Abstract
An integrated thermochemical-biochemical Biomass-to-Liquid (BtL) pathway for the production of aviation and maritime liquid fuels from biogenic residues is introduced. The presence of a semi-commercially proven technology like Dual Fluidized Bed Gasification (DFBG) ensures extended fuel flexibility, syngas of high quality, complete fuel conversion, and optimal heat integration while avoiding CAPEX (Capital Expenditure) intensive equipment like air separation unit. Then, a two-stage biochemical route is proposed: initially syngas fermentation (anaerobic) into acetate and subsequently acetate fermentation (aerobic) into targeted triglycerides (TAGs) that will be finally purified and hydrotreated to form the desired drop-in biofuels. The tolerance of the bacteria to syngas contaminants minimizes the gas cleaning requirements. Moreover, the low-pressure requirements (1–10 bar) along with the mild operating temperatures (30–60 °C) reduce drastically the capital and operational cost of the process. The biological process of syngas fermentation inherently has limited side products, a fact that reduces the risk of deactivation of hydrotreatment catalysts. Heat and mass balances are calculated for the proposed concept via full-scale process simulations in Aspen Plus™ assuming a thermal input of 200 MWth with crushed bark as feedstock. Three different operational scenarios are examined mainly through overall performance indicators such as carbon utilization (CU) and energetic fuel efficiency (EFE). Competitive performance compared to technologies that exploit similar feedstock (i.e., biogenic residues) was noticed, since values in the range of 22–27% and 31–37% were obtained for the CU and EFE, respectively. The aim of this study is to determine the appropriate key process specifications and assess the potential of the proposed concept compared to other competitive technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Paris Agreement’s objectives related to climate change put aviation and shipping sectors, along with other industries, under great pressure and environmental inspection. According to a study based on different business-as-usual scenarios [1], maritime CO2 emissions in 2050 have been projected to increase by 50–250% compared to 2012. In 2018, the International Maritime Organization (IMO) adopted a preliminary strategy to mitigate the shipping sector’s GHG emissions and reduce its contribution to climate change. The main goals of this strategy were to reduce the carbon intensity of international shipping by 70% and the total annual GHG emissions by at least 50% by 2050, compared to 2008 [2]. As for the aviation sector, projections suggest that by 2050 air passengers could exceed 10 billion per year. Based on a business-as-usual approach, the estimated 2021–2050 carbon emissions are approximately 21.2 Gt of CO2. At the 77th Annual General Meeting of International Air Transport Association (IATA) in 2021, IATA member airlines agreed to commit to net-zero carbon emissions by 2050 to limit the aviation industry’s contribution to the global warming [3].
Biofuels have recently started to attract great interest and have been identified by IATA and IMO as a promising strategy to reduce CO2 emissions in the aviation and shipping sector, respectively. The International Energy Agency (IEA) claims that biofuels could provide 27% of total transport fuel by 2050, mainly replacing diesel, kerosene, and jet fuel [4]. Lignocellulosic biomass conversion into liquid biofuels through thermochemical routes has been considered as a favorable option that offers several advantages. The main challenge for these pathways is to develop advanced technologies with reduced energy consumption in a cost-effective way. The low energy density (due to high oxygen content) and the corrosive nature of pyrolysis bio-oil or the high costs (catalysts, high pressures) of liquefaction have established biomass gasification as the most cost-effective and efficient technology for lignocellulosic biomass conversion to bio-energy [5, 6].
Today, the main types of synthesized paraffinic kerosene, approved by ASTM (ASTM D7566-20) as blending components for conventional jet fuel (Jet A1) to make up bio-jet fuels, are the Fischer–Tropsch synthetic paraffinic kerosene (FT-SPK), Fischer–Tropsch synthetic kerosene with aromatics (FT-SKA), Hydroprocessed Esters and Fatty Acids (HEFA), synthesized isoparaffins (SIP), and Alcohol-to-Jet (ATJ) [7]. Aviation biofuels must comply almost entirely with conventional jet fuel specifications, since blending regulations for aviation sector are stricter than other transportation. Currently, the vast majority of bio-jet fuel is produced via the HEFA route using fats, oils, and greases (FOGs) as feedstock since it is the most technically mature technology. Neste and World Energy are two of the world’s largest companies for commercial-scale HEFA production from vegetable oils, used cooking oils (UCO), animal fat, non-edible oils, and waste [8]. However, the major obstacle for such technologies is the high feedstock cost and low sustainability [9]. All the other SAF (Sustainable Aviation Fuel) routes continue to grow, but have not reached commercialization yet. Indicatively, Sasol and Syntroleum that produce aviation fuel through the FT process using coal and natural gas have now turned their attention to the production of biomass-derived jet fuel [10]. LanzaTech and Gevo, companies specializing in advanced biochemically engineered fermentation processes, have developed processes for the production of ATJ using gas-fermented ethanol and isobutanol, respectively [11]. A collaboration between Total and Amyris has resulted in the development of a SIP production route through the conversion of sugarcane into farnesene and subsequent hydroprocessing into jet fuel [12]. In the marine sector, there are two types of fuels, distillate (e.g., marine gas oil (MGO)) and residual fuels (e.g., heavy fuel oil). The most common bio variants of MGO are fatty acid methyl ester (FAME/biodiesel) and hydrotreated vegetable oil (HVO). There are several companies producing FAME and HVO on a commercial scale, with Renewable Energy Group and Neste being the world’s leading producers, respectively [13, 14]. In general, sustainability issues (e.g., food vs. fuel and costly feedstock) and uncertainty over cost reduction related to most of the mentioned current biofuels impose the incorporation of alternate approaches in the current biofuel production pathways that target to more competitive drop-in biofuel prices [15].
In this study, an alternative Biomass-to-Liquid (BtL) route for the production of drop-in aviation and maritime fuels is introduced. The proposed concept aims to establish a combined thermochemical–biochemical pathway for the treatment of biogenic residues that minimizes the shortcomings of the existing technologies and takes advantage of their strong aspects in order to produce elevated yields of the desired fuels with limited energy consumption. The suggested process chain can be divided into three distinct parts: the thermochemical, the biological, and the thermocatalytic. Concerning the first (thermochemical) part, a Dual Fluidized Bed Gasification (DFBG) unit is considered for the syngas production from biogenic residues followed by a catalytic tar reformer, while for the second (biological) part a double-stage syngas-to-acetate-to-triglyceride (TAG) fermentation unit is involved accompanied by a lipid extraction and purification system. The last (thermocatalytic) part refers to the hydrotreatment unit where the obtained TAGs are converted into drop-in liquid fuels. The proposed concept from start to end is presented in Fig. 1.
