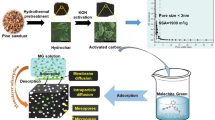
Abstract
Zinc chloride–activated carbon (AC) and nano zinc oxide/activated carbon composite (NZAC) as solid adsorbents were synthesized from date palm fronds (DPF) as a green material of activated carbon, while zinc oxide nanoparticles (NZ) were prepared in the presence of polyethylene glycol as capping agent. All the prepared solid adsorbents were investigated by several characterization techniques such as thermogravimetric analysis (TGA), nitrogen adsorption/desorption isotherm, Fourier transform infrared spectroscopy (FTIR), pH of point of zero charges (pHPZC), scanning electron microscopy (SEM), and transmission electron microscope (TEM). The NZAC sample had the highest total pore volume (0.960 cm3/g), specific surface area (2085.47 m2/g), and with pHPZC of 7.8. Adsorption of methylene blue was tested by Langmuir, Freundlich, and Temkin adsorption isotherms. NZAC had the highest Langmuir adsorption capacity (456.62 mg/g) at 40 °C with a dimensionless separation factor (RL) of 0.030. MB adsorption matched well with pseudo-second-order and Elovich kinetic models. The entropy, free energy, and heat changes for MB adsorption onto NZAC were found to be 0.0243 (kJ/mol. K), − 3.5589 (kJ/mol), and 3.561 (kJ/mol), respectively. Thermodynamic analysis demonstrated that the adsorption process of MB is favorable, spontaneous, physisorption, and endothermic. Desorption studies showed that HCl (0.01 mol/L) achieved the higher desorption percent (87%) compared with the other eluents.






Similar content being viewed by others
Data availability
Not applicable.
References
Somsesta N, Sricharoenchaikul V, Aht-Ong D (2020) Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: equilibrium and kinetic studies. Mater Chem Phys 240:122221. https://doi.org/10.1016/j.matchemphys.2019.122221
Son G, Kim D, Lee JS, Kim H, Lee C, Kim S-R, Lee H (2018) Synchronized methylene blue removal using Fenton-like reaction induced by phosphorous oxoanion and submerged plasma irradiation process. J Environ Manage 206:77–84. https://doi.org/10.1016/j.jenvman.2017.10.024
Lokhande KD, Pethsangave DA, Kulal DK, Some S (2020) Remediation of toxic dye pollutants by using graphene-based adsorbents. Mater Sci Inc. Nanomater Polym 5(27):8062–8073. https://doi.org/10.1002/slct.202002130
Yadav S, Yadav A, Bagotia N, Sharma AK, Kumar S (2022) Novel composites of Pennisetum glaucum with CNT: preparation, characterization and application for the removal of safranine O and methylene blue dyes from single and binary systems. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02240-z
Soares SF, Simões TR, Trindade T, Daniel-da-Silva AL (2017) Highly efficient removal of dye from water using magnetic carrageenan/silica hybrid nano-adsorbents. Water, Air, Soil Pollut 228:87. https://doi.org/10.1007/s11270-017-3281-0
Shahrul AM, Syarifah Adilah MY, Radzali R, Malek MF, Isa IS, Rusop M, Damanhuri NS, Abdullah MH (2022) Low-cost coagulation treatment of dye sensitizer for improved time immersion of dye-sensitized solar cells (DSSC). Microelectron Eng. 262:111832. https://doi.org/10.1016/j.mee.2022.111832
Zhao J, Liu H, Xue P, Tian S, Sun S, Lv X (2021) Highly-efficient PVDF adsorptive membrane filtration based on chitosan@CNTs-COOH simultaneous removal of anionic and cationic dyes. Carbohydr. Polym. 274:118664. https://doi.org/10.1016/j.carbpol.2021.118664
