versus H/C ratio for torrefied SS and BH are 0.958867 and 0.864904, respectively, and that for CPI versus crystallinity index are 0.908732 and 0.883227 respectively. During the torrefaction treatment, the atomic H/C ratio and CRI are predicted using CPI because of its obviously linear nature. A higher CPI also indicates a simpler and quicker pyrolysis process [49].
The profiles of torrefaction severity index (TSI) and energy yield% for torrefied SS and BH versus (CPI) are shown in Fig. 15a and b. It appears that both the EY% linearly increases with rising CPI; on the other hand, the (TSI) linearly decreases with increasing CPI [63]. The reactivity of torrefied biomasses steadily reduces with the torrefaction severity rising, indicating an increase in the thermal stability of torrefied SS and BH. Such a finding indicates the reduced reactivity of torrefied biomass compared to related studies [26, 44]. This provides a quick and easy method for designing and operating torrefaction reactors, which improves the accuracy and reliability of predictions.
5 Conclusion
This research evaluated the impact of torrefaction on sesame stalks and bean husk as agricultural residues. Five operating temperatures (200, 225, 250, 275, and 300 °C) and two residence durations (30 and 60 min) were studied. The experimental findings showed that for these materials, the ideal operating condition is of 275 °C and 30 min for further studies. The results obtained for torrefied SS and BH indicated a reduction in the amount of volatile matter, hydrogen, oxygen contents, mass yield, energy yield, and a rise in the fixed carbon, HHV, fuel ratio, and on the other hand, bulk density of biomasses did not change remarkably after torrefaction process. The torrefaction process modified the ultimate analysis of the wastes getting them closer to coal.
Under inert conditions, the thermal decomposition of SS and BH shows four different stages of mass loss. Coats and Redfern (CR) and Direct Arrhenius (DA) approaches are used to compute the thermal kinetic parameters. All (R2) values were higher than 0.9, indicating the activation energy’s dependability and accuracy. The CR approach yields kinetics values (Ea and A) higher than their corresponding values when derived using the DA method’s calculations. As the operating temperature rises, Ea and A decreases; however, at 200 °C, both Ea and A increase as the thermal resistance increases.
Torrefaction treatment process considerably modifies the FTIR spectra. The majority of FTIR spectra changes occur between 1200 and 800 cm−1. The strength of the peak reduces as the temperature of torrefaction rises. As the operating temperature increased, the crystallinity index (CRI) of SS and BH progressively decreased. The work that has been suggested draws attention to the potential of an agricultural biomass that is not being fully exploited and provides a chance to generate products with additional value.
Data availability
The data will be available upon request.
References
Wang Y, Dai L, Wang R et al (2016) Hydrocarbon fuel production from soapstone through fast microwave-assisted pyrolysis using microwave absorbent. J Anal Appl Pyrolysis 119:251–258. https://doi.org/10.1016/j.jaap.2016.01.008
Dai L, Wang Y, Liu Y et al (2019) Bridging the relationship between hydrothermal pretreatment and co-pyrolysis: effect of hydrothermal pretreatment on aromatic production. Energy Convers Manag 180:36–43. https://doi.org/10.1016/j.enconman.2018.10.079
Deng W, Feng Y, Fu J et al (2022) Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. https://doi.org/10.1016/j.gee.2022.07.003
El-sattar HA, Kamel S, Sultan HM, Zawbaa HM (2022) Optimal design of photovoltaic, biomass, fuel cell, hydrogen tank units and electrolyzer hybrid system for a remote area in Egypt. Energy Rep 8:9506–9527. https://doi.org/10.1016/j.egyr.2022.07.060
