Abstract
The cornerstones of an efficient circular waste management strategy aiming for enhanced resource efficiency are maximizing organic waste valorization and improving residual conversion to biochemicals. In this regard, this study focuses on the production of volatile fatty acids (VFAs) from the effluent of fungi biomass cultivation on low-grade residues from the potato starch industry with batch and semi-continuous membrane bioreactors (MBRs) containing the effluent of already fermented potato protein liquor (FPPL) inoculated with chicken and cow manure. The effect of pH in the batch experiments on the production and yield of VFAs during acidogenic digestion was evaluated. Rapid generation of VFAs at a concentration of up to 11.8 g/L could be successfully achieved in the MBR. Under the optimal conditions, a high yield of 0.65 g VFAs/g VSfed was obtained for the organic loading rate (OLR) of 1 g VS/L/d using FPPL substrate and chicken manure as inoculum. The results show that the application of sequential multi-step bioconversion of potato starch industry residues has the potential to increase the variety of value-added products generated from a single organic residue while enhancing nutrient recovery capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Potato can be grown under a wide range of conditions, making it a common crop in all EU countries and worldwide. The global annual potato production is now over 370 million tons, of which, in 2021, 826,400 tons was produced in Sweden, corresponding to an area under cultivation of 23,750 ha [1]. The yield per hectare for starch potato processing in Sweden is estimated at 41,950 kilos [2]. However, the potato industry entails side streams that take up a significant part of the original biomass. Especially in southern Sweden, potato starch production generates a large amount of low-value residuals consisting of potato liquor and potato pulp [3]. The potato liquor is treated with steam to coagulate the protein fraction, which is separated using a decanter. The remaining potato liquor, which mainly consists of the soluble fraction of the waste from potato starch production, is concentrated to form potato protein liquor (PPL). Together with the processing costs and stricter EU requirements, the potato and starch industry is forced to use valuable resources responsibly and develop a more efficient approach that can produce more value-added products from the by-products [4]. Increasing resource efficiency by maximizing the utilization of nutrients in a used resource is a step further than converting organic waste into bio-based resources and is a major contribution to sustainable production with higher circularity. Therefore, efficient utilization of protein-rich side streams is important for the starch industry.
In current practices, residual effluents from the potato starch industry usually end up as fertilizer [5]. This common scenario has been compared with other scenarios in different literatures [5] and the use of PPL for the cultivation of fungal biomass and proteins [6, 7], yeast biomass [8], and enzyme production [9] was investigated. PPL has been valorized through conversion of a high fraction of its nutrients into food and feed grade fungal biomass in a previous study [7]. This partial valorization approach generates another type of effluent stream that can be used to maximize value creation from PPL. In this regard, the well-known valorization method of anaerobic digestion can use the effluent from fungal cultivation that still contains high chemical oxygen demand (COD), unconverted organics, and fungal metabolites.
A modified version of anaerobic digestion, acidogenic fermentation, or acidogenic digestion which provides conditions for the production of volatile fatty acids (VFAs) rather than biogas (mainly methane) can help with further value creation from such effluents. VFAs including fatty acids with 2 to 6 carbon chains (acetic acid, propionic acid, butyric acid, etc.) are important platform chemicals produced in the anaerobic digestion process as an intermediate metabolites [10]. Traditionally, VFAs are synthesized commercially through petrochemical pathways. Due to the continuously rising environmental awareness on the excessive application consequences and resource scarcity of global oil sources, new alternative approaches such as VFA generation from low or negative value organic waste has attracted great attention. The generated VFAs through acidogenic fermentation or digestion have market demands in applications such as bioplastics and bioenergy production, external carbon sources for biological nutrient removal, and usage in pharmaceutical and food industries [11,12,13]. Different sources of organic waste including food waste [12, 14, 15], lignocellulosic materials [16], waste activated sludge [17, 18], and various animal manures [19,20,21] have been used as feed stock for acidogenic digestion and VFAs production. The addition and type of inoculum are also essential for the effectivity of the anaerobic process because they provide the initial microbial population [22]. The main benefit of employing inoculum from manure with AD process is related to have a faster starting time and well optimized digestion process [23].
This study focuses on a sequential biological treatment strategy for the production of different value-added products from potato starch industry residues. In this regard, the waste generated from the fungal cultivation of PPL was further valorized for the production of volatile fatty acids via acidogenic fermentation. The effects of pH adjustment through changes in the inoculum to substrate ratio, inoculum type (cow manure (CM) and chicken manure (CKM)), and methane production inhibition were initially evaluated in batch assays. In order to benefit from continuous VFA production from this rather low organic bearing effluent and in situ recovery of the particle- and microorganism-free VFA bearing stream, two immersed membrane bioreactors (iMBRs) operating with different inoculum type and at different organic loading rate were applied. Changes in the VFAs production and distribution, MBR performance (transmembrane pressure (TMP) and flux), medium characterization (viscosity, pH, extracellular polymeric substance (EPS), soluble microbial products (SMP), solid content), and inoculum activities were analyzed to determine the underlying mechanism for efficient VFA production by anaerobic digestion of FPPL. The introduced approach in this study targets maximum nutrient recovery and enhanced resource efficiency for industries generating organic residues through bioconversion approaches.