Zhang X, Jiang S, Sun LX, Xing YH, Bai FY (2022) Synthesis and structure of a 3D supramolecular layered Bi-MOF and its application in photocatalytic degradation of dyes. J Mol Struct 1270:133895. https://doi.org/10.1016/j.molstruc.2022.133895
Lu C, Yang J, Khan A, Yang J, Li Q, Wang G (2022) A highly efficient technique to simultaneously remove acidic and basic dyes using magnetic ion-exchange microbeads. J. Environ. Manage. 304:114173. https://doi.org/10.1016/j.jenvman.2021.114173
Bravo-yumi N, Alvarez MP, Bandala ER (2022) Studying the influence of different parameters on the electrochemical oxidation of tannery dyes using a Ti/IrO2-SnO2-Sb2O5 anode. Chem Eng Process - Process Intensif 109173. https://doi.org/10.1016/j.cep.2022.109173
Shajeelammal J, Mohammed S, Prathish KP, Jeeva A, Asok A, Shukla S (2022) Treatment of real time textile effluent containing azo reactive dyes via ozonation, modified pulsed low frequency ultrasound cavitation, and integrated reactor. J Hazard Mater Adv 7:100098. https://doi.org/10.1016/j.hazadv.2022.100098
Lv B, Dong B, Zhang C, Chen Z, Zhao Z, Deng X, Fang C (2022) Effective adsorption of methylene blue from aqueous solution by coal gangue-based zeolite granules in a fluidized bed: fluidization characteristics and continuous adsorption. Powder Technol 408. https://doi.org/10.1016/j.powtec.2022.117764
Wan X, Rong Z, Zhu K, Wu Y (2022) Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int J Biol Macromol 222:725–735. https://doi.org/10.1016/j.ijbiomac.2022.09.213
Wu T, Yang G, Cao J, Xu Z, Jiang X (2022) Activation and adsorption mechanisms of methylene blue removal by porous biochar adsorbent derived from eggshell membrane. Chem Eng Res Des 188:330–341. https://doi.org/10.1016/j.cherd.2022.08.042
Alam S, Ullah B, Khan MS, Ur Rahman N, Khan L, Shah LA, Zekker I, Burlakovs J, Kallistova A, Pimenov N, Yandri E, Setyobudi RH, Jani Y, Zahoor M (2021) Adsorption kinetics and isotherm study of basic red 5 on synthesized silica monolith particles. Water (Switzerland) 13:1–13. https://doi.org/10.3390/w13202803
Zambare R, Song X, Bhuvana S, Prince JSA, Nemade P (2017) Ultrafast dye removal using ionic liquid−graphene oxide sponge. ACS Sustain Chem Eng 5(7):6026–6035. https://doi.org/10.1021/acssuschemeng.7b00867
Gao Q, Xu J, Bu XH (2019) Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord Chem Rev 378:17–31. https://doi.org/10.1016/j.ccr.2018.03.015
Singh AR, Dhumal PS, Bhakare MA, Lokhande KD, Bondarde MP, Some S (2022) In-situ synthesis of metal oxide and polymer decorated activated carbon-based photocatalyst for organic pollutants degradation. Sep Purif Technol 286:120380–120388. https://doi.org/10.1016/j.seppur.2021.120380
Ahmad A, Al-Swaidan HM, Alghamdi AH (2015) Production of activated carbon from raw date palm fronds by ZnCl2 activation. J Chem Soc Pakistan 37:1081–1087
Ozdemir I, Şahin M, Orhan R, Erdem M (2014) Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process Technol 125:200–206. https://doi.org/10.1016/j.fuproc.2014.04.002
Ferrera-Lorenzo N, Fuente E, Suárez-Ruiz I, Ruiz B (2014) KOH activated carbon from conventional and microwave heating system of a macroalgae waste from the Agar-Agar industry. Fuel Process Technol 121:25–31. https://doi.org/10.1016/j.fuproc.2013.12.017
Gerçel Ö, Özcan A, Özcan AS, Gerçel HF (2007) Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl Surf Sci 253:4843–4852. https://doi.org/10.1016/j.apsusc.2006.10.053