Said N, El-Shatoury SA, Díaz LF, Zamorano M (2013) Quantitative appraisal of biomass resources and their energy potential in Egypt. Renew Sustain Energy Rev 24:84–91. https://doi.org/10.1016/j.rser.2013.03.014
Chen WH, Cheng CL, Show PL, Ong HC (2019) Torrefaction performance prediction approached by torrefaction severity factor. Fuel 251:126–135. https://doi.org/10.1016/j.fuel.2019.04.047
Riaz S, Oluwoye I, Al-Abdeli YM (2022) Oxidative torrefaction of densified woody biomass: Performance, combustion kinetics and thermodynamics. Renew Energy 199:908–918. https://doi.org/10.1016/j.renene.2022.09.023
Granados DA, Ruiz RA, Vega LY, Chejne F (2017) Study of reactivity reduction in sugarcane bagasse as consequence of a torrefaction process. Energy 139:818–827. https://doi.org/10.1016/j.energy.2017.08.013
Chen Y, Mu T (2019) Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ 4:95–115. https://doi.org/10.1016/j.gee.2019.01.012
Chen WH, Peng J, Bi XT (2015) A state-of-the-art review of biomass torrefaction, densification and applications. Renew Sustain Energy Rev 44:847–866. https://doi.org/10.1016/j.rser.2014.12.039
Niu Q, Ronsse F, Qi Z, Zhang D (2022) Fast torrefaction of large biomass particles by superheated steam: enhanced solid products for multipurpose production. Renew Energy 185:552–563. https://doi.org/10.1016/j.renene.2021.12.070
Li MF, Chen CZ, Li X et al (2015) Torrefaction of bamboo under nitrogen atmosphere: influence of temperature and time on the structure and properties of the solid product. Fuel 161:193–196. https://doi.org/10.1016/j.fuel.2015.08.052
Mi B, Liu Z, Hu W et al (2016) Investigating pyrolysis and combustion characteristics of torrefied bamboo, torrefied wood and their blends. Bioresour Technol 209:50–55. https://doi.org/10.1016/j.biortech.2016.02.087
Niu Y, Lv Y, Lei Y et al (2019) Biomass torrefaction: properties, applications, challenges, and economy. Renew Sustain Energy Rev 115:109395. https://doi.org/10.1016/j.rser.2019.109395
Kanwal S, Chaudhry N, Munir S, Sana H (2019) Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag 88:280–290. https://doi.org/10.1016/j.wasman.2019.03.053
Zhang C, Ho SH, Chen WH et al (2019) Oxidative torrefaction of biomass nutshells: evaluations of energy efficiency as well as biochar transportation and storage. Appl Energy 235:428–441. https://doi.org/10.1016/j.apenergy.2018.10.090
Sukiran MA, Wan Daud WMA, Abnisa F et al (2021) A comprehensive study on torrefaction of empty fruit bunches: characterization of solid, liquid and gas products. Energy 230:120877. https://doi.org/10.1016/j.energy.2021.120877
Vashishtha M, Patidar K (2021) Property enhancement of mustard stalk biomass by Torrefaction: characterization and optimization of process parameters using response surface methodology. Mater Sci Energy Technol 4:432–441. https://doi.org/10.1016/j.mset.2021.08.002
Amer M, Nour M, Ahmed M et al (2019) The effect of microwave drying pretreatment on dry torrefaction of agricultural biomasses. Bioresour Technol 286:121400. https://doi.org/10.1016/j.biortech.2019.121400
Ashraf A, Sattar H, Munir S (2019) A comparative applicability study of model-fitting and model-free kinetic analysis approaches to non-isothermal pyrolysis of coal and agricultural residues. Fuel 240:326–333. https://doi.org/10.1016/j.fuel.2018.11.149
Amer M, Nour M, Ahmed M et al (2021) Kinetics and physical analyses for pyrolyzed Egyptian agricultural and woody biomasses: effect of microwave drying. Biomass Convers Biorefinery 11:2855–2868. https://doi.org/10.1007/s13399-020-00684-3
Huang YF, Chen WR, Chiueh PT et al (2012) Microwave torrefaction of rice straw and pennisetum. Bioresour Technol 123:1–7. https://doi.org/10.1016/j.biortech.2012.08.006