2 Materials and methods
2.1 Substrate and inoculums
Potato protein liquor (PPL) was obtained from Lyckeby Starch AB (Kristianstad, Sweden) and used for fungal cultivation study in a pilot-scale reactor with 1200 L capacity (Process& Industriteknik AB, Kristianstad, Sweden) with results presented in another study by Sar et al. [24]. After completion of cultivation, the fermented broth was passed through a sieve in order to collect the fungal biomass that is a product of fungi fermentation process. The already fermented residues and a waste liquid (FPPL) from the pilot-scale reactor that were commonly drained to the sewage system were collected and stored at −18 °C to be used as substrate in this study. The composition of substrate used is shown in Table 1. The same batch of liquid was used for all experiments described in this article. For inoculum, cow manure (CM) was provided by Hushållningssällskapet Sjuhärad (Länghem, Sweden) and chicken manure (CKM) was collected from Sjömarkens Höngårds (Sjömarken, Sweden). The as-received total solids (TS) of CKM and CM were 87.0 ± 0.47 and 45.2 ± 0.17 g/L), respectively, while volatile solids (VS) were 51.4 ± 0.77 and 32.3 ± 1.23 (g/L), respectively.
2.2 Batch digestion
CKM, CM, and FPPL used in the anaerobic batch experiments were thermally treated at 80 °C for 15 min (thermal shock) using a water bath to inhibit the activity and growth of methanogens [25]. The batch assays were carried out under mesophilic conditions (37 ± 1 °C) for 23 days, using 120 mL glass serum bottles as bioreactors. Batch acidogenic digestion experiments were performed in triplicate with a working volume of 80 mL and inoculum to substrate ratios of 1:1 and 1:4 (on VS basis) (Table 2). A total of 1 g/L VS including the substrate and inoculum mixture was loaded to each bioreactor. The inoculum controls contained the same VS loading as in the mixture. Tap water was added to fill the serum bottles to the same working volume for each condition, and then the initial pH was measured. Based on the as-mixed pH of the inoculum and substrate, the pH of the controls (inoculum only) was defined. Prior to digestion, all bottles were tightly sealed and purged with nitrogen gas for 2 min to remove any residual oxygen and create anaerobic conditions. The reactors were placed in water bath shakers at 37 ± 1 °C and 110 rpm for incubation. Liquid and gas samples were collected on days 1, 2, 3, 5, 7, 9, 11, 13, 15, 18, and 23. During incubation, 250 μL of biogas was sampled using a gas-tight syringe (VICI, Precision Sampling Inc., USA) to analyze the volume and composition of the biogas. A 1 mL aliquot was taken from the digestion medium using a syringe to analyze the VFAs content and distribution.
2.3 Anaerobic immersed membrane bioreactor (iMBR) setup and operation
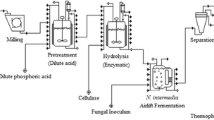
Two identical iMBRs that house second generation Integrated Permeate Channel (IPC) membrane panels were used (Fig. 1). The MBRs were operated semi-continuously at 37 °C with a working volume of 3.5 L. The IPC flat-sheet membranes were developed and supplied by the Flemish Institute for Technological Research (VITO NV, Mol, Belgium) [26]. Flat sheet membrane panels were made of hydrophilic polyethersulfone (PES)/PVP membranes coated on both sides of a spacer fabric with a total effective area of 205.8 cm2. Membranes had an average pore size of 0.3 μm and two different membranes were used in this regard in each MBR. Membrane specifications were the same, but the membrane age was different in the MBRs. MBR2 had a lower permeability from the beginning as they were not virgin membranes and have been used in different practices. Hence, the membranes were exposed to application and cleaning cycles which could not fully regenerate the capacity of the membrane. Each membrane panel had a total of 12 built-in air/gas diffusers on each side of the panel with a diameter of 0.5 mm. This allows effective membrane surface scouring during digestion and filtration and helps better mixing and mass transfer in the reactor using nitrogen gas. The iMBRs benefited from a gas flow meter (Dwyer Instruments, USA) to control the nitrogen flow. The nitrogen flow was set at 5 L/min during the filtration cycle and 3 L/min when filtration was not applied. During filtration, permeate flow was measured using Atrato 710-V11-D ultrasonic flowmeters (Titan Enterprises Ltd, Dorset, England), while liquid pressure on the permeate line was measured using a PMC131 pressure sensors (Endress+Hauser AB, Solna, Sweden). Temperature and pH in the bioreactor were monitored real-time using a probe combined with a transmitter via fermenter PLC (Micro DCU-300, Germany). Peristaltic pumps (Watson Marlow 400) were used separately to withdraw permeate from the membrane modules in the reactors. The filtration process consisted of a 4-min filtration cycle followed by a 30-s backwash cycle. Data logging and control of the permeate pump, pressure sensors, and flow meters were centrally controlled and automated with LabView-based MeFIAS® software [27] kindly customized and developed for these specific lab-scale iMBRs by VITO NV. MBR1 was initially loaded with a total of 3.5 L of thermally treated FPPL (4 g VS/L) and chicken manure as inoculum (4 g VS/L). For MBR2, cow manure was used instead as inoculum with the same loading ratios. During the start-up phase (days 1–6), iMBRs were operated in batch mode. Then, the iMBRs were switched to semi-continuous mode by performing the filtration cycle before the daily feeding of the reactor. Separation of the VFA-bearing effluent was performed by filtering out 350 mL of permeate a specific initial flowrate of 0.3–0.4 L/h (gives the average flux of 20 L/m2/h (LMH)) before feeding the same volume of the thermally treated FPPL. To compare membrane filtration performance at a different organic loading and VFA production at different solids concentrations, feeding regime began with an organic loading rate (OLR) of 1 gVS /L/d on day 6th, and later increased to 2 g VS/L/d from day 26th (i.e., after approximately 2 hydraulic retention times (HRT) at which the VFA concentration remained stable or unchanged) until day 48th. The initial pH of the MBRs was 8.15 and 8.28 for MBR1 and MBR2, respectively, and the pH during the digestion process was not controlled. Samples were collected every 24 h from the MBRs’ bulk medium for viscosity, COD, total solids (TS), total suspended solids (TSS), ammonium, extracellular polymeric substances (EPS), and soluble microbial product (SMP) analysis.