Alvarez P, Blanco C, Granda M (2007) The adsorption of chromium (VI) from industrial wastewater by acid and base-activated lignocellulosic residues. J Hazard Mater 144:400–405. https://doi.org/10.1016/j.jhazmat.2006.10.052
Sivaraj R, Rajendran V, Gunalan GS (2010) Preparation and characterization of activated carbons from parthenium biomass by physical and chemical activation techniques. E-Journal Chem 7:1314–1319. https://doi.org/10.1155/2010/948015
Ncibi MC, Ranguin R, Pintor MJ, Jeanne-Rose V, Sillanpää M, Gaspard S (2014) Preparation and characterization of chemically activated carbons derived from Mediterranean Posidonia oceanica (L.) fibres. J Anal Appl Pyrolysis 109:205–214. https://doi.org/10.1016/j.jaap.2014.06.010
Haghbin MR, Niknam Shahrak M (2021) Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technol 377:890–899. https://doi.org/10.1016/j.powtec.2020.09.051
Debnath P, Mondal NK (2020) Effective removal of congo red dye from aqueous solution using biosynthesized zinc oxide nanoparticles. Environ Nanotechnol Monit Manag 14:100320. https://doi.org/10.1016/j.enmm.2020.100320
Sanjid Qais D, Nazrul Islam M, Hafiz Dzarfan Othman M, Ekramul Mahmud HNM, Emran Quayum M, Anwarul Islam M, Mohammad Ibrahim Ismail I, Habib A (2021) WITHDRAWN: Synthesis and characterization of nano-zinc oxide: adsorption of acid blue 92 dye, isotherms, thermodynamics and kinetics. Arab J Chem 103627. https://doi.org/10.1016/j.arabjc.2021.103627
Eskikaya O, Ozdemir S, Tollu G, Dizge N, Ramaraj R, Manivannan A, Balakrishnan D (2022) Synthesis of two different zinc oxide nanoflowers and comparison of antioxidant and photocatalytic activity. Chemosphere 306:135389. https://doi.org/10.1016/j.chemosphere.2022.135389
Norouzi A, Nezamzadeh-Ejhieh A, Fazaeli R (2021) A Copper(I) oxide-zinc oxide nano-catalyst hybrid: brief characterization and study of the kinetic of its photodegradation and photomineralization activities toward methylene blue. Mater Sci Semicond Process 122:105495. https://doi.org/10.1016/j.mssp.2020.105495
Youssef A, EL-Didamony H, Sharabasy SEL-, Sobhy M, Hassan A, Buláneke R (2017) Adsorption of 2, 4 dichlorophenoxyacetic acid on different types of activated carbons based date palm pits: kinetic and thermodynamic studies. Int Res J Pure Appl Chem 14:1–15. https://doi.org/10.9734/irjpac/2017/33073
Elsayed MS, Ahmed IA, Bader DMD, Hassan AF (2021) Green synthesis of nano zinc oxide/nanohydroxyapatite composites using date palm pits extract and eggshells: adsorption and photocatalytic degradation of methylene blue. Nanomater (Basel, Switzerland) 12. https://doi.org/10.3390/nano12010049
Altıntıg E, Yenigun M, Sarı A, Altundag H, Tuzen M, Saleh TA (2021) Facile synthesis of zinc oxide nanoparticles loaded activated carbon as an eco-friendly adsorbent for ultra-removal of malachite green from water, Environ. Technol. Innovation 21:101305–101319. https://doi.org/10.1016/j.eti.2020.101305
Hassan AF, Mustafa AA, Esmail G, Awad AM (2022) Adsorption and photo-fenton degradation of methylene blue using nanomagnetite/potassium carrageenan bio-composite beads. Arab J Sci Eng. https://doi.org/10.1007/s13369-022-07075-y
Shaltout WA, El-Naggar GA, Esmail G, Hassan AF (2022) Synthesis and characterization of ferric@nanocellulose/nanohydroxyapatite bio-composite based on sea scallop shells and cotton stalks: adsorption of Safranin-O dye. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02753-1