The proposed BtL concept from start to end [16]
The European project BioSFerA [16, 17] has undertaken the realization, technical maturation, and implementation of the mentioned novel concept for the production of drop-in biofuels that has never been studied before as a whole. DFBG is a semi-commercially proven technology that has already been tested with a wide variety of different feedstocks, like wood pellets/chips, bark, straw, and sewage sludge [18]. However, so far, no previous research has examined the connection of a DFBG unit with a double-stage fermentation system. Hu et al. [19] introduced a two-stage integrated bioprocess for the conversion of syngas to acetate and finally to lipids and performed bench-scale experiments demonstrating the potential of such a system. As for the lipid extraction, in the present study, a novel method is proposed based on the steam explosion technique that has never been tested before for the extraction of TAGs from yeasts; this technology has only been investigated for the cell disruption of microalgae resulting in high lipid extraction yields [20, 21]. To date, no known study has focused on the hydrotreatment of this type of microbial oil deriving from yeast, although there is a vast variety of oils that have been hydroprocessed for the production of advanced liquid fuels (e.g., vegetable oils, fats, or waste cooking oils). Thus, indubitably, this is a highly innovative concept for the production of jet-like and bunker-like fuels, involving technologies with very strong prospect in an integrated scheme.
In this paper, a conceptual design based on the aforementioned process chain is developed and presented. Heat and mass balances are calculated for the integrated scheme via full-scale process simulations in Aspen Plus™ assuming a thermal input of 200 MWth with crushed bark as feedstock. Three different operational scenarios have been examined and assessed mainly through overall performance indicators: carbon utilization (CU), energetic fuel efficiency (EFE), liquid fuel mass yield, and overall energetic/exergetic efficiency. Design considerations and their impact on process efficiency were performed within the assumed scenarios, including parameters such as internal/external hydrogen securement via pressure swing adsorption (PSA)/water electrolysis and oxy-/air-acetate fermentation as well as autothermal/allothermal operation of the catalytic reformer. The development of this preliminary process design is based on available literature data and relevant experimental studies of the main individual sub-processes. Planned experimental trials of the proposed integrated concept will shed more light in selected process aspects and will feed with confidence the simulation results.
2 Materials and methods
2.1 Concept description
2.1.1 Feedstock selection and handling
Thanks to the DFBG technology, the process can be driven feedstock-flexible using a broad and variable portfolio of biogenic residues, which may be carbon sources of lower quality compared to the sugar, starch, and oil plants used for conventional liquid biofuels, but do not come in conflict with food production and tend to avoid land use restrictions. Using biogenic residues also has the advantage of being in line with the EU’s biofuel policy documented in the RED II directive, mentioning the promotion of residue-based biofuels (or so-called advanced biofuels). Within this study, an extended feedstock screening around Europe was performed and the most promising types of feedstock from each residual biomass category involving agricultural residues (prunings, straw), forestry residues (logging, bark), and wood industry residues (sawdust) as well as biogenic wastes from airports/ports or other “waste-productive” fields were selected. The selection was also based on the pretreatment requirements of each feedstock in order to optimize the process performance. These pretreatment requirements are more intense in feedstock exhibiting high contaminant concentrations, low energy densities, or low ash melting temperatures [22].
In general, each feedstock involvement should be assessed in terms of gasification requirement fulfillment as well as supply chain economic optimization, and subsequently, the appropriate pretreatment pathway should be applied including from the mildest (e.g., drying and chipping) to more energy and cost-intensive measures (e.g., torrefaction and pelletizing). It has to be noticed that the pretreatment requirements for the selected DFBG technology are expected to be rather limited in commercial scale, and therefore, extended costs related to them can be avoided [23].
2.1.2 Dual Fluidized Bed Gasification (DFBG) and gas cleaning
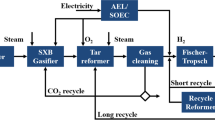
The conversion of the biomass feedstock into syngas is carried out with the DFBG technology. The DFBG system consists of two interconnected CFB (Circulating Fluidized Bed) reactors, the gasifier where gasification takes place and the oxidizer where partial combustion of the char or supplementary fuel combustion takes place in order to secure the heat requirements of the gasifier. In particular, the produced char, other residues (i.e., ash), and part of the bed material are transported to the combustor where they react with the oxidizing medium to produce heat. The (hotter) bed material returns to the gasifier, serving as the heating medium for the endothermic pyrolysis and steam gasification reactions, leading to higher carbon conversion rate and thermal efficiency. Raw syngas of moderate heating value and relatively low tar levels is produced filtered at gasifier exit temperature and subsequently is catalytically reformed. The reformer is heated by partial combustion with oxygen or air, and in addition, the reforming reactions consume steam and/or CO2. The primary function of the catalytic reformer may be to convert tars and hydrocarbon gases to H2 and CO, but it can also be modified to attain several targets relating to the syngas purification requirements for the subsequent fermentation process. Depending on the gas cleaning needs, different catalyst loadings and reactor design can be applied. For example, HCN contents can be reduced to 1–10 ppm by using calcium-based bed materials in the gasifier followed by a reformer that is also active for NH3 decomposition. Beyond that, depending on the purity level target, additional scrubbers and adsorbents can be implemented for the efficient removal of other syngas contaminants (e.g., H2S, HCl, and COS) prior to the fermentation unit [23]. A typical layout of a DFBG configuration that contains the filter and the catalytic reformer at the exit of the gasifier as well as an indicative gas cleaning section is presented in Fig. 2. As when this study was conducted, it was not clear yet which of the contaminants are harmful for the fermentation, and thus, a complete gas cleaning chain is considered. A future, more advanced study of the concept may exclude some of these cleaning steps as unnecessary.
DFBG typical scheme (CFB gasifier–CFB oxidizer) accompanied by the catalytic reformer and a mild gas cleaning section. Raw syngas is produced, filtered at gasifier exit temperature and catalytically reformed for tar conversion in the presence of steam and/or CO2. Then, removal of contaminants (e.g., H2S) prior fermentation is applied. Preheating of air and steam is performed via the hot outlet gas streams
2.1.3 Syngas fermentation
In the first step of the biological part of the process, syngas is converted into acetate under anaerobic conditions. Several anaerobic bacteria (Clostridium, Acetobacterium, and Eubacterium) have shown their ability to ferment single carbon gases such as CO and CO2 plus H2 into chemicals, usually acetate, through the acetyl-CoA pathway. These bacteria are named acetogens. The acetyl-CoA pathway (Wood-Ljungdahl pathway) can utilize both CO and H2 as a source of electrons and CO and CO2 as a source of carbon [24].
Two critical factors, which highly influence the fermentation kinetics and consequently the acetate productivity, are the gas solubility and the ratios of CO2/CO/H2; especially CO and H2 present low solubility in water. By recirculating the off-gas back to the fermenter, the unconverted syngas components can be recovered and recycled. At the same time, the broth containing the produced acetate in low concentration is extracted in a continuous way, and the liquid volume is kept constant by adding fresh culture medium. Increasing the pressure improves the gas solubility and consequently the acetate production yield. A cell recycling system (hollow fiber membrane) is also required to retain the cells while extracting the liquid effluent from the fermenter.
2.1.4 Acetate fermentation
The second fermentation step refers to the production of TAGs through an aerobic fermentation process. The production of lipids from acetate has been described in different microbial species. So far, the most efficient microorganisms in carrying out this conversion are the so-called oleaginous yeasts, such as Yarrowia lipolytica and Cutaneotrichosporon oleaginosus. In order to obtain strains that exhibit high lipid concentration, yield, and acetate conversion, a metabolic engineering strategy of Y. lipolytica can be adopted. The produced intracellular microbial oil mainly consists of fatty acids like oleate, stearate, and palmitate [25].