2.4 Analytical methods
Concentration and distribution of VFAs (acetate, propionate, iso-butyrate, butyrate, iso-valerate, valerate, and caproate) in the permeate and gas volume and compositions (H2, CH4, and CO2) from the batch digestion were analyzed using gas chromatography (GC) (Clarus 550; Perkin-Elmer, Norwalk, CT, USA) as described by Jomnonkhaow, Uwineza [20]. The volumetric gas measurements were transformed to standard conditions at 1.01 bar of pressure and 273.2 K of temperature and presented as NmL as in Fig. 3. Prior to analysis, the liquid samples were mixed with an acid mixture (25% (v/v) formic acid and 25% (v/v) ortho-phosphoric acid in a 1:3 ratio) to protonate the -COOH groups of the VFAs needed to facilitate extraction in the sample, and then centrifuged at 8000 rpm for 5 min and the supernatant was then filtered through 0.2 μm syringe filter to remove particulate matter before GC analysis. Butanol solution with a concentration of 1 g/L was used as an internal standard. The pH was determined using a pH meter (Mettler Toledo F20 FiveEasy, OH, USA). Viscosity measurements were carried out with a Vibro viscometer (SV -10, A&D Co., Ltd., Tokyo, Japan). TS, VS, TSS, and volatile suspended solids (VSS) were measured using an oven and a muffle furnace at 105±1 °C and 550±1 °C, respectively, according to the American Public Health standard methods [28]. Total chemical oxygen demand (tCOD) and soluble chemical oxygen demand (sCOD) were measured using COD 15,000 test kits (range of 1.0–15.0 g/L O2) and ammonium nitrogen (NH4+-N) was analyzed using ammonium 100 test kits (range of 0–80 mg/L) (Nanocolor, MACHEREY-NAGEL GmbH & Co. KG, Germany). sCOD samples were filtered through a 0.45 μm syringe filters before analysis. Concentrations of tCOD, sCOD, and NH4+-N were measured using Nanocolor 500D photometer (MACHEREY-NAGEL GmbH & Co. KG, Germany). For EPS and SMP analysis, protein content of EPS and SMP was measured according to the modified Lowry method [29, 30], and carbohydrate content was analyzed according to Dubois, Gilles [31] by using glucose (0–200 mg/L) as a polysaccharide standard and bovine serum albumin (0–200 mg/L) as a protein standard for calibration. SMP, loosely bound EPS (LB-EPS), and tightly bound EPS (TB-EPS) were extracted according to the method described by Li and Yang [32].
For comparison of VFA yield data from batch experiments, statistical significance was determined using MINITAB® software package (version 17.1.0, Minitab Inc., State College, PA, USA) using one-way analysis of variance (ANOVA, p<0.05) with a 95% confidence interval. All experiments were performed in duplicates and the error bars indicated in the graphs represent two standard deviations.
3 Results and discussion
In this study, the valorization of the effluent from fungal cultivation on PPL to VFAs using CKM and CM as inoculum was investigated using both batch assays and semi-continuous iMBRs.
3.1 Batch digestion of FPPL to VFAs
In order to investigate the potentials of VFAs generation from FPPL, batch tests were conducted considering thermal shocking, different types of inoculums (CKM and CM), and inoculum to substrate ratios. When exposed to extreme conditions such as high temperatures, acidogenic bacteria can generate spores, whereas methanogens cannot [15, 33, 34]. The ability of acidogens to form spores has been exploited as a strategy to kill methanogens by applying 80 °C for 15 min thermal shock pretreatment. The obtained maximum VFA productions together with calculated yields are summarized in Table 3.
The results of VFA concentration and distribution during batch digestion under each of the planned conditions are shown in Fig. 2. The maximum VFA concentration of 5.89 ± 0.10 g/L and 5.76 ± 0.20 g/L was obtained from the conditions with CKM as inoculum with an I:S ratio of 1:4 (initial pH of 7.56) and CM as inoculum with an I:S ratio of 1:1 (initial pH of 7.41), respectively. Normally, the higher the VFA production, the lower the final pH of the medium; however, this is not the case with CKM batch digestion. When comparing CKM + FPPL (1:4) and CKM + FPPL (1:1) results, the former happens to have a slightly higher final pH value. This can be explained by the difference in the starting I:S ratio and the buffering capacity of CKM in the I:S ratio of 1:4 during acidogenic digestion (Table 2). For both conditions, a total of 1 g VS was used in batch reactors and the yields obtained under these conditions were 0.47 g VFA/g VSadded and 0.46 g VFA/g VSadded, respectively. Both inoculums showed similar maximum yields for FPPL at similar pH. The yield for the CKM-only reactors was about 0.75 g VFA/g VSadded, while the VFA production was only 1.88 ± 0.10 g VFA/L produced only by 2 g VS of CKM at set pH of 7.56 as the inoculum itself was highly biodegradable compared to FPPL, resulting in higher bioconversion. The maximum VFA production yield for CM was 0.46 g VFA/g VSadded at initial pH 7.41. The reason for CKM-only batch fermentation produces a considerable amount of VFAs can be attributed to its substantial sCOD content.