Gohar H, Khoja AH, Ansari AA et al (2022) Investigating the characterisation, kinetic mechanism, and thermodynamic behaviour of coal-biomass blends in co-pyrolysis process. Process Saf Environ Prot 163:645–658. https://doi.org/10.1016/j.psep.2022.05.063
Saeed S, Saleem M, Durrani A (2020) Thermal performance analysis and synergistic effect on co-pyrolysis of coal and sugarcane bagasse blends pretreated by trihexyltetradecylphosphonium chloride. Fuel 278:118240. https://doi.org/10.1016/j.fuel.2020.118240
Hakeem IY, Amin M, Zeyad AM et al (2022) Effects of nano sized sesame stalk and rice straw ashes on high-strength concrete properties. J Clean Prod 370:133542. https://doi.org/10.1016/j.jclepro.2022.133542
Odetoye TE, Ocheni ML (2022) Thermochemical characterization of African oil bean husk for potential biofuel production. Chem Africa 5:1097–1101. https://doi.org/10.1007/s42250-022-00372-x
Aied A (2021) In-vitro and in-vivo studies on most common straws in Egypt after differed treatments to improve its nutritive value fed to Ossimi rams. Sci J Agric Sci 3(2):284–291. https://doi.org/10.21608/sjas.2021.81339.1117
Collard FX, Blin J (2014) A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:594–608. https://doi.org/10.1016/j.rser.2014.06.013
Silva JE, Calixto GQ, de Almeida CC et al (2019) Energy potential and thermogravimetric study of pyrolysis kinetics of biomass wastes. J Therm Anal Calorim 137:1635–1643. https://doi.org/10.1007/s10973-019-08048-4
Ong HC, Yu KL, Chen WH, et al (2021) Variation of lignocellulosic biomass structure from torrefaction: A critical review. Renew Sustain Energy Rev 152:111698. https://doi.org/10.1016/j.rser.2021.111698
Wada M, Hori R, Kim UJ, Sasaki S (2010) X-ray diffraction study on the thermal expansion behavior of cellulose Iβ and its high-temperature phase. Polym Degrad Stab 95:1330–1334. https://doi.org/10.1016/j.polymdegradstab.2010.01.034
Li MF, Li X, Bian J et al (2015) Influence of temperature on bamboo torrefaction under carbon dioxide atmosphere. Ind Crops Prod 76:149–157. https://doi.org/10.1016/j.indcrop.2015.04.060
Bach QV, Chen WH, Chu YS, Skreiberg Ø (2016) Predictions of biochar yield and elemental composition during torrefaction of forest residues. Bioresour Technol 215:239–246. https://doi.org/10.1016/j.biortech.2016.04.009
Haseli Y (2018) Process modeling of a biomass torrefaction plant. Energy Fuels 32:5611–5622. https://doi.org/10.1021/acs.energyfuels.7b03956
Nepal R, Kim HJ, Poudel J, Oh SC (2022) A study on torrefaction of spent coffee ground to improve its fuel properties. Fuel 318:123643. https://doi.org/10.1016/j.fuel.2022.123643
Abdullah I, Ahmad N, Hussain M et al (2022) Conversion of biomass blends (walnut shell and pearl millet) for the production of solid biofuel via torrefaction under different conditions. Chemosphere 295:133894. https://doi.org/10.1016/j.chemosphere.2022.133894
Tian X, Dai L, Wang Y et al (2020) Influence of torrefaction pretreatment on corncobs: A study on fundamental characteristics, thermal behavior, and kinetic. Bioresour Technol 297:122490. https://doi.org/10.1016/j.biortech.2019.122490
El-Sayed SA, Mostafa ME (2015) Kinetic parameters determination of biomass pyrolysis fuels using TGA and DTA techniques. Waste Biomass Valorization 6:401–415. https://doi.org/10.1007/s12649-015-9354-7
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74. https://doi.org/10.1016/j.biortech.2017.12.029
Bach QV, Chen WH, Lin SC et al (2017) Wet torrefaction of microalga Chlorella vulgaris ESP-31 with microwave-assisted heating. Energy Convers Manag 141:163–170. https://doi.org/10.1016/j.enconman.2016.07.035
Silveira EA, Luz SM, Leão RM, et al (2021) Numerical modeling and experimental assessment of sustainable woody biomass torrefaction via coupled TG-FTIR. Biomass and Bioenergy 146:105981. https://doi.org/10.1016/j.biombioe.2021.105981