During the continuous acetate fermentation process, the dilute acetate effluent stream from the syngas fermentation enters the aerobic fermenter, where the targeted TAGs are produced in the presence of oxygen, additional nutrients, salts, and the oleaginous yeast (Y. lipolytica). A cell recycle system (hollow fiber membrane) can be installed to recirculate the cellular biomass in the bioreactor while extracting the effluent. During the continuous feed of the diluted acetate into the reactor, metabolic reactions take place and lipids are formed as intracellular products. At the same time, a gaseous CO2-rich stream is formed and leaves the reactor from the top. Depending on the oxygen content of this stream, the resulting CO2 can be partially recycled back to the inlet of the gas fermenter or cover other CO2 needs of the plant (e.g., gasifier and reformer). The complete double-stage fermentation scheme, containing both the anaerobic syngas fermentation and the aerobic acetate fermentation, is presented in Fig. 3.
The double-stage fermentation scheme. Fresh syngas is converted into acetate under anaerobic conditions (gas fermenter) in the presence of acetogens. A cell recycling system is required to retain the cells while extracting the continuous liquid effluent from the fermenter. During the continuous feed of the diluted acetate into the liquid aerobic fermenter, lipids are formed as intracellular products via oleaginous yeast
2.1.5 Triglyceride (TAG) purification
Lipid extraction from the oleaginous yeasts is an important step before hydrotreatment and the final liquid biofuel formation. As oleaginous yeasts store lipids in intracellular form, an extraction technique is required to obtain TAGs. Cell disruption and lipid extraction steps are critical for large-scale biofuel production in terms of cost adequacy. Mechanical processes generally provide high product recovery yields with good management and scalability, but they are energy intensive. Steam explosion is an innovative method with reduced environmental impact, lower costs, and energy demand, compared to other techniques that are widely used. In steam explosion, raw material is exposed to steam at 180–240 °C for several minutes, and then, it is subjected to depressurization under ambient conditions. This generates an explosion that causes cell-wall disruption [20]. In a context in which heat flows are available as downstream of other processes and so steam could be generated at low cost, steam explosion should be considered as a potential technology for the recovery of intracellular products reaching high yields. The process converts thermal energy into mechanical energy, and the shear forces caused by the expansion of water vapor lead to the disruption of cell wall. Centrifugation could then be applied for the separation of lipids from the exploded material. Using centrifugation, a lipid fraction can be efficiently separated at least from water. Lipids are partially separated as a top layer and partially form an oil-in-water emulsion. After this, if purification of a single lipid category is needed, the oil fraction could be further processed in a membrane plant. Membrane separation is well suited for such purposes and is, therefore, a promising option for downstream processing. The proposed microbial oil purification and recovery process is presented in Fig. 4.
2.1.6 Triglyceride hydrotreatment
The final stage of the value chain includes the upgrading of microbial oil into drop-in aviation and marine biofuel through TAG hydrotreatment. The catalytic hydrotreatment process is generally divided into three main steps.
The first two steps refer to hydrogenation and subsequent hydrodeoxygenation and decarboxylation. In particular, unsaturated fatty acids and triglycerides are converted into saturated fatty acids by catalytic hydrogenation. Then, the saturated fatty acids are converted to straight-chain alkanes by deoxygenation (decarboxylation/decarbonylation), co-producing propane, water, CO, and CO2. The desired products from these two steps are mainly straight-chain paraffins containing no oxygen. In the last step, the deoxygenated straight-chain paraffins are selectively hydrocracked or isomerized yielding highly branched alkanes. This step is essential to improve the cold properties of the product. The common catalysts for this step are Pt, Ni, or other metals based on Al2O3 or zeolite molecular sieves. The resulted organic product is a mixture of straight and branched CnH2n+2 that can be suitably used as drop-in liquid fuel. The hydrotreatment unit is presented in Fig. 5.
TAGs to drop-in liquid fuels via hydrotreatment. TAGs are converted to saturated fatty acids by catalytic hydrogenation. Then, the fatty acids are converted to straight-chain alkanes by deoxygenation co-producing propane, water, CO, and CO2 (purge gas). In the last step, the deoxygenated paraffins are selectively hydrocracked or isomerized to improve the cold properties of the final product
2.2 Model description
The proposed BtL value chain could be separated in three main parts: the thermochemical part, the biological part, and the thermocatalytic part. The thermochemical part refers to the DFBG unit as well as the following syngas cleaning and conditioning that will secure the smooth transition to the biological part, which contains the double-stage syngas fermentation scheme. The thermocatalytic part refers to the TAG hydrotreatment unit and the fractionation in order for the final liquid fuels to emerge. Two additional units, which could potentially interact with the BtL value chain and determine the plant operation mode, were investigated. The first one is a RES-based water electrolysis unit that will be able to secure the hydrogen and pure oxygen requirements of the plant, while the second one is a Heat Recovery Steam Generator (HRSG) unit for the efficient heat recovery and steam generation from the thermochemical part. The described concept is illustrated in a block form in Fig. 6.
The process model was developed in the commercial software Aspen Plus™. The simulations were performed at full scale (200 MWth), and crushed bark was selected as feedstock. The main specifications of the feedstock used in the process simulations are presented in Table 1.
An important aspect for the correct operation and integration of the individual units in the simulation environment is the definition of the appropriate property methods for the efficient estimation of the thermophysical properties of the components and streams. The IDEAL property method was selected for the thermochemical part, while the Predictive Soave–Redlich–Kwong (PSRK) method was used for the biological and thermocatalytic parts. For the development of the HRSG model, IAPWS-95 property method was used for the water side and IDEAL property method for the flue gases side. A heat to power conversion efficiency equal to 45% is applied in case of a steam turbine (ST) coupling with the HRSG. The water electrolysis unit was modelled in a simplified way that includes the mass balance of the water electrolysis reaction (2 H2O → 2 H2 + O2) as well as an average required electricity demand equal to 180 MJ/kg of produced hydrogen that reflects to an electrolyzer efficiency of 70–80% [26].
2.2.1 Model development of the thermochemical part
The thermochemical part of the process consists of the DFBG unit and the catalytic reformer as well as the gas cleaning steps required for a subsequent efficient syngas fermentation. Equilibrium models have been used for the implementation of the gasification and the reforming reactions, while for kinetically and hydrodynamically controlled phenomena that cannot be predicted with the rules of chemical equilibrium (e.g., unconverted solid carbon and formation of gaseous hydrocarbons), fitting of selected parameters with experimental data was followed. The selected parameters and the fitting of the model are based on previous steam DFBG pilot tests with crushed bark [23, 27].
For the DFBG unit, a gasifier operating with 100% steam at 780 °C and an oxidizer operating with air at 880 °C were considered. Char is the main fuel source of the oxidizer, but also off-gases from other sub-units of the integrated BtL scheme can be used as supplementary fuel. Filtration of syngas takes place at gasifier outlet temperature, while the filter ashes are also directed to the oxidizer. A mixture of sand and calcium carbonate was used to represent the bed material. The governing reactions in the gasifier are the steam gasification reaction, the water–gas shift (WGS) reaction, the Boudouard reaction, the homogeneous gas reactions that form hydrocarbons, and the partial combustion reactions. The main input and process parameters for the DFBG unit are gathered in Table 2 (left side).