The different digestion substrates/inoculums used affect the optimal pH in the VFA production system. In a batch digestion study [21] investigating the effects of initial pH (5.0, 6.0, 7.0, and 9.0) on VFA production from CKM, the highest yield was found to be 0.27 ± 0.01 at an initial pH of 6.0. In the same study, the highest net yield of VFAs was reported to be 0.53 g VFA/g VS (23.11 g/L) using heat shocked inoculum and substrate, and without pH control [21]. The maximum yield found in the current study for the substrate + inoculum conditions, however, was comparatively lower at 0.47 ± 0.01 g VFA/g VSadded with an I:S ratio of 1:4 (initial pH of 7.56). The reason for that could be the initial microbial characteristics of the inoculums used. In another study on anaerobic co-digestion of sewage sludge and cattle manure, it was found that the initial pH of 9.0 significantly increased VFA production and provided a yield of 67.99 ± 1.44 g/kg TS (with a VS ratio of sewage sludge to cattle manure of 3:7) [35]. Cow manure was also used as an inoculum for acidogenic digestion of food waste, yielding a VFA production yield of 0.43 g VFAs/g VS where an initial pH of 9 dropped to 7 during VFA fermentation [36]. In the current study, the slighter lower yields achieved compared to the abovementioned studies could be due to the type of substrate and load of soluble organic matter and the non-optimal initial as-mixed pH values.
In addition to the efficiency in VFA production, the change in VFA composition due to use of 2 different inoculums (CKM and CM) was also investigated. As expected, acetic acid was the predominant VFA in most conditions, followed by propionic and butyric acids, except for days 2 and 3 in for the FPPL + CM assays. The results indicate that the VFA composition was not affected by the difference in inoculum. A similar conclusion was drawn by Atasoy, Eyice [37], who worked with three different inoculum types (large and small granular, and slurry) and emphasized that inoculum type did not significantly affect the VFA composition. This shows that the microorganisms in inoculums are more acclimated to produce similar VFA patterns.
Figure 2a, c, e, and g show results acquired from batch reactors loaded only with inoculum (as control) along with the ones with a mixture of substrate and inoculum. The maximum total VFA (tVFA) concentrations of 5.56 ± 0.10 g/L and 5.89 ± 0.10 g/L were achieved for CKM + FPPL (1:1) and CKM + FPPL (1:4), respectively, on day 18. When CM was used as inoculum, 5.76 ± 0.20 g/L and 5.68 ± 0.40 g/L of VFAs were detected on day 11 for CM + FPPL (1:1) and CM + FPPL (1:4), respectively. As expected, the reactors operating with inoculum only had lower concentrations of VFAs than the reactors to which FPPL was added. According to the findings, substrate is crucial to the production of VFAs. The difference between the two shows the contribution of FPPL to the VFA generated.
The cumulative gas productions for different conditions in batch experiments are presented in Fig. 3 presenting changes in H2, CH4, and CO2 generation volumes. According to the obtained CH4 production results for CKM (CKM + FPPL, pH 7.90 and CKM + FPPL, pH 7.56), it was found that methane production was almost completely inhibited in heat shocked assays as they showed almost no production and remained stable during digestion (Fig. 3b). However, for the inoculums (CKM, pH 7.90 and CKM, pH 7.56), signs of methane production were observed from day 13 and day 9, respectively. Moreover, in the case of the reactors inoculated by CM, thermal pretreatment was able to hinder methanogens during almost the entire digestion period under all conditions, except for CM with pH 7.41, where 0.7 mL CH4/g VS production started on the last day of digestion (day 23) (Fig. 3e). The effectiveness of heat shock treatment at different temperatures on methane inhibition has been confirmed in different literature, with some reporting methanogens recovering in long runs [20, 38] which can back up what has been happening in this study. Carbon dioxide and hydrogen production commenced on day 1 in all batch fermenters. Carbon dioxide content appears to be higher for CM compared to CKM (Fig. 3c and f). The CO2 yields for FPPL at initial pH of 7.90, FPPL at pH 7.56 together with CKM as inoculum and FPPL at pH 7.41, FPPL at pH 7.02 together with CM were similar under the tested conditions and ranged from 7.5 to 14 mL/g VS. Hydrogen gas that is produced and VFAs have some parallels as they are both mediators and precursors to biogas synthesis. As a result, hydrogen gas is typically created as a byproduct of bioprocesses that are advantageous for high-yielding VFA generation, and vice versa [25]. CKM only at pH 7.9 showed the highest H2 yield of 11.7 mL/g VS. Stabilization of H2 production occurred shortly after for FPPL with CKM and CM batch reactors (Fig. 3a). Stabilization of the cumulative production data could be explained by gas production having come to a halt, interaction of other operating factors or the amount of gas produced being equal to the conversion of it in the complex anaerobic metabolism [39].
3.2 VFAs production and recovery using iMBR
Semi-continuous anaerobic iMBRs were used for acidogenic fermentation and in situ recovery of VFA-bearing permeate using FPPL as feed and CKM (MBR1) and CM (MBR2) as inoculums. They were run for 48 days with 6 days of startup. The concentrations and compositions of VFAs in the iMBR during the anaerobic digestion process are shown in Fig. 4.
Acetic acid is often the main component of the tVFAs during anaerobic digestion [40]. This was also the case in the present study, where acetic acid comprised 59–97% of the tVFAs in both reactors and at both operating OLRs. The features of the substrate and inoculum, the operating conditions, and the initial pH and pH changes during digestion by the microbial community responsible for the specific acid type all have an impact on VFA distribution [41, 42]. Following acetic acid, butyric acid (1–21%) and propionic acid (6–18%) were the second and third largest VFA components in the digestion of FPPL (Fig. 4). Longer-chain fatty acids such as valeric and caproic acids were produced at very low concentrations. This is in line with the results from batch experiments (Fig. 2).