Li L, Huang Y, Zhang D et al (2018) Uncovering Structure-reactivity relationships in pyrolysis and gasification of biomass with varying severity of torrefaction. ACS Sustain Chem Eng 6:6008–6017. https://doi.org/10.1021/acssuschemeng.7b04649
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data [12]. Nature 201:68–69. https://doi.org/10.1038/201068a0
Coats and Redfern (1964) Kinetic parameter_Coats and Redfern. Nature 201:68–69
Lee BH, Jeong TY, Trinh VT, Jeon CH (2021) Thermal degradation of kenaf (Hibiscus cannabinus L.): Impact of torrefaction on pyrolysis kinetics and thermal behavior. Energy Rep 7:951–959. https://doi.org/10.1016/j.egyr.2021.01.012
Tadesse Y, Kassahun SK, Kiflie Z (2021) Effects of operational parameters on torrefaction performance of coffee husk and cotton stalk mixed biomass: a surface response methodology approach. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01649-w
Patidar K, Vashishtha M (2020) Impact of torrefaction conditions on the physicochemical properties of mustard crop residue. Mater Today Proc 44:4072–4078. https://doi.org/10.1016/j.matpr.2020.10.445
Chen D, Zhou J, Zhang Q et al (2014) Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 9:5893–5905. https://doi.org/10.15376/biores.9.4.5893-5905
Dai L, Wang Y, Liu Y et al (2019) Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: a state-of-the-art review. Renew Sustain Energy Rev 107:20–36. https://doi.org/10.1016/j.rser.2019.02.015
EL-Sayed SA, Mostafa ME, (2021) Kinetics, thermodynamics, and combustion characteristics of Poinciana pods using TG/DTG/DTA techniques. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02021-8
Mlonka-Mędrala A, Magdziarz A, Gajek M et al (2020) Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 261:116421. https://doi.org/10.1016/j.fuel.2019.116421
Ma C, Weiland F, Hedman H et al (2013) Characterization of reactor ash deposits from pilot-scale pressurized entrained-flow gasification of woody biomass. Energy Fuels 27:6801–6814. https://doi.org/10.1021/ef401591a
Ivanovski M, Goricanec D, Krope J, Urbancl D (2022) Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 240:122483. https://doi.org/10.1016/j.energy.2021.122483
Adeleke AA, Odusote JK, Ikubanni PP et al (2020) The ignitability, fuel ratio and ash fusion temperatures of torrefied woody biomass. Heliyon 6:e03582. https://doi.org/10.1016/j.heliyon.2020.e03582
Sarker TR, Azargohar R, Dalai AK, Meda V (2021) Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep 7:6338–6353. https://doi.org/10.1016/j.egyr.2021.09.068
Thengane SK, Kung KS, Gomez-Barea A, Ghoniem AF (2022) Advances in biomass torrefaction: parameters, models, reactors, applications, deployment, and market. Prog Energy Combust Sci 93:101040. https://doi.org/10.1016/j.pecs.2022.101040
Amer M, Brachi P, Ruoppolo G et al (2021) Pyrolysis and combustion kinetics of thermally treated globe artichoke leaves. Energy Convers Manag 246:114656. https://doi.org/10.1016/j.enconman.2021.114656
Ibarra JV, Muñoz E, Moliner R (1996) FTIR study of the evolution of coal structure during the coalification process. Org Geochem 24:725–735. https://doi.org/10.1016/0146-6380(96)00063-0
Pathomrotsakun J, Nakason K, Kraithong W et al (2020) Fuel properties of biochar from torrefaction of ground coffee residue: effect of process temperature, time, and sweeping gas. Biomass Convers Biorefinery 10:743–753. https://doi.org/10.1007/s13399-020-00632-1
Zhang C, Ho SH, Chen WH et al (2018) Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl Energy 220:598–604. https://doi.org/10.1016/j.apenergy.2018.03.129
Deng S, Tan H, Wang X et al (2017) Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char. Bioresour Technol 239:302–310. https://doi.org/10.1016/j.biortech.2017.04.067