For the catalytic reformer, there are two design options (see Sect. 3.4). On the one hand, there is the autothermal reforming (ATR) where the reformer operates under autothermal conditions with the addition of oxygen or air as oxidation media and steam or carbon dioxide as reforming agent, while on the other hand there is the steam methane reformer (SMR) that is heated externally with the assistance of an air-heated combustor where purge gases are burnt in order to cover the energy requirements of the steam reforming reactions. The main input and process parameters for the reforming unit are shown on the right side of Table 2.
2.2.2 Model development of the biological part
The core of the biological part of the process model is the two fermenters where syngas and acetate fermentation take place, respectively. Both fermenters were modelled as stoichiometric reactors (RStoic), with specific reaction stoichiometry and fixed conversions.
For the syngas fermentation stage, Moorella thermoacetica was used as the reference acetogenic bacterium, and thus, an anaerobic reactor operating at 55 °C was considered, since the optimal temperature range for these strains is 55–60 °C [28]. The operating pressure of the reactor was considered to be 5 bar in order to achieve higher solubility of the reacting gases in the liquid phase. Syngas derived from the reforming and purification units (plus the recycle gas) enters the fermenter where syngas is mainly converted into acetate. The only by-product considered is ethanol, yet with very low production. The 97.5% of the bioreactor’s off-gas, which mainly consists of the unreacted syngas and the produced CO2 is recycled back to the fermenter. Equations (1–4) were selected as the key reactions occurring during syngas fermentation.
For modelling purposes, the acetate which is the real product of gas fermentation is represented by acetic acid. Additionally, it was considered that the H2 and CO utilization of the syngas inlet stream (fresh plus recycled gas) by the bacteria in each pass is 43% and 61%, respectively. The selected values were based on literature data [24, 29]. The main input and process parameters for the syngas fermentation unit are presented in Table 3.
The aerobic fermenter, where the acetate fermentation takes place, operates at 30 °C under atmospheric pressure. The acetate extracted by the first fermenter reacts with oxygen for the production of TAGs and non-lipid biomass. Dilinoleylpalmitin (C55H98O6) and tripalmitin (C51H98O6) were selected as the two representative TAGs produced during the lipid accumulation phase. Equations (5) and (6), which represent the intracellular lipid formation by the yeast, were based on previous studies [19, 30]. The conversion rates of the reactions were selected in such a way that the subsequent decomposition of TAGs simulates the targeted fatty acid distribution, as reported in the literature [19]. The main input and process parameters for the acetate fermentation unit are presented on the right side of Table 3.
In order to extract the lipids from the yeast cells, the fermentation broth containing the cells undergoes some lipid purification steps. The estimated energy demand for conventional lipids purification techniques (i.e., bead milling, ultrasound, and microwave) for Y. lipolytica is in the range of 115–194 MJ/kg of extracted oil [31]. However, with the novel steam explosion-based technology that is proposed for the described value chain, the corresponding energy demands are expected to be remarkably lower.
2.2.3 Model development of the thermocatalytic part
The thermocatalytic part of the process refers to the hydrotreatment of the produced TAGs to obtain the willing liquid fuels [32]. Initially, the decomposition of the two representative triglycerides (C51H98O6 and C55H98O6) is taken into account to simulate the fatty acid distribution that contains palmitic acid (C16H32O2), oleic acid (C18H34O2), stearic acid (C18H36O2), and linoleic acid (C18H32O2). Total conversion of the triglycerides into acids and propane (C3H8) is assumed. Then, an equilibrium reactor is employed for the simulation of the hydrotreating reactor involving hydrogenation, deoxygenation, and reduction reactions. The product yield is determined by the equilibrium state of the occurred reactions in it [33, 34]. The formed light gases, mainly containing propane, are sent back to the DFBG unit to be used as supplementary fuel for the oxidizer. The main process parameters for the hydrotreatment reactor are presented in Table 4.
The hydrotreated microbial oil is separated from the gas phase (unreacted hydrogen, light hydrocarbons, and produced CO/CO2) and sent to a distillation column in order to retrieve the targeted drop-in biofuels. The last part of the process (i.e., isomerization and fractionation) was not modeled in detail, and the produced alkanes were considered as the final product in this analysis.
2.2.4 Process configurations and examined scenarios
Apart from the nutrients and microorganisms for the biological step, the process has also heat, electricity, steam, air/oxygen, and hydrogen requirements. The overall plant efficiency, its operation mode, and its full spectrum of capabilities are highly dependent on the effective securement and integration of all these parameters in the BtL scheme. The oxygen-based components (i.e., autothermal reformer and aerobic fermenter) have been identified as key aspects concerning the overall process character and functionality.
An oxy-blown autothermal reformer covers its heat requirements for the reforming reactions with partial oxidation of syngas. The high-quality syngas along with the relatively low content of light hydrocarbons derived from the DFBG unit makes the energy degradation of the gas that takes place with its partial oxidation affordable, since the gas that leaves the reformer is a nitrogen-free gas which still maintains a high energetic content that can be used entirely for the liquid fuel production. An ATR can be operated also with air instead of oxygen, but the extended presence of nitrogen in the reformed gas may cause problems in the biological part and its handling in general. On the other hand, an allothermal steam reformer can be operated with external heating from a combustor that utilizes air and not necessarily oxygen. The impact of WGS reaction in this case, due to the excess steam in the reformer and absence of oxidation, may be stronger creating a local energetic upgrade of the reformed syngas, but the external heat requirements are larger and remarkable part of the syngas should be used for combustion instead of fermentation. The latter is rather inefficient from the overall BtL point of view. The two different operations of the catalytic reformer are illustrated in Fig. 7. The other procedure that has oxygen requirements is the aerobic fermentation of acetate. The process can be driven as oxy-fermentation or air fermentation (Fig. 7). The difference is that fermentation with pure oxygen will lead to the formation of a quite pure CO2 stream in the fermenter outlet and consequently strengthen the carbon capture and storage or utilization (CCS and CCU) ability of the plant.
There are also hydrogen requirements in the process chain and in particular in the hydrotreatment unit, but they are expected to be low. The disproportionately lower hydrogen requirements in comparison with the oxygen requirements of the plant mean that potential oxygen securement via water electrolysis would be accompanied with excess of pure hydrogen. The establishment of an electrolysis unit to cover primarily oxygen demands instead of hydrogen seems rather unreasonable and inefficient for the plant. However, in this way, two valuable off-gases (i.e., pure CO2 from oxy-fermentation of acetate and pure H2 from the water electrolysis) are produced that are capable of completely upgrading the plant either via their reutilization in the biological part (i.e., gas fermentation) or via other catalytic routes of fuel synthesis. If there is no electrolysis implementation, then the required hydrogen for the hydrotreatment procedure can be obtained from syngas via PSA. Finally, the steam requirements of the plant can be covered with a HRSG section that utilizes the waste heat from the DFBG unit and produces steam. A steam turbine (ST) system for power production could be applied also in the end of the HRSG unit in case of excess heat in high temperatures. After taking all the above mentioned points into consideration, the following scenarios have been developed and simulated (Fig. 8).