The iMBRs were initiated in batch mode and accumulation of VFAs during batch mode reached 5.39 ± 0.23 g/L for MBR1 and 2.93 ± 0.02 g/L for MBR2 on day 5 and stabilized thereafter. Afterwards on day 6, batch mode was switched to semi-continuous mode with an OLR of 1 g VS/L/d. The VFA production in this OLR increased to a maximum of 8.25 g/L for MBR1 and 7.61 g/L for MBR2. The average of tVFAs on MBR1 and MBR2 was 5.84 ± 1.03 g/L and 5.26 ± 1.31 g/L, respectively, during the period of day 6 to day 26. In order to enhance production and yield of VFAs, OLR is one of the most important operational parameters to be focused on. Applied OLR also has a great impact on the microbial community structure [43]. The change in the microbial community can lead to changes in the metabolic pathways, which means a different distribution of the individual VFAs [44]. Therefore, the OLR was doubled to 2 g VS /L/d on day 26, which required feeding and filtration twice a day (one in the morning and one in the evening) due to the low VS content of the feedstock (9.2 ± 0.08 g/L). VFA samples were taken daily before the first and second filtration and feedings, and the results of both VFA profiles are shown in Fig. 2. When the OLR was doubled, the average total production of VFAs in both reactors increased to 10.40 ± 0.79 g/L for MBR1 and 10.22 ± 0.86 g/L for MBR2, which is an increase in VFA production of almost 44% and 48% for MBR1 and MBR2, respectively. The systems stabilized during days 40–48 in terms of VFA production. Further increases in the various OLRs could not be attempted due to the low VS of the feedstock. It was observed that doubling the OLRs resulted in a higher increase in MBR2, although the average concentrations for MBR2 were always lower compared to MBR1. Moreover, the maximum VFA yield was calculated as 0.65 g VFAs/g VSfed and 0.36 g VFAs/g VSfed for OLR 1 and 2 g VS/L/d in MBR1, and 0.54 g VFAs/g VSfed and 0.35 g VFAs/g VSfed for OLR 1 and 2 g VS/L/d, respectively in MBR2. The reduction of the VFA yield by increasing substrate loading, and hence the presence of an optimum OLR value, has already been described in the literature [20]. When this point is achieved, the first phase of acidogenic digestion becomes restricted. These studies attribute this point of inflection to the system’s hydrolytic capacity with increased concentrations of different compounds such as K+, Na+, chlorophenols, and heavy metals (Cu>Zn>Cr>Cd>Ni>Pb) that are responsible for acidogenic inhibition might be an influencing parameter that leads to an inhibition of the acidogenic step [45]. The pH of the reactors was not controlled or adjusted during the digestion which can be another reason of this reduction on VFA yields. Methanogens should be kept in an environment with controlled pH and temperature because these factors are essential for the anaerobic bacteria’s ability to function. Methanogenesis requires pH levels of 6.5–7.5, while acidogenesis and acetogenesis need pH levels of 5–5.5 [46]. The average pH during the whole semi-continuous process in the present study was 8.10 ± 0.13 and 8.31 ± 0.15 at MBR1 and MBR2, respectively. In literature, the productions of VFAs from potato-related residues are limited to potato peel waste or waste potato [47,48,49,50]. In one of these studies [49], the waste of peeled potatoes from a student cafeteria was used to produce VFAs and the highest VFA production was found to be 41.9 g COD /L and 632.2 mg COD /g VSfed at a pH of 7.0. Li, Zhang [48] also reported the importance of pH control and microbial diversity analysis for feasible and efficient acidogenic digestion in a study using potato waste from a vegetable processing center and an anaerobic sludge as inoculum for VFA production. The highest concentration and proportion of VFAs were observed as 7.8 g/L and 49.7% for butyric acid at pH 6.0 [48].
3.3 iMBRs performance during anaerobic digestion and filtration
In the current research, two identical iMBRs were used to conduct in situ recovery of VFA-containing effluent during acidogenic digestion of FPPL. Semi-continuous production and recovery of VFAs was performed at an HRT of 10 days by filtering out 350 mL of the liquid medium as permeate before feeding on daily basis. Membrane filtration was performed over a 48-day period at a set initial permeate flux of 20 LMH. After approximately 2 HRTs of filtration and feeding at OLR of 1 g VS/L/d, the OLR was increased to 2 g VS/L/d (day 26). During filtration, membrane filtration performance, and the changes in the membrane surface condition and the extent of fouling, was tracked by observing changes in the filtration parameters (TMP and flux) and medium characteristics (SS, viscosity, etc.).
As shown in Fig. 5a, the starting TMP for the MBR2 was slightly higher than that of MBR1 which is due to different ages (consecutive experiments that the membranes were exposed to previously) of the membranes in the MBRs. Another reason may be due to the characteristics of the inoculum (CKM and CM) that may have affected the initial TMP through initial adsorption of a layer of foulants to the membrane surface at first contact. TMP fluctuated between 0.011 and 0.103 bar throughout the digestion period for MBR1 (Fig. 5a). The trend for the increase in the concentrations of TSS in MBR1 indicated accumulation rates of 0.13 g TSS/L/d. The average TSS at OLR of 1 g VS/L/d was 4.71 ±1 g/L. Although this value increased to 8.25 ± 2 g/L at OLR 2, an unlimited solid retention was considered where no drainage of the accumulated sludge was performed throughout the digestion. The average viscosity for MBR1 was 0.93 ± 0.12 mPa.s during OLR1 and 0.99 ± 1.10 mPa.s during OLR2. The difference in viscosity could not have been the cause of TMP difference as the viscosities are low and rather in the same range during the digestion period. TMP fluctuated between 0.064 and 0.219 bar throughout the digestion period for MBR2 (Fig. 5a). The trend for the increase in the concentrations of TSS in MBR2 indicated accumulation rates of 0.17 g TSS/L/d. The average TSS at OLR of 1 g VS/L/d was 5.16 ± 1.5 g/L in MBR2. Similarly, as seen in MBR1, TSS value increased to 9.10 ± 2.3 g/L at OLR 2 for MBR2, an unlimited solid retention was considered where no drainage of the accumulated sludge was performed throughout the digestion. The low accumulation of TSS compared to similar studies [20, 51, 52] is due to the low solid content (TS and VS) of the fed substrate in addition to the ease of breakdown and solubilization of organic suspended solids (Table 1).