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Teodoro FS, Elias MMC, Ferreira GMD, Adarme OFH, Savedra RML, Siqueira MF, da Silva LHM, Gil LF, Gurgel LVA (2018) Synthesis and application of a new carboxylated cellulose derivative. Part III: Removal of auramine-O and safranin-T from mono- and bi-component spiked aqueous solutions. J. Colloid Interface Sci. 512:575–590. https://doi.org/10.1016/j.jcis.2017.10.083
Ghori SW, Rao GS (2021) Mechanical and thermal properties of date palm/kenaf fiber-reinforced epoxy hybrid composites. Polym Compos 42:2217–2224. https://doi.org/10.1002/pc.25971
Kumar A (2020) Sol gel synthesis of zinc oxide nanoparticles and their application as nano-composite electrode material for supercapacitor. J Mol Struct 1220:128654. https://doi.org/10.1016/j.molstruc.2020.128654
Moharram AH, Mansour SA, Hussein MA, Rashad M (2014) Direct precipitation and characterization of ZnO nanoparticles. J Nanomater 2014 https://doi.org/10.1155/2014/716210
Lonkar SP, Pillai V, Abdala A (2019) Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl Surf Sci 465:1107–1113. https://doi.org/10.1016/j.apsusc.2018.09.264
Ramya V, Murugan D, Lajapathirai C, Sivasamy A (2018) Activated carbon (prepared from secondary sludge biomass) supported semiconductor zinc oxide nanocomposite photocatalyst for reduction of Cr(VI) under visible light irradiation. J Environ Chem Eng 6:7327–7337. https://doi.org/10.1016/j.jece.2018.08.055
Montalvo Andia J, Larrea A, Salcedo J, Reyes J, Lopez L, Yokoyama L (2020) Synthesis and characterization of chemically activated carbon from Passiflora ligularis, Inga feuilleei and native plants of South America. J Environ Chem Eng 8:103892. https://doi.org/10.1016/j.jece.2020.103892
Yun SI, Kim SH, Kim DW, Kim YA, Kim B-H (2019) Facile preparation and capacitive properties of low-cost carbon nanofibers with ZnO derived from lignin and pitch as supercapacitor electrodes. Carbon N Y 149:637–645. https://doi.org/10.1016/j.carbon.2019.04.105
Gomez-Eerrano V, Valenzuela-Calahorro C, Pastor- Villegas J (1993) Characterization of rockrose wood and activated carbon. Biomass Bioenergy 4(5):35S364. https://doi.org/10.1016/0961-9534(93)90052-6
Akpomie KG, Ghosh S, Gryzenhout M, Conradie J (2021) One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci Rep 11:1–17. https://doi.org/10.1038/s41598-021-87819-2
Hassan HS, Abol-Fotouh D, Salama E, Elkady MF (2022) Assessment of antimicrobial, cytotoxicity, and antiviral impact of a green zinc oxide/activated carbon nanocomposite. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-12648-w
Zheng Y, Wu B (2021) Preparation process and characterization of activated carbon from mango kernels. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/2021/1/012061
Umamaheswari A, Prabu SL, John SA, Puratchikody A (2021) Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol Reports 29:e00595. https://doi.org/10.1016/j.btre.2021.e00595
Nourmoradi H, Ghiasvand AR, Noorimotlagh Z (2015) Removal of methylene blue and acid orange 7 from aqueous solutions by activated carbon coated with zinc oxide (ZnO) nanoparticles: equilibrium, kinetic, and thermodynamic study. Desalin Water Treat 55:252–262. https://doi.org/10.1080/19443994.2014.914449
Vu DL, Seo JS, Lee HY, Lee JW (2017) Activated carbon with hierarchical micro-mesoporous structure obtained from rice husk and its application for lithium-sulfur batteries. RSC Adv 7:4144–4151. https://doi.org/10.1039/C6RA26179E