1st scenario
In this case study, the establishment of an electrolysis unit is assumed for hydrogen production. This means that pure oxygen can be available also for the autothermal reformer as well as for the aerobic fermentation of acetate. The produced syngas is utilized entirely for the final fuel production, meaning that the efficiency of the BtL plant is high and it can be further enhanced from the emerging pure streams of H2 and CO2. Of course, since water electrolysis is a rather expensive choice, it can be considered only in the case of low-cost RES electricity. Otherwise, this scenario refers to a scheme with high electricity demands.
2nd scenario
In this case study, electrolysis unit is not involved. Pure industrial oxygen can be purchased externally for oxy-autothermal reforming or oxy-fermentation of acetate. Otherwise, autothermal reforming with limited air can be applied and, respectively, air fermentation that will lead to a N2/CO2 mixture in the fermenter gas outlet. The chemical energy of the produced syngas is utilized once again almost entirely for the biofuel production, apart from a small portion of hydrogen that is extracted via PSA from the recirculating off-gases of the anaerobic fermenter in order to secure the hydrotreatment hydrogen requirements.
3rd scenario
In this case study, no use of pure oxygen is considered neither in the reformer nor in the aerobic fermenter. The technology of allothermal steam reforming is applied, which imposes an assisting combustor that utilizes air and part of the syngas to provide the appropriate heat to the reformer. This is achieved by extracting a portion of the recirculating off-gases of the anaerobic fermenter and sending them to the SMR combustor. The hydrogen requirements are covered again by the same stream via PSA, and therefore, the syngas “losses” in terms of fuel production are expected remarkable and the BtL plant’s efficiency low. However, the flue gases stemming from the SMR combustor in this case are an additional hot source that can be thermally exploited. The primary objective is the steam generation for the reforming, but its further thermal utilization could boost a potential power generation of the plant with the addition of a ST.
The examined scenarios are presented in Table 5 in a more concise form.
3 Results and discussion
The heat and mass balances are performed for each case study, and indicators for the overall plant performance are assessed. The following critical factors are introduced:
-
Total carbon utilization factor is the fraction of carbon in initial feedstock that is converted to the final fuels, hereinafter referred to as carbon utilization (CU) and calculated as
$$\mathrm{CU}=\frac{{\dot m}_{\mathrm c,\mathrm{liquid}\;\mathrm{fuel}}}{{\dot m}_{\mathrm c,\mathrm{crushed}\;\mathrm{bark}}}$$(7) -
Liquid fuel to feed energy ratio is the fraction of the chemical energy in the initial feedstock that is transferred to the final fuels, hereinafter referred to as energetic fuel efficiency (EFE) and calculated as
$$\mathrm{EFE}=\frac{{\dot m}_{\mathrm{liquid}\;\mathrm{fuel}}\cdot{\mathrm{LHV}}_{\mathrm{liquid}\;\mathrm{fuel}}(E_{\mathrm{liquid}\;\mathrm{fuel}})}{{\dot m}_{\mathrm{crushed}\;\mathrm{bark}}\cdot{\mathrm{LHV}}_{\mathrm{crushed}\;\mathrm{bark}}(E_{\mathrm{feed}})}$$(8) -
Liquid fuel mass yield is the mass flow ratio of liquid fuels to solid feedstock (crushed bark) and calculated as
$${\mathrm{yield}}_{\mathrm{liquid\;fuel}}= \frac{{\dot{m}}_{\mathrm{liquid\;fuel}} }{{\dot{m}}_{\mathrm{crushed\;bark}}}$$(9) -
Energetic efficiency (ηE) describes the overall performance of the system considering any involved electrical power generation/consumption as well and can be calculated as
$$\eta_{\mathrm E}=\frac{{\mathrm E}_{\mathrm l\mathrm i\mathrm q\mathrm u\mathrm i\mathrm d\;\mathrm f\mathrm u\mathrm e\mathrm l}+{\mathrm{Pel}}_{\mathrm{produced}}}{{\mathrm E}_{\mathrm{feed}}+{\mathrm{Pel}}_{\mathrm{consumed}}}$$(10) -
Exergetic efficiency (ηEx) is used for a more holistic assessment of the examined schemes in terms of performance and energy quality. It is expressed as the ratio of total exergy output to total exergy input:
$$\eta_{\mathrm{Ex}}=\frac{{\mathrm{Ex}}_{\mathrm{liquid}\;\mathrm{fuel}}+{\mathrm{Pel}}_{\mathrm{produced}}}{{\mathrm{Ex}}_{\mathrm{feed}}+{\mathrm{Pel}}_{\mathrm{consumed}}}$$(11)
where Exliquid fuel is the chemical exergy of the final liquid fuels calculated according to the following equation [35]:
where N is the molar flow in kmol/s and xi the molar fraction of each component I and R is the gas constant. The term Exfeed is the chemical exergy of the solid feedstock (crushed bark) calculated according to [36] (the corresponding physical exergies are assumed equal to zero and thus are neglected). The standard chemical exergy εo,I (kJ/kmol) for each component i that exists in the final fuel stream at reference conditions (T0 = 298.15 K, p0 = 1.013 bar) is obtained from [37]. The standard chemical exergy of any component that is not apparently given from [37] was calculated according to [38] by means of the contribution method of simple chemical groups.
3.1 Carbon balance
Cold gas efficiency (CGE) is the fraction of the chemical energy in the initial feedstock that is transferred to syngas in the gasifier. This is measured to be 85% after the hot filtration of syngas, while it drops to 80% after the ATR since partial oxidation of syngas takes place at the auto-reforming procedure. The acetate concentration in the effluent stream (Broth 1) of the gas fermenter is around 30 g/L, while the TAGs are obtained in the effluent stream (Broth 2) of the aerobic fermenter in a concentration of 100 g/L as a result of the cell recycle system applied in the fermenter. According to the model, the decomposition of triglycerides leads to the formation of palmitic acid, oleic acid, linoleic acid, and stearic acid as well as propane. The dominant species in the final liquid products are C16 and C18 alkanes. The light gases leaving the hydrotreatment reactor consisting of propane, carbon dioxide, and any remaining hydrogen are directed to the oxidizer of the DFBG unit in order to boost the gas production efficiency. The main stream results for the 1st case study are attached in the Supplementary Material (SI). The obtained carbon balances for all three simulated scenarios are presented in Fig. 9.
In the 1st case study, a water electrolysis unit feeds the BtL plant with oxygen and hydrogen, while the HRSG unit exploits the thermal load of the hot gases to cover the process steam requirements (i.e., gasification, reforming, and lipid purification). In case all the oxygen requirements are covered from the electrolyzer, excess of hydrogen and a quite pure stream of CO2 are obtained along with the final liquid products. The CU of the BtL plant, meaning the carbon content of the final liquid fuels, has been calculated equal to 26.44%. A high carbon content (43.37%) is found among the outlet gas streams of the biological part, mainly through the CO2-rich stream that leaves the aerobic fermenter (36.94%) and secondly through the purge gas (bleed stream) extracted from the recirculation gases of the anaerobic fermenter (6.43%). Further utilization of this CO2-rich stream along with the hydrogen excess sourcing from the electrolyzer can remarkably increase the CU of the BtL plant and reach values greater than 37%. The rest carbon “expenses” of the process are the flue gases leaving the oxidizer (24.23%) and the carbon utilized for the cellular biomass formation in both fermenters (5.22%) as well as the low organic content of wastewaters (0.74%).