Considering the changes in the membrane surface condition and resistance to liquid permeation, the TMP of MBR1 and MBR2 maxed at 0.10 bar and 0.22 bar on days 42 and 32, indicating the highest membrane fouling throughout the period (Fig. 5a and b). At a relatively high flux of 20 LMH, a constant but gradual increase in TMP was observed even though TMP levels are very different in both MBRs. This increase can also be attributed to the changes in the membranes surface condition due to cake layer build up and its subsequent resistance to permeate flow [53]. The increase in the TMP together with medium TSS content shows the gradual deposition of foulants on the membrane surface gradually adding cake layer resistance to membrane hydraulic resistance. As presented in Fig. 5b, c, and d which illustrate TMP patterns at different intervals, MBR1 using CKM as inoculum was the least problematic when it comes to the changes in membrane hydraulic resistance. The first and second filtering cycles on day 36 appear to be perfect repeats in both MBRs, with no decrease in performance (Fig. 5c and d). As indicated in Fig. 5a with red arrows, membrane chemical cleaning was performed in two occasions when the TMP had values between 0.15 and 0.20 bar. Although chemical cleaning temporarily reduced the TMP, fouling re-occurred progressively as the conventional physical fouling preventive method of gas scouring and backwashing proved rather ineffective. Although TSS has been quite stable, the development of a biofilm and the deposition of biopolymers (proteins, polysaccharides) that cannot be easily removed by backwashing could have contributed to hydraulic resistance [54, 55]. For this reason, the concentrations of carbohydrates, proteins, and humic like substances in EPS and SMP were analyzed to determine their contribution to the rise in TMP observed during filtration.
Figure 6 show the content of TB-EPS, LB-EPS, and SMP from the initial sample, the average of 3 consecutive days in OLR 1 and 2 gVS/L/d when the reactor was stable, plus the final day of digestion. In general, marginal increase in the concentration of EPS and SMP was observed by increasing OLR in both MBRs. However, the protein and carbohydrate content of TB-EPS and SMP in MBR1 and MBR2 were generally similar with discrete differences. The MBR2 started with 1.33 times more SMP proteins plus humic-like substances and slightly higher SPM carbohydrates compared to MBR1, which may be attributed to the complexity of the cow manure used as inoculum in MBR2. It was observed that much more SMP proteins and humic-like substances were produced in MBR1 when the OLR increased at 2 gVS/L/d, resulting in similar SMP protein at the same OLR in MBR2. In general, the LB-EPS content of the digestion medium was higher than the TB-EPS content. The LB-EPS protein and carbohydrate content were 1- to 4-times higher than the TB-EPS content in MBR2, while the LB-EPS content in MBR2 was 1–2 times higher than the LB-EPS content in MBR1. Moreover, the EPS content increased from 3 to 5% in OLR 1 and 2 in MBR2, respectively, and only up to 3% in MBR1 in both OLR 1 and 2. As reported in other studies, the EPS content strongly contributes to the formation of biofilms leading to membrane fouling; therefore, EPS and SMP can be deposited and/or adsorbed on the membrane surface and in membrane pores, which promote the formation of a gel layer or biofilm and blockage of the pores [56, 57]. It has also been reported that LB-EPS helps to increase the resistance of the cake layer, leading to an increase in TMP, while TB-EPS could reduce the tendency of the membrane to foul [56]. In a study investigating the dynamic changes of EPS and its role in fouling of membranes in municipal wastewater treatment, LB-EPS was found to have higher fouling potential than TB-EPS [58]. However, in this study, the EPS and SMP results did not show any variances in MBRs, and they also did not show changes in a single MBR as a result of such components. Zhang [59] studied the influence of EPS and SMP on membrane fouling in membrane bioreactor operation and found that both EPS and SMP contribute more to cake layer resistance to total resistance ranging from 82.89 to 90.44% and negatively affecting membrane filtration, while the concentration of SMP contributes more to pore blocking resistance to total resistance, ranging from 7.31 to 13.46%. The initial content of protein and humic-like substances, and carbohydrates in EPS and SMP can be one of the reasons for the high initial TMP in MBR2 compared to MBR1. The increase in these substances reflects the gradual increase in TMP over time (Fig. 6a), confirming the relationship between bound EPS and SMP and membrane fouling in MBR2 compared to MBR1. In addition, as the membranes used for these experiments are not new membranes, the initial state of each membrane panel may have differed from one another based on the irreversible fouling induced in previous applications.
4 Conclusions
The effluent from fungal cultivation on PPL was used to further maximize value creation from the potato starch industry through acidogenic digestion of VFAs. The effect of inoculum type and substrate to inoculum ratio on VFA yield on FPPL was investigated. iMBRs inoculated with either cow or chicken manure were used to generate a particle- and microorganism-free VFA-bearing effluent with a maximum yield of 0.65 g VFAs/g VSfed recovered at a flux of 20 LMH in a long-term (48-day) digestion and filtration process. The findings prove that a multi-stage bioconversion of potato starch industry residues to value added products with the aim of nutrient reuse and resource circularity can be realized.
References
Jordbruksverket. Harvesting of agricultural plants. 2022 [cited 2022 -01-12].