Bach QV, Chen WH (2017) Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): a state-of-the-art review. Bioresour Technol 246:88–100. https://doi.org/10.1016/j.biortech.2017.06.087
Zhang C, Yang W, Chen WH, et al (2022) Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy 240:122470. https://doi.org/10.1016/j.energy.2021.122470
Manatura K (2020) Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud Therm Eng 19:100623. https://doi.org/10.1016/j.csite.2020.100623
Kaur R, Gera P, Jha MK, Bhaskar T (2018) Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol 250:422–428. https://doi.org/10.1016/j.biortech.2017.11.077
Jayaraman K, Kok MV, Gokalp I (2017) Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew Energy 101:293–300. https://doi.org/10.1016/j.renene.2016.08.072
El-Sayed SA, Mostafa ME (2014) Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers Manag 85:165–172. https://doi.org/10.1016/j.enconman.2014.05.068
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis 91:1–33. https://doi.org/10.1016/j.jaap.2011.01.004
Aslam U, Aslam Z, Ashraf M, Kamal MS (2022) Influence of pretreatments on the fuel properties and pyrolytic kinetics of biomass. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02235-w
Hu J, Song Y, Liu J et al (2020) Combustions of torrefaction-pretreated bamboo forest residues: physicochemical properties, evolved gases, and kinetic mechanisms. Bioresour Technol 304:122960. https://doi.org/10.1016/j.biortech.2020.122960
Gajera B, Tyagi U, Sarma AK, Jha MK (2022) Impact of torrefaction on thermal behavior of wheat straw and groundnut stalk biomass: kinetic and thermodynamic study. Fuel Commun 12:100073. https://doi.org/10.1016/j.jfueco.2022.100073
Wang S, Dai G, Ru B et al (2016) Effects of torrefaction on hemicellulose structural characteristics and pyrolysis behaviors. Bioresour Technol 218:1106–1114. https://doi.org/10.1016/j.biortech.2016.07.075
Wang S, Dai G, Ru B et al (2017) Influence of torrefaction on the characteristics and pyrolysis behavior of cellulose. Energy 120:864–871. https://doi.org/10.1016/j.energy.2016.11.135
Ma Z, Yang Y, Ma Q et al (2017) Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J Anal Appl Pyrolysis 127:350–359. https://doi.org/10.1016/j.jaap.2017.07.015
Ma Z, Wang J, Li C et al (2019) New sight on the lignin torrefaction pretreatment: Relevance between the evolution of chemical structure and the properties of torrefied gaseous, liquid, and solid products. Bioresour Technol 288:121528. https://doi.org/10.1016/j.biortech.2019.121528
Ma Z, Wang J, Yang Y et al (2018) Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J Anal Appl Pyrolysis 134:12–24. https://doi.org/10.1016/j.jaap.2018.04.002
Mei Y, Che Q, Yang Q et al (2016) Torrefaction of different parts from a corn stalk and its effect on the characterization of products. Ind Crops Prod 92:26–33. https://doi.org/10.1016/j.indcrop.2016.07.021
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M. Khairy: methodology, formal analysis, writing—original draft, writing—review & editing. M. Amer: conceptualization, writing—original draft—review—supervision & editing. M. Ibrahim: Writing—review & editing. S. Ookawara: writing—review & editing. H. Sekiguchi: writing—review & editing. A. Elwardany: conceptualization, methodology, resources, writing—original draft, writing—review & editing.
Corresponding author
Ethics declarations
Ethical approval
This declaration is “not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khairy, M., Amer, M., Ibrahim, M. et al. The influence of torrefaction on the biochar characteristics produced from sesame stalks and bean husk. Biomass Conv. Bioref. 14, 17127–17148 (2024). https://doi.org/10.1007/s13399-023-03822-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03822-9