In the absence of an electrolysis unit in the context of the 2nd case study, autothermal reforming is performed with the assistance of externally purchased industrial oxygen, while the hydrogen requirements of the hydrotreatment unit are covered via PSA with extraction from the off-gases of the anaerobic fermenter. Industrial oxygen could be purchased also for the aerobic fermentation in order to achieve high CO2 purity in the off-gases, but this would lead to remarkably higher operational costs. The internal H2 securement, which can be considered as a small syngas “loss” for the BtL plant, affects the efficiency of the syngas fermentation and is translated to slightly lower syngas conversion to acetate and consequently lower liquid fuel production and carbon conversion to biofuels. In particular, the obtained CU for the 2nd scenario is measured at 25.19%. Since the plant’s hydrogen requirements are not extended, PSA technology might be preferable in terms of pure hydrogen generation in comparison with the establishment of a whole electrolysis unit.
In the integrated concept of the 3rd case study, there is not any pure oxygen involvement since the reforming (i.e., allothermal) as well as the aerobic fermentation procedures are performed with air utilization. The required heat input for the allothermal reformer is secured with partial gas extraction from the recirculating gases of the anaerobic fermenter. The same goes for hydrogen, which is extracted via PSA from the same stream. The reformer operates at 900 °C and the assisting combustor at 950 °C. The obtained CU for this case study is equal to 22.86%. A remarkable carbon content (20.52%) is transferred to the supporting combustor of SMR and ends up as an additional CO2 emission from the thermochemical part. Therefore, in terms of carbon, an increase in the carbon content that is released from the thermochemical unit is observed due to the presence of two flue gas sources now (i.e., DFBG oxidizer and SMR combustor). The allothermal operation of the reformer seems to have a notable negative impact on the overall performance of the BtL plant, since a non-negligible amount of syngas ends up as flue gas in the SMR combustor instead of acetate and subsequently liquid fuel.
The CU factors of the investigated scenarios are relevant with the calculated liquid fuels mass yields that are presented in Table 6. The highest liquid fuel yields are obtained for the 1st scenario where the supply of pure hydrogen and oxygen can potentially boost the liquid fuel production, while the 2nd scenario achieves competitive numbers without the energy consuming electrolysis addition. The 3rd scenario, due to the remarkable syngas losses in the allothermal reforming, presents the lowest fuel yields.
3.2 Energy balance
The obtained energy balances for all three simulated scenarios are presented in Fig. 10. The heating value of the obtained raw mixture of jet/diesel paraffins, which is considered as the final product of the present simulation study, was measured in the range of 44–45 MJ/kg (LHV-based) in every case.
The EFE for the 1st scenario is measured at 37%. Heat recovery for steam generation and the oxidizer’s air preheating is performed from the hot streams of the DFBG unit (i.e., syngas and flue gases) (15.35%). The main energy losses are observed in the biological synthesis of TAGs via double-stage fermentation (42.25%), while the losses from the syngas cooling to the operating temperatures of the biological part (7.5%) and the hydrotreatment unit (1.5%) are lower. The electrolysis power consumptions only for the hydrogen requirements of the hydrotreatment unit have been considered as well. The redirection and the reutilization of the quite pure CO2 stream sourcing from potential oxy-fermentation of acetate will enhance the CU as well as the EFE of the plant in a remarkable way (i.e., CU > 37% and EFE > 45%). However, a prerequisite of this strategy is the extended electrolysis operation (i.e., higher power consumptions) for pure oxygen supply.
Within the 2nd scenario, the impact of the internal hydrogen extraction in the energy balance of the process can be observed. An EFE equal to 35% is obtained. The lower acetate production leads to lower energy content of the produced TAGs. The observed decrease in CU and EFE of the BtL plant can be characterized as affordable. The involvement of the PSA technology and the internal securement of the limited hydrogen needs of the process seem to have a controllable effect on the process performance. The avoidance of an electrolysis unit would drastically reduce the capital and operational costs of the plant. However, the main shortcoming of a scheme without the capability of pure oxygen is that the off-gases of the aerobic fermenter will be a mixture of CO2 and N2, and therefore, their carbon reutilization will be difficult.
The decreased fuel production of the BtL plant in the 3rd scenario is also reflected in the EFE that is calculated at 31.5%. The purge gas that is transferred to the reforming combustor contains a remarkable energy content (25%) that does not participate in the CU or EFE enhancement. However, the flue gases of the SMR combustor are a hot stream that updates the heat recovery and steam generation capability of the plant. For this reason, this is the only case study that the addition of a steam turbine could make sense in terms of power production (> 10% of thermal input). It has to be mentioned that this is the only case that seems to have the potential to offer power independence of the plant via a polygeneration scheme of power, heat, and fuel production.
Aiming to obtain a performance overview of the investigated concept in terms of energy quantity and quality distribution in the examined scenarios, the corresponding energetic and exergetic efficiencies have been calculated (Table 7).
The 3rd scenario may present the higher overall energetic/exergetic efficiencies (~ 40%) due to its polygeneration scheme, but on the other hand, it is the scenario with the lowest performance indicators concerning liquid fuel production (EFE and CU). On the contrary, the 1st and the 2nd scenarios come up with lower and similar overall energy efficiency (~ 35%) since power production is not envisaged in these cases, but present higher liquid fuel productivity factors (EFE and CU). Another important aspect that has already been mentioned and is proven from the performed energetic/exergetic analysis is the inefficiency of potential extended electrolysis to cover pure oxygen demand further than the hydrogen requirements of the hydrotreatment unit. In other words, the higher the electrolysis involvement in the 1st scenario, the lower the overall system performance in terms of energy quality (~ 30%) despite the increased fuel productivity.
A short description of the three scenarios along with the identified pros and cons and the calculated KPIs are included in Table 8.
Taking for granted that the priority of a BtL concept is the high liquid fuel productivity, the competitiveness of the 2nd scenario in all aspects by avoiding the establishment of an electrolysis unit turns the combination of internal H2 extraction via PSA (for hydrotreatment) and the potential limited purchase of industrial O2 (for reforming) as an attractive possibility in terms of cost and performance. Moreover, the external purchase of O2 is rather an operational option rather than an inherent drawback of the 2nd scenario since air reforming can be functional as well despite the unwilling N2 presence. A thorough techno-economic analysis, which is expected to be a follow-up work of the present study, will serve the optimization of the proposed concept and along with dedicated lab and pilot tests will verify its potential.
3.3 Comparison with other certified biofuel pathways
Within this section, it is aimed the comparative assessment of the proposed concept against other certified biofuel production pathways in terms of liquid fuel productivity. Thus, the focus is given on the corresponding performance indicators such as EFE, CU, and liquid fuel yield. In particular, the established technologies of HEFA/HVO, the Fischer–Tropsch Synthesis (FT), and the Alcohol-to-Jet (ATJ) are selected for comparison.