Agriculture, SBo (2022) Harvest of potatoes 2021. Preliminary statistics, S. Sweden, Editor. The Swedish Board of Agriculture’s statistical reports
Kuktaite, R., et al., How the unuseful can be turned into sustainable and useful: novel potato protein bioplastics with unusual strength. 2015.
Ahokas M et al (2014) Resource assessment for potato biorefinery: side stream potential in Northern Ostrobothnia. Agron Res 12(3):695–704
Souza Filho PF et al (2017) Techno-economic and life cycle assessment of wastewater management from potato starch production: present status and alternative biotreatments. Fermentation 3(4):56. https://doi.org/10.3390/fermentation3040056
Schügerl K, Rosen W (1997) Investigation of the use of agricultural byproducts for fungal protein production. Process Biochem 32(8):705–714. https://doi.org/10.1016/S0032-9592(97)00033-2
Souza Filho PF, Zamani A, Taherzadeh MJ (2017) Production of edible fungi from potato protein liquor (PPL) in airlift bioreactor. Fermentation 3(1):12. https://doi.org/10.3390/fermentation3010012
Lotz M et al (1991) Bakers’' yeast cultivation on by-products and wastes of potato and wheat starch production on a laboratory and pilot-plant scale. Process Biochem 26(5):301–311. https://doi.org/10.1016/0032-9592(91)85018-J
Klingspohn U, Vijai Papsupuleti P, Schügerl K (1993) Production of enzymes from potato pulp using batch operation of a bioreactor. J Chem Technol Biotechnol 58(1):19–25. https://doi.org/10.1002/jctb.280580104
Zhang L et al (2022) Acidogenic fermentation of organic wastes for production of volatile fatty acids. In: Biomass, Biofuels, Biochemicals. Elsevier, pp 343–366
Agnihotri S et al (2022) A glimpse of the world of volatile fatty acids production and application: a review. Bioengineered 13(1):1249–1275. https://doi.org/10.1080/21655979.2021.1996044
Sapmaz T et al (2022) Potential of food waste-derived volatile fatty acids as alternative carbon source for denitrifying moving bed biofilm reactors. Bioresour Technol 364:128046
Wainaina S et al (2019) Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Bioresour Technol 274:329–334. https://doi.org/10.1016/j.biortech.2018.11.104
Shewa WA et al (2020) Valorization of food waste and economical treatment: Effect of inoculation methods. J Clean Prod 261:121170. https://doi.org/10.1016/j.jclepro.2020.121170
Wainaina S et al (2019) Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: a critical review. Bioengineered 10(1):437–458. https://doi.org/10.1080/21655979.2019.1673937
Sun J, Zhang L, Loh K-C (2021) Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresources and Bioprocessing 8(1):1–21. https://doi.org/10.1186/s40643-021-00420-3
Yuan Y et al (2019) Advances in enhanced volatile fatty acid production from anaerobic fermentation of waste activated sludge. Sci Total Environ 694:133741. https://doi.org/10.1016/j.scitotenv.2019.133741
Fang W et al (2020) Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J Environ Sci 87:93–111. https://doi.org/10.1016/j.jes.2019.05.027
Huang W et al (2016) Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res 90:344–353. https://doi.org/10.1016/j.watres.2015.12.044
Jomnonkhaow U et al (2021) Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour Technol 321:124456. https://doi.org/10.1016/j.biortech.2020.124456
Yin D-M et al (2021) The effect of mono-and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour Technol 330:124992
Rajput AA, Sheikh Z (2019) Effect of inoculum type and organic loading on biogas production of sunflower meal and wheat straw. Sustainable Environment Research 29(1):1–10
Paes JL et al (2020) Use of inoculum in biodigesters with cattle manure under conventional and organic production systems. Engenharia Agrícola 40:146–153
Sar, T., et al., Scale production of protein-rich fungal biomass from potato protein liquor for use as innovative food and feed products. Food Bioscience, 2022: p. 101637 https://doi.org/10.1016/j.fbio.2022.101637.
Mondylaksita K et al (2021) Enhanced volatile fatty acid production from oil palm empty fruit bunch through acidogenic fermentation—a novel resource recovery strategy for oil palm empty fruit bunch. Fermentation 7(4):263
Doyen W et al (2010) Spacer fabric supported flat-sheet membranes: A new era of flat-sheet membrane technology. Desalination 250(3):1078–1082. https://doi.org/10.1016/j.desal.2009.09.112
Doyen, W., et al. (2003) A new approach to pilot installation automation, the ingeneous solution you have been looking for. 4-7.
Federation WE, A.P.H. Association (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC, USA
Frølund B et al (1996) Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res 30(8):1749–1758. https://doi.org/10.1016/0043-1354(95)00323-1
Griebe T, Nielsen PH (1995) Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 43(4):755–761. https://doi.org/10.1007/BF00164784
Dubois M et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Li XY, Yang SF (2007) Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res 41(5):1022–1030. https://doi.org/10.1016/j.watres.2006.06.037
Setlow P (2014) Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196(7):1297–1305. https://doi.org/10.1128/JB.01455-13
Valdez-Vazquez I, Poggi-Varaldo HM (2009) Hydrogen production by fermentative consortia. Renew Sust Energ Rev 13(5):1000–1013
Dai X et al (2016) High-solid anaerobic co-digestion of sewage sludge and cattle manure: the effects of volatile solid ratio and pH. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep35194
Tampio EA et al (2019) Volatile fatty acids (VFAs) and methane from food waste and cow slurry: comparison of biogas and VFA fermentation processes. GCB Bioenergy 11(1):72–84. https://doi.org/10.1111/gcbb.12556
Atasoy M et al (2019) Volatile fatty acids production via mixed culture fermentation: revealing the link between pH, inoculum type and bacterial composition. Bioresour Technol 292:121889. https://doi.org/10.1016/j.biortech.2019.121889
Mei R et al (2016) Effects of heat shocks on microbial community structure and microbial activity of a methanogenic enrichment degrading benzoate. Lett Appl Microbiol 63(5):356–362. https://doi.org/10.1111/lam.12629
Guo XM et al (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrog Energy 35(19):10660–10673. https://doi.org/10.1016/j.ijhydene.2010.03.008
Srisowmeya G, Chakravarthy M, Devi GN (2020) Critical considerations in two-stage anaerobic digestion of food waste–a review. Renew Sust Energ Rev 119:109587. https://doi.org/10.1016/j.rser.2019.109587
Bedaso, B., Volatile fatty acid production from co-fermentation of primary sludge and food waste without pH control. 2019.