Lee WH, Rhee CK, Koo J, Lee J, Jang SP, Choi SUS, Lee KW, Bae HY, Lee GJ, Kim CK, Hong SW, Kwon Y, Kim D, Kim SH, Hwang KS, Kim HJ, Ha HJ, Lee SH, Choi CJ, Lee JH (2011) Round-robin test on thermal conductivity measurement of zno nanofluids and comparison of experimental results with theoretical bounds. Nanoscale Res Lett 6:258. https://doi.org/10.1186/1556-276X-6-258
Geed SR, Samal K, Tagade A (2019) Development of adsorption-biodegradation hybrid process for removal of methylene blue from wastewater. J Environ Chem Eng 7:103439. https://doi.org/10.1016/j.jece.2019.103439
Hassan AF, Elhadidy H (2017) Production of activated carbons from waste carpets and its application in methylene blue adsorption: Kinetic and thermodynamic studies. J Environ Chem Eng 5:955–963. https://doi.org/10.1016/j.jece.2017.01.003
Tan CHC, Sabar S, Hussin MH (2018) Development of immobilized microcrystalline cellulose as an effective adsorbent for methylene blue dye removal, South African. J Chem Eng 26:11–24. https://doi.org/10.1016/j.sajce.2018.08.001
Başaran Kankılıç G, Metin AÜ (2020) Phragmites australis as a new cellulose source: Extraction, characterization and adsorption of methylene blue. J Mol Liq 312. https://doi.org/10.1016/j.molliq.2020.113313
Hassan AF (2019) Synthesis of carbon nano-onion embedded metal–organic frameworks as an efficient adsorbent for cadmium ions: kinetic and thermodynamic studies. Environ Sci Pollut Res 26:24099–24111. https://doi.org/10.1007/s11356-019-05581-5
Kamal KH, Dacrory S, Ali SSM, Ali KA, Kamel S (2019) Adsorption of Fe ions by modified carrageenan beads with tricarboxy cellulose: kinetics study and four isotherm models. Desalin Water Treat 165:281–289. https://doi.org/10.5004/dwt.2019.24560
Mok CF, Ching YC, Osman NAA, Muhamad F, Hai ND, Choo JH, Hassan CR (2020) Adsorbents for removal of cationic dye: nanocellulose reinforced biopolymer composites. J Polym Res 27. https://doi.org/10.1007/s10965-020-02347-3
Duman O, Polat TG, Diker CÖ, Tunç S (2020) Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int J Biol Macromol 160:823–835. https://doi.org/10.1016/j.ijbiomac.2020.05.191
Üner O, Geçgel Ü, Bayrak Y (2016) Adsorption of methylene blue by an efficient activated carbon prepared from Citrullus lanatus rind: kinetic, isotherm, thermodynamic, and mechanism analysis. Water Air Soil Pollut 227(247). https://doi.org/10.1007/s11270-016-2949-1
Vargas AM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730. https://doi.org/10.1016/j.cej.2011.01.067
Sangon S, Hunt AJ, Ngernyen Y, Youngme S, Supanchaiyamat N (2021) Rice straw-derived highly mesoporous carbon-zinc oxide nanocomposites as high performance photocatalytic adsorbents for toxic dyes. J Clean Prod 318:128583–128595. https://doi.org/10.1016/j.jclepro.2021.128583
Acknowledgements
The authors would like to extend their sincere appreciation to University of Technology and Applied Sciences.
Funding
This study was funded by Ministry of Higher Education, Research and Innovation of Sultanate of Oman through the research group project no. BFI/URG/EBR/21/249.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by Asaad F. Hassa and Laila M. Alshandoudi. Data collection was performed by Said R. Alkindi and Tariq Y. Alhatmi.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alshandoudi, L.M., Alkindi, S.R., Alhatmi, T.Y. et al. Synthesis and characterization of nano zinc oxide/zinc chloride–activated carbon composite based on date palm fronds: adsorption of methylene blue. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03815-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03815-8