HEFA/HVO fuels are produced by the hydrogenation of vegetable oils, animal fats, or waste oils. The HEFA technology is currently the most mature one, with HEFA fuels being the only alternative already used commercially (TRL 8–9). However, it should be mentioned that the feedstock for HEFA is usually costly, and it often raises the question of food vs. fuel, as cultivating bigger amounts of its feedstock would severely change land use. Fischer–Tropsch liquids are produced through bio-based gasification with FT synthesis using lignocellulosic biomass as feedstock. This technology is now just approaching commercialization (TRL 7–8) and has received growing attention since it offers clean and potentially carbon neutral fuels directly usable in the transportation sector. Alcohol-to-Jet is a pathway that produces fuels from sugary, starchy, and lignocellulosic biomass, such as sugarcane, corn grain, and switchgrass, via fermentation of sugars to ethanol or other alcohols. This technology is still on a demonstration level (TRL 6–7), partly because of the alcohol production cost.
An estimation of the performance range of the mentioned technologies has been carried out by utilizing data from previous related studies reported in the literature [10, 39,40,41,42] and is contained in Table 9 and Fig. 11 along with the corresponding performance indicators extracted by the present study.
The proposed BtL pathway is able to achieve competitive values in terms of liquid fuel productivity (EFE, CU, and yield) in comparison with already certified technologies that exploit similar feedstock (i.e., FTS and ATJ). However, the favorable position of the suggested concept lies on its ability to reach decent efficiency levels by avoiding the strict specifications of FTS that usually require costly and energy-demanding equipment or the several unit operations (pretreatment, hydrolysis, fermentation, dehydration, and oligomerization) of the ATJ route that raise the total production costs [43].
HEFA/HVO technology, as expected, presents high efficiency numbers in the selected performance indicators due to the more straightforward chemical structure of the involved feedstock (i.e., oils) compared to the other routes. However, it should not be ignored that the HEFA/HVO feedstock is significantly more expensive than the feedstock used in the other technologies (i.e., lignocellulose and energy crops).
4 Conclusions
Within this study, a basic definition of a novel integrated thermochemical-biochemical BtL process has been performed. The extended feedstock flexibility and the limited gas cleaning requirements as well as the low-pressure and mild operating temperatures of the biological part turn the proposed pathway into a promising BtL technology. The standalone sub-technologies may have already been tested and involved in medium/large-scale applications, but the major technical challenges of the proposed concept are the efficient coupling of the thermochemical part with the biological part as well as the avoidance of expensive purification techniques for microbial oil recovery. An overall process model was developed, and process simulations were performed at full scale (200 MWth) for the BtL plant with crushed bark as feedstock. Design parameters like PSA/water electrolysis, oxy-/air-acetate fermentation, or autothermal/allothermal reformer operation were investigated, and their compatibility with the system was assessed via dedicated operational scenarios. The heat and mass balances for the examined configurations were solved and evaluated via overall performance indicators (i.e., CU and EFE).
Values between 22 and 27% and between 31 and 37% were obtained for the CU and EFE, respectively. Reutilization of the CO2 stream deriving from the oxy-fermentation of acetate could enhance the CU and EFE of the plant reaching values of 37% and 45%, respectively. The major carbon and energy losses were observed in the biological part. The optimization of the double-stage syngas fermentation (recirculation rates, gas solubility, optimum parameters, etc.) is expected to reduce these losses and enhance the overall performance of the plant. The limited H2 requirements of the plant cannot probably justify the presence of such an energy-consuming unit like the electrolyzer while internal H2 extraction via PSA seems the most efficient option in terms of cost-performance balance. The scheme with the allothermal SMR seems inappropriate for this concept, since notable decrease in the performance indicators of the BtL plant is observed. A primary placement of the suggested concept among other certified biofuel pathways (i.e., HEFA/HVO, FTS, and ATJ) was attempted. Competitive performance indicators were achieved compared to technologies that refer to similar feedstock. Of course, the concept of the present study is subject to optimization and a subsequent techno-economic assessment is expected to properly define its encouraging potential.
The main objective of the present study is to define the key process specifications and evaluate the potential of the proposed concept compared to other competitive technologies. The investigated scenarios and the obtained primary conclusions can act as a benchmark for the further development and optimization of the integrated concept. The more in-depth techno-economic assessment of multiple aspects (water electrolysis/PSA, air/oxy blown fermentation, CO2 exploitation, etc.) is a necessary follow-up work that is due to be performed after the conduction of further lab and pilot tests concerning the feasibility and sustainability of the examined process configurations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
An initial presentation of the investigated novel concept, without the parametric analysis and impact assessment of the present study, has been performed within ECOS 2021 (34th International Conference on Efficiency, Cost, Optimization, Simulation, and Environmental Impact of Energy Systems) [17], while the feedstock selection was based on a dedicated work presented within GCGW 21 (9th Global Conference on Global Warming) [22].
References
IMO (2015) Third IMO GHG study 2014 executive summary and final report. https://www.imo.org/. Accessed 9 Jan 2023
IMO (2018) Initial IMO Strategy on reduction of GHG emissions from ships. https://www.imo.org/. Accessed 9 Jan 2023
IATA (2021) Net-zero carbon emissions by 2050. https://www.iata.org/en/pressroom/2021-releases/2021-10-04-03/. Accessed 9 Jan 2023
IEA (2011) Technology roadmap: biofuels for transport. https://www.iea.org/. Accessed 9 Jan 2023
de Lasa H, Salaices E, Mazumder J, Lucky R (2011) Catalytic steam gasification of biomass: catalysts, thermodynamics and kinetics. Chem Rev 111:5404–5433. https://doi.org/10.1021/cr200024w
Sikarwar VS, Zhao M, Fennell PS et al (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248. https://doi.org/10.1016/j.pecs.2017.04.001
EASA Bio-based aviation fuels. https://www.easa.europa.eu/eaer/topics/sustainable-aviation-fuels/bio-based-aviation-fuels. Accessed 30 May 2022
IRENA (2021) Reaching zero with renewables: biojet fuels. Abu Dhabi. https://www.irena.org/publications/2021/Jul/Reaching-Zero-with-Renewables-Biojet-Fuels. Accessed 9 Jan 2023
Doliente SS, Narayan A, Tapia JFD, et al (2020) Bio-aviation fuel: a comprehensive review and analysis of the supply chain components. Front Energy Res 8: https://doi.org/10.3389/fenrg.2020.00110