Lee WS et al (2014) A review of the production and applications of waste-derived volatile fatty acids. Chem Eng J 235:83–99. https://doi.org/10.1016/j.cej.2013.09.002
De Groof V et al (2021) Selecting fermentation products for food waste valorisation with HRT and OLR as the key operational parameters. Waste Manag 127:80–89. https://doi.org/10.1016/j.wasman.2021.04.023
Li B-Y et al (2021) Production of volatile fatty acid from fruit waste by anaerobic digestion at high organic loading rates: performance and microbial community characteristics. Bioresour Technol:126648. https://doi.org/10.1016/j.biortech.2021.126648
Magdalena JA, Greses S, González-Fernández C (2019) Impact of organic loading rate in volatile fatty acids production and population dynamics using microalgae biomass as substrate. Sci Rep 9(1):1–11
Osman AI et al (2021) Conversion of biomass to biofuels and life cycle assessment: a review. Environ Chem Lett 19(6):4075–4118
den Boer E et al (2016) Volatile fatty acids as an added value from biowaste. Waste Manag 58:62–69. https://doi.org/10.1016/j.wasman.2016.08.006
Li Y et al (2019) Acidogenic properties of carbohydrate-rich wasted potato and microbial community analysis: effect of pH. J Biosci Bioeng 128(1):50–55. https://doi.org/10.1016/j.jbiosc.2018.12.009
Lu Y et al (2020) Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour Technol 316:123851. https://doi.org/10.1016/j.biortech.2020.123851
Ma H et al (2017) Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnology for biofuels 10(1):1–15. https://doi.org/10.1186/s13068-017-0821-1
Barreto CM et al (2017) Assessing the performance of an MBR operated at high biomass concentrations. Int Biodeterior Biodegradation 119:528–537. https://doi.org/10.1016/j.ibiod.2016.10.006
Wainaina S et al (2020) Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew Energy 152:1140–1148. https://doi.org/10.1016/j.renene.2020.01.138
Judd S (2010) The MBR book: principles and applications of membrane bioreactors for water and wastewater treatment. Elsevier
Meng F et al (2017) Fouling in membrane bioreactors: an updated review. Water Res 114:151–180. https://doi.org/10.1016/j.watres.2017.02.006
Sambusiti C et al (2020) Influence of HRT reduction on pilot scale flat sheet submerged membrane bioreactor (sMBR) performances for Oil&Gas wastewater treatment. J Membr Sci 594:117459. https://doi.org/10.1016/j.memsci.2019.117459
Deng L et al (2016) Biofouling and control approaches in membrane bioreactors. Bioresour Technol 221:656–665. https://doi.org/10.1016/j.biortech.2016.09.105
Lin H et al (2014) A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J Membr Sci 460:110–125. https://doi.org/10.1016/j.memsci.2014.02.034
Zhang A et al (2014) Effects of EPS on membrane fouling in a hybrid membrane bioreactor for municipal wastewater treatment. Membrane and Water Treatment 5(1):1–14. https://doi.org/10.12989/mwt.2014.5.1.001
Zhang H-F (2009) Impact of soluble microbial products and extracellular polymeric substances on filtration resistance in a membrane bioreactor. Environ Eng Sci 26(6):1115–1122. https://doi.org/10.1089/ees.2008.0312
Acknowledgements
The authors are thankful to Sweden’s Innovation Agency, Swedish Research Council, and the University of Borås for the technical and financial support for this research work. We also gratefully acknowledge the Scientific and the Technical Research Council of Turkiye, TUBITAK (2211-A Internal Graduate Scholarship Programme) and the Council of Higher Education (CoHE) (100/2000 PhD Grant Programme).
Data availability
The datasets generated during the current study are available from the authors on reasonable request.
Funding
Open access funding provided by University of Boras.
Author information
Authors and Affiliations
Contributions
Tugba Sapmaz: data curation, formal analysis, methodology, validation, investigation, writing — original draft, writing — review and editing, and supervision. Clarisse Uwineza: data curation, formal analysis, investigation, and writing — review and editing. Amir Mahboubi: conceptualization, investigation, writing — review and editing, and supervision. Heleen De Wever: writing — review and editing and resources. Derya Y. Koseoglu-Imer: conceptualization and supervision. Mohammad J. Taherzadeh: conceptualization, funding acquisition, resources, and supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fungal cultivation effluent is a potential feedstock for VFA production.
• Having still unconverted metabolites, FPPL can be valorised into high value products.
• Challenges in VFA production and future perspectives were discussed.
• Multi-stage bioconversion of potato starch residue has shown resource circularity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sapmaz, T., Uwineza, C., Mahboubi, A. et al. Towards maximum value creation from potato protein liquor: volatile fatty acids production from fungal cultivation effluent. Biomass Conv. Bioref. 13, 16359–16372 (2023). https://doi.org/10.1007/s13399-023-03782-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03782-0