Atsonios K, Kougioumtzis M-A, Panopoulos KD, Kakaras E (2015) Alternative thermochemical routes for aviation biofuels via alcohols synthesis: process modeling, techno-economic assessment and comparison. Appl Energy 138:346–366. https://doi.org/10.1016/j.apenergy.2014.10.056
Geleynse S, Brandt K, Garcia-Perez M et al (2018) The alcohol-to-jet conversion pathway for drop-in biofuels: techno-economic evaluation. Chemsuschem 11:3728–3741. https://doi.org/10.1002/cssc.201801690
Wormslev EC, Pedersen JL, Eriksen C, et al (2016) Sustainable jet fuel for aviation: Nordic perpectives on the use of advanced sustainable jet fuel for aviation. Nordic Council of Ministers. https://doi.org/10.6027/TN2016-538. Accessed Jan 2023
Renewable Energy Group. https://www.regi.com/. Accessed 30 May 2022
Neste. https://www.neste.com/. Accessed 30 May 2022
Hirani AH, Javed N, Asif M, et al (2018) A review on first- and second-generation biofuel productions. In: Biofuels: Greenhouse Gas Mitigation and Global Warming. Springer India, New Delhi, pp 141–154
BioSFerA project. https://biosfera-project.eu/. Accessed 4 Jul 2022
Detsios N, Maragoudaki L, Atsonios K, et al (2022) Aviation and maritime biofuels production via a combined thermochemical/biochemical pathway: a conceptual design and process simulation study. In: 34th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems (ECOS 2021). ECOS 2021 Program Organizers, Tokyo, Japan, pp 1246–1257
Pfeifer C, Koppatz S, Hofbauer H (2011) Steam gasification of various feedstocks at a dual fluidised bed gasifier: impacts of operation conditions and bed materials. Biomass Convers Biorefin 1:39–53. https://doi.org/10.1007/s13399-011-0007-1
Hu P, Chakraborty S, Kumar A et al (2016) Integrated bioprocess for conversion of gaseous substrates to liquids. Proc Natl Acad Sci 113:3773–3778. https://doi.org/10.1073/pnas.1516867113
Nurra C, Torras C, Clavero E et al (2014) Biorefinery concept in a microalgae pilot plant. Culturing, dynamic filtration and steam explosion fractionation. Bioresour Technol 163:136–142. https://doi.org/10.1016/j.biortech.2014.04.009
Lorente E, Farriol X, Salvadó J (2015) Steam explosion as a fractionation step in biofuel production from microalgae. Fuel Process Technol 131:93–98. https://doi.org/10.1016/j.fuproc.2014.11.009
Gavidou V, Detsios N, Atsonios K, Panagiotis G (2021) Screening of biogenic residues and setting up sustainable scenarios for commercial biorefineries around Europe. https://biosfera-project.eu/wp-content/uploads/2022/02/Conference-Paper_CERTH-at-GCGW-2021.pdf. Accessed 9 Jan 2023
Kurkela E, Kurkela M, Tuomi S, et al (2019) Efficient use of biomass residues for combined production of transport fuels and heat. https://doi.org/10.32040/2242-122X.2019.T347. Accessed 9 Jan 2023
Stoll IK, Boukis N, Sauer J (2019) Syngas fermentation at elevated pressure - experimental results. In: 27th European Biomass Conference and Exhibition. pp 1255–1261
Wei S, Jian X, Chen J et al (2017) Reconstruction of genome-scale metabolic model of Yarrowia lipolytica and its application in overproduction of triacylglycerol. Bioresour Bioprocess 4:51. https://doi.org/10.1186/s40643-017-0180-6
Shiva Kumar S, Himabindu V (2019) Hydrogen production by PEM water electrolysis – a review. Mater Sci Energy Technol 2:442–454. https://doi.org/10.1016/j.mset.2019.03.002
Hannula I, Kurkela E (2012) A parametric modelling study for pressurised steam/O2-blown fluidised-bed gasification of wood with catalytic reforming. Biomass Bioenergy 38:58–67. https://doi.org/10.1016/j.biombioe.2011.02.045
Drake HL, Daniel SL (2004) Physiology of the thermophilic acetogen Moorella thermoacetica. Res Microbiol 155:869–883. https://doi.org/10.1016/j.resmic.2004.10.002
Almeida Benalcázar E, Noorman H, Maciel Filho R, Posada JA (2020) Modeling ethanol production through gas fermentation: a biothermodynamics and mass transfer-based hybrid model for microbial growth in a large-scale bubble column bioreactor. Biotechnol Biofuels 13:59. https://doi.org/10.1186/s13068-020-01695-y
Xu J, Liu N, Qiao K, et al (2017) Application of metabolic controls for the maximization of lipid production in semicontinuous fermentation. Proceedings of the National Academy of Sciences 114: https://doi.org/10.1073/pnas.1703321114
Zainuddin MF, Fai CK, Ariff AB, et al (2021) Current pretreatment/cell disruption and extraction methods used to improve intracellular lipid recovery from oleaginous yeasts. Microorganisms 9: https://doi.org/10.3390/microorganisms9020251
Bezergianni S (2013) Catalytic hydroprocessing of liquid biomass for biofuels production. In: Fang Z (ed) Liquid, Gaseous and Solid Biofuels. IntechOpen, Rijeka
Atsonios K, Panopoulos KD, Nikolopoulos N et al (2018) Integration of hydroprocessing modeling of bio-liquids into flowsheeting design tools for biofuels production. Fuel Process Technol 171:148–161. https://doi.org/10.1016/j.fuproc.2017.11.009
Guzman A, Torres JE, Prada LP, Nuñez ML (2010) Hydroprocessing of crude palm oil at pilot plant scale. Catal Today 156:38–43. https://doi.org/10.1016/j.cattod.2009.11.015
Szargut J (2005) Exergy method: technical and ecological applications. WIT Press, UK
Zaleta-Aguilar A, Correas-Uson L, Kubiak-Szyszka J, Sierra-Espinosa FZ (2007) Concept on thermoeconomic evaluation of steam turbines. Appl Therm Eng 27:457–466. https://doi.org/10.1016/j.applthermaleng.2006.06.018
Kotas TJ (1985) The exergy method of thermal plant analysis. Elsevier
Szargut J, Morris DR, Steward FR (1987) Exergy analysis of thermal, chemical, and metallurgical processes. Hemisphere, United States
Pearlson M, Wollersheim C, Hileman J (2013) A techno-economic review of hydroprocessed renewable esters and fatty acids for jet fuel production. Biofuels, Bioprod Biorefin 7:89–96. https://doi.org/10.1002/bbb.1378
Diederichs GW, Ali Mandegari M, Farzad S, Görgens JF (2016) Techno-economic comparison of biojet fuel production from lignocellulose, vegetable oil and sugar cane juice. Bioresour Technol 216:331–339. https://doi.org/10.1016/j.biortech.2016.05.090
Vela-García N, Bolonio D, García-Martínez M-J et al (2021) Biojet fuel production from oleaginous crop residues: thermoeconomic, life cycle and flight performance analysis. Energy Convers Manag 244:114534. https://doi.org/10.1016/j.enconman.2021.114534
Leibbrandt NH, Aboyade AO, Knoetze JH, Görgens JF (2013) Process efficiency of biofuel production via gasification and Fischer-Tropsch synthesis. Fuel 109:484–492. https://doi.org/10.1016/j.fuel.2013.03.013
Doliente SS, Narayan A, Tapia JFD, et al (2020) Bio-aviation fuel: a comprehensive review and analysis of the supply chain components. Front Energy Res 8: https://doi.org/10.3389/fenrg.2020.00110
Funding
Open access funding provided by HEAL-Link Greece. This research was performed as part of the project “BioSFerA” (https://biosfera-project.eu) funded by the European Union’s Horizon 2020 research and innovation program (Grant Agreement 884208).
Author information
Authors and Affiliations
Contributions
Nikolaos Detsios: original draft preparation, methodology, software. Leda Maragoudaki: methodology, writing. Konstantinos Atsonios: conceptualization, review, and editing. Panagiotis Grammelis: project administration. Nikolaos G. Orfanoudakis: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Detsios, N., Maragoudaki, L., Atsonios, K. et al. Design considerations of an integrated thermochemical/biochemical route for aviation and maritime biofuel production. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03754-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03754-4